Abstract
Vaccinia virus early gene transcription requires the vaccinia termination factor, VTF, nucleoside triphosphate phosphohydrolase I, NPH I, ATP, the virion RNA polymerase, and the motif, UUUUUNU, in the nascent RNA, found within 30 to 50 bases from the poly A addition site, in vivo. In this study, the relationships among the vaccinia early gene transcription termination efficiency, termination motif specificity, and the elongation rate were investigated. A low transcription elongation rate maximizes termination efficiency and minimizes specificity for the UUUUUNU motif. Positioning the termination motif over a 63 base area upstream from the RNA polymerase allowed efficient transcript release, demonstrating a remarkable plasticity in the transcription termination complex. Efficient transcript release was observed during ongoing transcription, independent of VTF or UUUUUNU, but requiring both NPH I and either ATP or dATP. This argues for a two step model: the specifying step, requiring both VTF and UUUUUNU, and the energy-dependent step employing NPH I and ATP. Evaluation of NPH I mutants for the ability to stimulate transcription elongation demonstrated that ATPase activity and a stable interaction between NPH I and the Rap94 subunit of the viral RNA polymerase are required. These observations demonstrate that NPH I is a component of the elongating RNA polymerase, which is catalytically active during transcription elongation.
Keywords: Vaccinia, transcription termination, transcript release, RNA polymerase
Background
Vaccinia virus, a member of the poxvirus family, contains a linear double stranded covalently closed DNA genome that encodes about 200 genes (Moss, 2007). Vaccinia genes can be divided into three transcriptional classes that differ based on their promoter sequences, transcription initiation and termination factors and on the conformation of the template employed (Broyles, 2003). Early genes are transcribed in the virion core, which is deposited in the cytoplasm immediately after infection. Intermediate and late genes are transcribed on replicating DNA templates. The multi-subunit RNA polymerase, transcription initiation, elongation and termination factors, and RNA processing enzymes are all encoded by the virus.
Early genes are the only class that exhibit sequence dependent transcription termination (Rohrmann et al. 1986; Condit et al, 1996). Early mRNA possesses the sequence motif UUUUUNU that is located 30 to 50 bases upstream from the site of poly A addition (Yuen and Moss, 1986). Mutations in this sequence reduce or eliminate transcription termination (Yuen and Moss, 1987). Chemical modification of the base, the 2’ position of the sugar or the phosphodiester bond impedes termination, as well, demonstrating the remarkable specificity for a U-rich sequence (Shuman and Moss, 1988; 1989; Deng and Shuman, 1997a; Mohamed et al, 2006). UV photo crosslinking studies demonstrated that the UUUUUNU motif is capable of interacting with both Rap94 (Christen et al, 2008), an early gene specific RNA polymerase subunit that is required for both early gene transcription initiation (Ahn et al, 1994; Kane and Shuman, 1992; Ahn and Moss, 1992; Deng and Shuman, 1994) and termination (Mohamed and Niles, 2000, 2001; Mohamed et al, 2002), and with VTF, the vaccinia virus early gene transcription termination factor (Hagler et al, 1994;Christen et al, 2008). VTF is a heterodimeric enzyme that also catalyzes the initial three steps in mRNA cap formation (Ensinger et al, 1975; Venkatesan et al, 1980) and serves as an essential intermediate gene transcription initiation factor (Vos et al, 1991). Early gene termination requires ATP hydrolysis (Shuman et al, 1987; Hagler et al, 1994), which is catalyzed by nucleoside triphosphate phosphohydrolase I, NPH I, a ssDNA dependent ATPase, that possesses motifs commonly associated with superfamily II RNA helicases (Christen et al, 1998; Deng and Shuman, 1998). An interaction between the C-terminal end of NPH I and the N-terminal end of Rap94 is required for transcription termination (Mohamed and Niles, 2001; Piacente et al, 2003). At low UTP levels, NPH I is able to stimulate transcription elongation through A-rich sequences in the template strand, indicating that NPH I also serves as a transcription elongation factor (Deng and Shuman, 1998). It is clear, however, that NPH I is not essential for elongation, in an in vitro assay (Christen et al, 1998). The termination event is also linked to the concentration of NTPs in the transcription reaction, exhibiting a positive correlation between the length of the terminated transcript and the NTP levels (Hagler et al. 1994; Deng and Shuman, 1997b). Thus, it was noted that vaccinia early gene transcription termination is kinetically coupled to transcription elongation.
The specificity of the transcription termination motif was evaluated by mutagenesis revealing a range of activities permitting the definition of the minimal sequence UUUUUNU (Yuen and Moss, 1987). In this report, the efficiency of transcription termination, mediated by both wild type and various mutant termination motifs, is shown to be inversely proportional to the transcription elongation rate. Furthermore, during transcription, RNA is readily released in a process that requires NPH I and either ATP or dATP and is independent of either added VTF or the UUUUUNU motif. Thus, the transcription termination pathway can be separated into two stages, a specifying step that requires VTF and the termination motif, UUUUUNU, and a release step that employs only NPH I and ATP.
Results
Measurement of early gene transcription termination and transcript release
Early gene transcription termination was measured in vitro using a linear bead-bound DNA template, Ter29 kas, modified from Ter29, described by Deng et al (1996), Fig. 1A. Ter29 kas contains a strong early promoter (P) that directs Step 1 transcription through a 20 base pair G-less cassette in the absence of GTP and the presence of 3’ O methyl GTP, forming a stable ternary complex containing a 21 nucleotide RNA, G21. Beads can be collected, washed and resuspended for a second transcription reaction, Step 2. If Step 2 transcription is carried out in the absence of ATP, the ternary complex moves to the end of the A-less cassette, generating a 77 base RNA, C77. If Step 2 transcription is conducted in the presence of all four NTPs and VTF, two products are formed: RT, a 189 nucleotide read through transcript, and Term, a 70 to 75 nucleotide RNA that is produced by transcription termination (Rohrmann et al., 1986). Efficient termination requires the addition of VTF, the vaccinia termination factor (Shuman et al, 1987). The results of a set of transcription assays are presented in Figs. 1B to E. A template which produces nascent RNA containing the wild type U9 motif was employed in Fig. 1B. Lane 1 contains the G21 RNA product of Step 1 transcription. The next two pairs of lanes contain RNA synthesized at either 50 µM or 1 mM UTP, CTP and GTP, plus 5 mM ATP, in the absence or presence of VTF. In lane 2, the prominent read through transcript, RT, is observed, in addition to a series shorter RNA products. The major truncated RNA, U9P, is formed by the slow elongation through the A9 stretch in the template strand of Ter29 kas (Hagler et al, 1994: Deng and Shuman, 1997b). When VTF is added, there is a reduction in the amount of RT formed and the appearance of the transcription termination product, Term. Note also that the length of the U9P product is increased in the presence of VTF indicating enhanced elongation in its presence. At 1 mM GTP, CTP, UTP and 5 mM ATP, the amount of U9P is reduced, lanes 4 and 5. Upon addition of VTF, transcription termination is readily apparent. Note that at the higher NTPs the length of the Term products is increased in relation to what is synthesized at 50 µM NTP. This is a reflection of the kinetic coupling previously described (Hagler et al, 1994) . Figs. 1 C,D show the results obtained when templates that direct the synthesis of RNA harboring mutant termination motifs are tested. In each case, a low level of VTF dependent transcription termination can be observed when transcription is conducted at 50 µM GTP, CTP, UTP and 5 mM ATP, lanes 7, 8 and 12, 13. Note that the U9P band is absent in these samples, due to the fact that the A9 sequence in the template strand has been altered. When transcription is conducted at 1 mM GTP, CTP, UTP and 5 mM ATP, there is little evidence for VTF dependent transcription termination. This observation extends to a more dramatically altered termination motif M29 mutation shown in Fig. 1E. Thus, the specificity of transcription termination appears to be directly related to the concentration of NTPs in the assay mix.
Figure 1. A schematic representing the Ter29 bead-bound template and possible transcription products is presented.


A. The thick line at the bottom represents the bead bound Ter29 kas template. The horizontal solid arrow signifies the early promoter (P). The dashed arrow is the position of the U9 termination motif (T). The positions of the G-less and A-less cassettes are demarked by vertical lines. K is the position of a Kas I recognition site. Step 1 transcription through the G-less cassette yields the G21 RNA. Elongation in Step 2 in the absence of ATP permits synthesis of the 77 base RNA which extends to the end of the A-less cassette. Elongation of G21 RNA in Step 2 in the presence of all four NTPs yields either the 189 base read through product (RT) or the product of transcription termination, Term, which measures about 70 to 75 bases in length. U9P represents a group of pause products formed during transcription through an oligo A sequence in the template strand.
1B to E. The transcription termination efficiency was measured on four templates that differ in the sequence of the termination motif. For panels B to D the migration position of the G21 Step 1 transcript is in the left lane: lanes 1, 6, 11. In each case, transcription was measured at 50 µM GTP, CTP, UTP and 5 mM ATP, in the absence or presence of 2 pmoles VTF: lanes 2, 3, 7, 8, 12, 13, or at 1 mM GTP, CTP, UTP and 5 mM ATP in the absence or presence of VTF: lanes 4, 5, 9, 10, 14, 15. E. Transcription termination was measured at 1 mM GTP, CTP, UTP and 5 mM ATP, using either a template with the wild type U9 termination motif, lanes 16, 17 or the M29 termination motif, lanes 18,19.
1F. Transcript release of ternary complexes containing either U9 or mutant termination motifs was evaluated in the absence or presence of 5 mM ATP and 2 pmoles of VTF. The percentage of released RNA is noted below each panel, which represents the average of two determinations.
To measure transcript release, bead-bound ternary complexes were formed by Step 2 transcription through the A-less cassette, in the absence of VTF and ATP. This yields ternary complexes that contain nascent RNA with the wild type or mutant U9 termination motif outside of the RNA polymerase, available to interact with Rap94 and VTF (Christen et al, 2008). The release efficiency for ternary complexes that contain transcripts possessing either wild type or mutant termination motifs was compared. Release was evaluated by incubating the ternary complexes in the absence or presence of ATP and VTF. Subsequent to the incubation, bead-bound and free transcripts were separated, purified and quantified after separation by gel electrophoresis. The results presented in the top panel of Fig. 1F demonstrate that efficient release of RNA that contains the wild type U9 motif requires both ATP and VTF. The low level of release observed in the presence of ATP alone reflects a low level VTF that remains after washing the Step 1 ternary complexes during the preparation of the release substrates. When release assays were conducted on ternary complexes containing mutant termination motifs, little VTF dependent release was observed. These results confirm that for the standard release assay, the U9 termination motif, VTF and ATP or dATP are required. (Deng et al, 1996).
Termination efficiency is inversely related to the transcription elongation rate
To extend these observations, the concentration of CTP, GTP and UTP (NTP) present in the Step 2 elongation reaction was varied, while ATP, required for termination/release, was kept constant at 5 mM. Assays were conducted in the presence of 2 pmoles VTF. Step 2 transcripts were resolved by gel electrophoresis and their size distribution was evaluated. In Fig. 2, panel A, transcripts produced from a template containing an A9 sequence in the template strand are displayed. On the left is the migration position of the Step 1 transcript, G21 RNA. The following lanes contain transcripts synthesized at different NTP levels indicated on the top of each lane. At low NTPs, one observes substantial level of U9P, as well as the termination product. Little read through transcript in seen demonstrating near complete termination. As the level of NTPs is increased the U9P is diminished and the read through transcript (RT) reappears, as the termination efficiency falls. Again, one can observe a minor lengthening of the termination product as the NTP level is increased. At higher NTP levels, termination reaches a minimum in efficiency. For each mutant template, Fig. 2 B to 2 D, significant transcription termination is observed at low NTPs. Termination efficiency is reduced as the NTP levels are increased, falling to a background level. Again, note that the U9P is not produced on each mutant template. These results were quantified and are presented as the percent transcription termination efficiency observed at each NTP level tested, Fig. 2E. For each template, the termination efficiency decreases to a plateau as the concentration of NTPs is increased.
Figure 2. Transcription termination measured at different Step 2 NTP levels on templates that possess either a wild type U9 motif or a mutant motif.


A to D. Transcription termination assays were conducted using a set of bead-bound templates that harbor either a wild type termination sequence or one of three mutant sequences. Step 2 transcription was carried out in the presence of 2 pmoles of VTF, at different levels of CTP, GTP and UTP, with 5 mM ATP. RT, read through transcript; Term, the product of transcription termination; U9P, a collection of transcripts that result from pausing in the T9 stretch found in the A-less region of the Ter29 template; G21, the product of Step 1 transcription; s, Step 1. The sequences of the wild type and mutant termination motifs are presented at the top of each panel. D is the M29 mutant sequence.
Figure 2E. The efficiency of transcription termination was calculated for each lane in A to D by dividing the radioactivity in the termination product by the sum of the counts in the read through transcript and Term. The results from two independent sets of assays were averaged and were plotted as the % termination versus the concentration of CTP, GTP and UTP in the Step 2 elongation reaction. WT U9 (△); AAUUAUUAU (○); GGUUGUUGU (▲); CGUGUGUUC (▽). Step 2 transcription elongation was measured at different levels of GTP, CTP, UTP and 5 mM ATP, in the absence of VTF using the bead-bound M29 template (GCUGUGUUC). The elongation rate (□) was measured by dividing the length of the longest RNA product synthesized at times after initiating Step 2 synthesis by the time of incubation. The average of two independent determinations is presented.
Measurement of the transcription elongation rate
To determine whether the reduced sequence specificity for transcription termination observed at low NTP levels is related to the transcription elongation rate, the rate of Step 2 transcription elongation was measured at each NTP concentration tested. 5 mM ATP was included in each elongation measurement. The M29 mutant bead-bound template was employed in order to eliminate the accumulation of the U9P transcript during elongation. Step 1 transcription was done in the absence of 3’ O methyl GTP, which reduced the length of Step 1 RNA to 20 bases. This was done to eliminate the need to hydrolyze the 3’ O methyl GMP from G21 RNA prior to elongation in Step 2 (Hagler and Shuman, 1993). Step 2 elongation was carried out at each NTP concentration. At times after initiating elongation of the isolated Step 1 ternary complex, samples were collected, the transcripts were isolated and separated by gel electrophoresis. The length of the longest extended product was measured by comparison to RNA size standards, the elongation rate was calculated and the results are included in Fig. 2E. The measured elongation rate in Step 2 transcription is dependent upon the NTP concentration, varying 10 fold over a concentrations from 10 µM to 500 µM. The maximum elongation rate observed at 1 mM NTP is 500 bases per minute or 8.3 bases per second. This rate is less than the 20 to 50 bases per second reported by Hagler and Shuman (1992) and 17 bases per second by Prins et al (2004). The difference may reflect the templates and the assay conditions employed in these analyses. None the less, these data demonstrate that at low NTP levels, the transcription elongation rate is inversely related to the termination efficiency seen for both wild type and mutant termination motifs.
Transcripts can be efficiently released during transcription in the absence of VTF
Two prior studies evaluated transcript release during Step 2 transcription. Deng and Shuman (1996) showed that U9P RNA remained template bound during the Step 2 RNA synthesis. In addition, Deng and Shuman (1997b) detailed the genesis of pause products produced during elongation at low NTP levels. They showed that the U9P pause products remained template bound. However, the latter studies were conducted at low levels of ATP, which would have precluded transcript release (Hagler et al, 1994). Furthermore, release was not assayed directly. However, the substantial accumulation of short RNA products observed during elongation at low NTP levels raised the possibility that the ternary complexes collected at the pause site may be unstable, thus releasing the short transcripts. For this reason, the efficiency of transcript release from ternary complexes present at the U9P site, the normal site of transcription termination and at the distal end of the linear template was evaluated, at different concentrations of CTP, GTP and UTP, plus 5 mM ATP, in the presence or absence of added VTF. Release was measured by separating free RNA from bead-bound ternary complexes during ongoing transcription. Transcripts synthesized on a wild type Ter29 kas template were compared to those synthesized on the M29 mutant template. In Fig. 3A, the results of the set of release assays derived using a wild type template are presented. On the left in the first lane is the migration position of the read through transcript marker, RT. The following lanes are in sets of four generated by transcription in different concentrations of GTP, CTP and UTP, plus 5 mM ATP. Each set contains pairs of bound (B) and free (F) transcripts synthesized in the absence or presence of VTF. The first set was prepared at low, 10 µM NTP. In the absence of VTF, one observes that little RNA remains in the bound lane, while 96% of the transcripts are released from the bead-bound template. The expected collection of transcripts: U9P, other minor pause products and RT, are in the free lane. When VTF is added, in the adjacent two lanes, again, one observes near complete release of the transcripts. As expected, the level of read through RNA is dramatically reduced while high levels of the termination product are found. Importantly, all transcripts are efficiently released during transcription at 10 µM NTP plus 5 mM ATP, whether VTF is present or absent. As the concentration of NTPs is increased one can see that the amount of U9P is reduced along with the other minor products yielding maximal levels of RT, in the absence of VTF. When VTF is added, termination results, and, as seen in Fig. 2, the termination efficiency is reduced at high NTPs. At all levels of the NTPs, the efficiency of release of all transcripts is high, 74% to 96%, and largely independent of added VTF.
Figure 3. Evaluation of transcript release during on going Step 2 transcription.

A,B. A series of transcription assays were conducted in the presence of 5 mM ATP, different levels of CTP, GTP and UTP, and the presence or absence of 2 pmoles of VTF. Bead-bound templates containing either the wild type U9 termination motif or the mutant M29 sequence were employed, A, B. At the end of the reaction, bead-bound ternary complexes were separated from free RNA and the transcripts were evaluated by autoradiography after separation by urea gel electrophoresis. A. Ter29 kas template with the wild type U9 motif. B. Ter29 kas template with the M29 mutant motif. RT, read through transcript; Term, the product of transcription termination; P1 and U9P, transcription pause products; B, bound RNA; F, free transcript. The percentage of released transcripts was calculated by dividing the density of the bands in the F lanes by the sum of the densities in the B and F lanes for each concentration of NTP. The average of two independent determinations is presented at the bottom of each panel.
Release of pause products does not require the UUUUUNU termination motif
The same analysis was carried out employing a template that contained the M29 termination motif mutation. Note that there are two prominent products, P1 and Term, in addition to RT, Fig. 3 A, B. The U9P transcript is absent. As the concentration of the NTPs is increased, the level of P1 and Term falls. In addition, P1, Term and RT were efficiently released in the presence or absence of added VTF. At each NTP level, transcripts are efficiently released from the ternary complex, 66% to 91%. Thus, during ongoing transcription, pause products or read through products can be efficiently released in the absence of added VTF or the termination motif U9.
Release of paused products requires either ATP or dATP
In the routine preparation of bead-bound ternary complexes to be employed in release assays, Step 2 transcription is conducted in the absence of ATP and the presence of the other three nucleoside triphosphates in order to translocate the ternary complex from the end of the G-less cassette to the end of the adjacent A-less cassette. This generates a nascent transcript with the termination motif positioned outside of the RNA polymerase and available to mediate transcript release in response to added factors (Deng et al, 1996). Importantly, the transcription-related release seen in Fig. 3 A,B was observed in the presence of 5 mM ATP. To determine whether release required added ATP the following analysis was conducted. Step 2 transcription was carried out employing either a wild type T9 template or the M29 mutant template, CTP, GTP and UTP, in the absence or presence of VTF and ATP or dATP. Bead-bound transcripts were separated from free RNA and the RNA was separated by gel electrophoresis. For the wild type T9 template, Fig. 4A, a 77 base transcript is observed, in lanes 1 and 2. C77 RNA is produced by elongation of the Step 1 G21 RNA in the absence of ATP to the end of the A-less cassette, Fig. 1A. This is similar in length but different from the normal termination product, Term. There is inefficient release of C77 RNA from the bead-bound ternary complex in the absence of added factors. The low level of free RNA may reflect incomplete separation of bound from free RNA. When ATP is included in Step 2 transcription, in the absence of VTF, lanes 3 and 4, primarily read through RNA is made due to elongation to the end of the linear DNA template in the presence of four NTPs, which is efficiently released from the end of the template. If ATP is replaced by dATP, lanes 5 and 6, again the C77 transcript predominates and release is highly efficient in the absence of added VTF. Minor elongation products longer than C77 are synthesized due to the presence of a low level of ATP, which contaminates commercially available ultra pure dATP. Addition of VTF alone does not enhance release, lanes 7 and 8, demonstrating the need for an energy source. If VTF is added in the presence of ATP or dATP, lanes 9 –12, both efficient transcription termination and an enhancement of the release of the termination products is seen. However, it is clear that release occurs independent of added VTF. Compare lanes 3 to 6 with 9 to 12 in Fig. 4A. Importantly, although release is observed, termination is not seen when ATP or dATP is added in the absence of VTF, lanes 3 to 6. In this assay, VTF appears to stimulate the release of the termination product as compared to release of either the C77 transcript or RT RNA. However, added VTF is not needed for efficient release of any transcript.
Figure 4. Release of paused, terminated or read through transcripts during Step 2 transcription does not require VTF or the U9 termination motif but does require ATP or dATP.

A,B. Either the wild type U9 motif or the M29 mutant motif containing bead-bound templates were employed. Step 2 transcription was carried out at 1 mM CTP, GTP and UTP in the absence or presence of VTF and either 1 mM dATP or 5 mM ATP. At the end of the reaction, bead-bound transcripts (B) were separated from free RNA (F) and analyzed by urea gel electrophoresis. RT, read through RNA; C77 the Step 2 RNA formed by extending the G21 transcript in the absence of ATP to the end of the A-less cassette. Termination products (Term) are also observed in lanes 10 and 12, which co-migrate with C77 RNA.
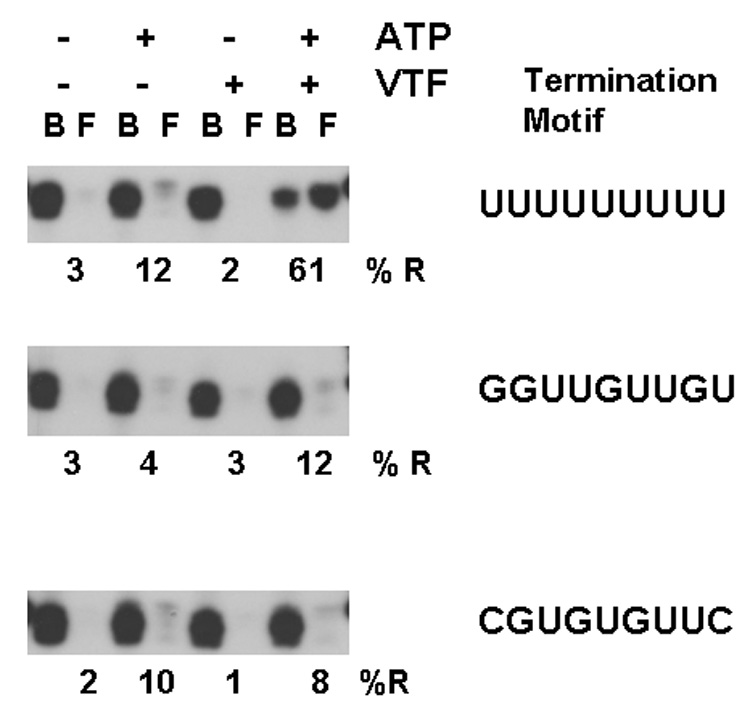
Similar results were observed when the M29 mutant template was employed. In the absence of added ATP or dATP release is inefficient, Fig. 4B, lanes 1 and 2. Addition of either ATP or dATP dramatically enhances release of transcripts paused on the template or bound at the end of the template, lanes 3 to 6. Added VTF has minimal influence on the release efficiency, lanes 7 to 12. Clearly, transcript release observed during ongoing transcription at 5 mM ATP is independent of the U9 termination motif. Thus, these results demonstrate that during ongoing transcription of a linear bead-bound template, transcript release of paused, stalled or terminal ternary complexes is dependent upon ATP or dATP, but independent of the U9 termination motif or the addition of VTF.
NPH I is required for ATP-dependent transcript release
NPH I is an essential early gene transcription termination factor that provides energy needed for transcript release through the hydrolysis of ATP or dATP (Deng and Shuman, 1998; Christen et al, 1998). Thus it was of interest to determine whether the ATP dependent step, essential for release of transcripts during transcription elongation, employs NPH I. Cts50 is a temperature sensitive mutation that maps to gene D11L, which encodes NPH I (Condit et al, 1983; Seto et al, 1987; Rodriguez et al, 1986; Broyles and Moss, 1987). Extracts prepared from Cts50 virus infected cells remain competent for early gene transcription yet fail to terminate due to their loss of active NPH I (Christen et al, 1998). Addition of NPH I restores termination activity, in vitro. To determine whether NPH I is involved with release of products formed during transcription, in vitro, transcript release activity of Cts50 virus infected cell extracts was evaluated in the absence and presence of NPH I. These results were compared to wild type virus extracts, in Fig. 5 A,B. Step 2 transcription was carried out in the presence of 1 mM GTP, CTP, UTP and the presence or absence of dATP, VTF and NPH I. C50 extracts lacking NPH I were evaluated and the results presented in Fig. 5A. Lanes 1 and 2 demonstrate little release in the absence of added factors. If dATP is added, lanes 3 and 4, a modest increase in release is seen, likely due to the incomplete elimination of NPH I activity. Addition of VTF or NPH I alone does not stimulate release, lanes 5 to 8. If both VTF and dATP are added, there is a measurable stimulation of release, lanes 9 and 10, again, likely due to the NPH I that remains. However, NPH I plus dATP addition results in a significant stimulation of release, lanes 11 and 12. Addition of VTF plus NPHI has no affect, lanes 13 and 14. However, release is restored by addition of VTF and NPH I in the presence of dATP, lanes 15 and 16. A comparison to a wild type virus extract, Fig. 5B, supports the conclusion that in addition to ATP or dATP, NPH I is required for efficient release of nascent transcripts from ternary complexes formed at the end of the A-less cassette.
Figure 5. Release of transcripts during Step 2 transcription requires NPH I.

A,B. A. Extracts were prepared from tsC50 virus infected cells under non-permissive conditions that yield a transcription competent extract reduced in NPH I activity. B. Partially purified wild type virus extracts were used. Transcription experiments employed bead-bound Ter29 kas DNA containing the wild type U9 termination motif. Step 2 transcription was carried out in the presence of 1 mM CTP, GTP and UTP and in the absence or presence of 1 mM dATP, 2 pmoles VTF and 0.1 pmole NPH I. Samples were analyzed as described in Figs. 4 A,B.
Transcription related release requires a ternary complex with an exposed nascent transcript
Formation of a bead bound ternary complex at the end of the A-less cassette used in transcript release assays (Deng et al, 1996) is done in the absence of ATP in Step 2. This explains why it is possible to efficiently form such a complex at the end of the A-less cassette. However, stable ternary complexes are efficiently formed at the end of the G-less cassette in a Step 1 transcription reaction where 1 mM ATP is present when the Ter29 template is employed. In this case, the nascent RNA is bound to the RNA polymerase and only a few bases at the 5’ end of the transcript are exposed in the ternary complex (Hagler et al, 1994). To determine whether the VTF and UUUUUNU independent transcript release is influenced by the length of the exposed transcript in the ternary complex a series of templates were prepared that possess G-less cassettes of different length. This permitted the synthesis of ternary complexes that harbor nascent transcripts of different lengths which lack the termination motif. Step 1 transcription was carried out in the presence of 1 mM ATP, CTP, 1 µM UTP, 5 µCi α32P UTP and 0.25 mM 3’ O methyl GTP, in the presence or absence of VTF. At the end of the reaction bead-bound and free RNA were isolated and transcript release was quantified. In Fig. 6, six sets of release assays are presented. Each set of assays was conducted on bead-bound ternary complexes that synthesize nascent RNA that vary in length from 21 bases to 76 bases. Inefficient release of the G21 transcript from the Ter29 ternary complex is seen confirming that it is possible to generate efficiently a stable ternary complex using this template. The Ter44 template, which harbors a 36 base transcript, is also quite stable with little evidence of transcript release. However, as the length of the nascent transcript is increased from 46 nucleotides to 76 nucleotides, efficient release is observed. Therefore, in order to observe efficient release during Step 1 transcription through a G-less cassette a transcript of greater than 36 bases must be in the transcribing complex. Since VTF is present in the partially purified virus extract during Step 1 transcription this experiment does not determine whether VTF is involved with the RNA length dependent transcript release. However, the length dependence is reminiscent of the observations of Hagler et al. (1994) who showed that ternary complexes that possessed a nascent RNA 51 bases in length but not complexes with a 31 nucleotide RNA formed stable complexes with VTF.
Figure 6. Release of transcripts from Step 1 ternary complexes containing RNA of different lengths.

A series of templates that possess G-less cassettes of different length were employed to generate ternary complexes that contain RNA of different lengths. The templates are identified by the position of the first U of the normal termination motif in comparison to Ter29. The length of the bound transcript is listed below. Transcript release was evaluated in the presence of 1 mM ATP, CTP, UTP, 0.25 mM 3’ O methyl GTP and the presence or absence of 2 pmoles of VTF. The percentage of released RNA listed below each pair of lanes is the average of three determinations.
Effect of the location of the U9 termination motif in relation to the RNA pol in the ternary complex on transcript release efficiency
The position of the termination event, as reflected by the length of the termination product, is influenced by the concentration of NTPs during Step 2 transcription (Hagler et al, 1994). It appears that the maximum length of the terminated transcript is obtained by 500 µM NTP, Fig. 2E, which coincides with the maximum transcription rate of 500 bases per minute. This suggests that if the U9 termination motif is positioned too far upstream from the RNA polymerase an effective termination complex can not be generated and read through will result. The location of the U9 motif in viral mRNA varies from 30 to 50 bases upstream from the site of poly A addition (Yuen and Moss, 1986). This suggests that there is an approximately 3 second window for a termination complex to assemble and to function on the elongating RNA polymerase. In order to attempt to determine the effect of the position of the U9 motif in relation to the RNA polymerase in the ternary complex on transcript release, a series of templates were generated in which the location of the T9 sequence in the A-less cassette was varied, Fig. 7A. Stable ternary complexes were prepared by conducting Step 2 transcription elongation reactions in the absence of ATP and VTF. Ternary complexes prepared on each template were employed in transcript release assays. Release was measured in the absence of added factors, or the presence of ATP and VTF. Apparent release observed in the absence of added factors (6% average) was subtracted from the other values and results were plotted as percent released in the presence of ATP and VTF, Fig. 7B. Positions of the U9 motif are defined by the distance from the 3’ nucleotide of the U9 termination motif to the 3’ end of the transcript in each ternary complex. Template 41 is the normal Ter29 kas template employed in Fig. 1 to Fig. 5. Template 41 exhibits more than 40% release when VTF and ATP are present. These values are within the expected range and demonstrate a requirement for VTF and ATP to observe efficient transcript release from an isolated ternary complex. In comparison, release is substantially reduced in M29 mutant, Fig. 7B, Mut 41. These results simply confirm the often described dependence of transcript release from a bead-bound stalled ternary complex, which requires both an intact termination motif in the nascent RNA in addition to VTF, Rap94, NPH I and ATP (Deng et al., 1996; Deng and Shuman, 1998; Mohamed and Niles, 2001). Movement of the termination signal downstream, on template 28, to a position 13 bases closer to the RNA polymerase, exhibits a wild type level of release upon addition of VTF and ATP. However, positioning the U9 motif closer to the RNA polymerase in templates 21 and 11 dramatically reduces transcript release. It is likely that the termination motif is at least partially occluded by its close proximity to the RNA polymerase and its ability to interact productively with VTF and Rap94 is impaired. Importantly, positioning the U9 motif 10 to 20 bases further upstream in templates 51 and 61, enhances VTF dependent release. However, locating the termination motif an additional 30 base pairs upstream, template 91, reduces release efficiency by about 50% in comparison to template 41. Nonetheless, this value is significantly above that observed for the mutant template. Thus, transcript release efficiency exhibits a measurable dependence upon the position of the U9 motif in relation to the RNA polymerase in the isolated ternary complex. However, efficient transcript release is observed with the termination motif positioned over a broad range of at least 63 bases. This demonstrates a remarkable plasticity in the termination mechanism.
Figure 7. Effect of the U9 location on transcript release.


A,B. A. A cartoon is presented that depicts the location of the wild type U9 termination motif in a series of ternary complexes generated by Step 2 transcription of templates that possess the T9 termination sequence in different positions. The positions of the termination motifs are denoted by a solid bar. The number above or below each bar is the name of the Ter29 kas derivative employed to generate the transcript. The number also defines the distance in bases from the 3’ nucleotide of the U9 sequence to the 3’ end of the transcript, C77, in the ternary complex. Template 91 is not shown is this drawing but the U9 motif would map an additional 30 bases to the left of template 61. The position of NPH I is based on its known interaction with Rap94 and on the possibility that it may interact with the non-template strand of the DNA template. Rap94 is the known early gene transcription specific RNA polymerase subunit. VTF is the vaccinia termination factor that is known to interact with the U9 termination motif.
B. The efficiency of transcript release was measured using ternary complexes formed at the end of the A-less cassette on a series of templates that would position the U9 termination motif at different distances from the RNA polymerase in the isolated ternary complexes. The results presented are the average of two independent determinations. Release was measured in the absence or presence of 5 mM ATP and 2 pmoles VTF. The low background value (average 6%) observed in the absence of added ATP or VTF was subtracted from the values obtained in their presence. Filled bars represent release seen in the presence 5 mM ATP and 2 pmoles VTF.
The position of the U9 motif in the nascent mRNA influences its ability to interact with Rap94 and with VTF in isolated ternary complexes
UV photo crosslinking of nascent Step 2 labeled RNA in the isolated ternary complex identifies several proteins that can be linked to the RNA (Hagler et al, 1994; Christen et al, 2008). RNA was shown to crosslink to the large 146 kDa and 133 kDa RNA polymerase subunits, and to a small RNA polymerase subunit, Rpo18. In addition, nascent RNA crosslinks to both the Rap94 subunit of the RNA polymerase and to VTF, both essential transcription termination factors. Further analyses showed that the 146 kDa subunit harbors the RNA product channel and crosslinks to the very 3’ terminal region of the transcript in the ternary complex. The minor crosslinking to the 133 kDa and 18 kDa polymerase subunits appears to be in the region of the U9 motif. Both VTF subunits and Rap94 crosslink to the U9 motif. The data support a model which posits that interactions among the U9 motif in the nascent RNA, VTF and Rap94 represent the initial steps in the termination pathway.
Ternary complexes were prepared in the presence or absence of VTF, on the set of templates described in Fig. 7A in which the U9 termination motif is located at different positions in the ternary complex in relation to the RNA polymerase. The amount and the integrity of the RNA in each ternary complex is shown Fig. 8C. Templates 51 and 61 produce products that are 10 and 20 bases longer, respectively, resulting in the reduced mobility of the RNA. Bead-bound ternary complexes were treated with UV light and after detergent disruption and nuclease treatment the proteins were separated by gel electrophoresis and observed by autoradiography. Fig. 8A presents the results for a series of cross linking analyses testing Step 1 salt washed ternary complexes radiolabeled in Step 2, in the absence of added VTF. Template 41 corresponds to the wild type Ter29 kas template. The slowest migrating band was shown previously to contain the two largest RNA polymerase subunits (Christen et al, 2008). In the absence of added VTF, the more rapidly migrating component is mostly Rap94, but a portion of the band is the large D1 subunit of VTF, which was not removed entirely by salt washes. In comparison, cross linking to proteins in a ternary complex prepared on a template that contains the M29 mutant termination motif is reduced, M, Fig. 8A, lane 2. The decrease in cross linking is due in part to a reduction in the number of U residues in the mutant termination motif, reducing both the cross linking efficiency and the number of radioactive phosphates present in the cross linked product. Relative to the large RNA polymerase subunits, there is a substantial reduction in crosslinking to the Rap94/VTF-D1 band in lane 2. When the U9 motif is moved farther upstream, templates 51 and 61, a pattern is observed that is similar to that seen with template 41, Fig. 8A. As the U9 motif is moved downstream, closer to the RNA polymerase, template 28, the Rap94/VTF-D1 band becomes fuzzy giving the appearance of reduced cross linking. It will be seen below that upon immunoprecipitation with antibodies directed against the VTF-D1 subunit that the crosslinked band is more diffuse, perhaps due to multiple conformations in this cross linked product. The four templates, 41, 28, 51, 61, which exhibit high efficiency transcript release, demonstrate similar relative levels of cross linking to Rap94/VTF-D1 in comparison to the large RNA polymerase subunits. In ternary complexes in which the U9 motif is moved close to the RNA polymerase, templates 21 and 11, cross linking to the large RNA polymerase subunits is enhanced relative to Rap94 and VTF-D1, Fig. 8A. This is likely due to the proximity of the U9 motif to the RNA polymerase in the ternary complex. Crosslinking to the Rap94/VTF-D1 band in template 11 is reduced in relation to crosslinking to the large RNA polymerase subunits. This may be related to the reduced transcript release exhibited by this template.
Figure 8. UV photocrosslinking of nascent RNA radiolabeled in Step 2 transcription of templates with termination motifs located at different distances from the RNA polymerase in the isolated ternary complex.

A,B,C. Isolated ternary complexes were treated with UV light in the absence (A) or presence (B) of 2 pmoles of VTF. After nuclease treatment and denaturation with SDS samples were separated by denaturing gel electrophoresis and observed by autoradiography. C. Gel electrophoresis of the transcripts synthesized on each template is presented.
When VTF is added to the isolated Step 2 labeled ternary complexes prior to treatment with UV light one observes enhanced photo cross linking of the VTF-D1 subunit and the appearance of a low level of cross linking to the small VTF-D12 subunit, when template 41 is considered, Fig. 8B. There is a reduction in cross linking to the RNA synthesized on the M29 mutant template, consistent with the demonstrated interaction between VTF and the U9 motif (Christen et al, 2008). Again, for templates that exhibit efficient transcript release in the presence of VTF, templates 41, 28, 51, 61, similar high levels of cross linking to the two VTF subunits relative to the large RNA polymerase subunits are observed. Template 28 exhibits a disperse Rap94/VTF-D1 cross linked product, as well as a VTF-D12 subunit of altered mobility. For template 11, which exhibits little VTF dependent transcript release, one observes reduced cross linking to the VTF-D1 subunit relative to the large RNA polymerase subunits. Notably, no cross linking to the D12 subunit is seen with ternary complexes prepared on this template. The RNA synthesized on template 21 represents an interesting contrast. In this case, RNA exhibits strong cross linking to both VTF subunits in the absence or presence of added VTF, yet transcript release is inefficient with this template. These data indicate that an interaction between VTF with the U9 sequence alone is not sufficient to stimulate transcript release.
In order to ensure the identity of the cross linked proteins, a series of immunoprecipitation analyses were conducted. Ternary complexes were prepared on each template in the absence or presence of added VTF. After UV irradiation and nuclease treatment, the cross linked ternary complexes were disrupted in SDS and the proteins were collected after precipitation with polyclonal antibodies. For template 41, Fig. 9A, one observes the expected 146 kDa subunit of the RNA polymerase along with Rap94 and the large VTF subunit, D1. When VTF is added prior to UV light treatment there is an enhancement in the amount of the cross linked VTF D1 subunit and also the appearance of the VTF D12 subunit. In the case of M29 mutant template, Fig. 9B, there is a reduction in the radioactivity in both Rap94 and the VTF large subunit in relation to the 146 kDa RNA pol subunit. As shown previously, VTF and Rap94 cross link to both mutant and wild type termination motifs (Christen et al, 2008). For templates that exhibit efficient transcript release, templates 41, 28, 51, 61, there is a similar relative level of cross linking to both Rap94 and VTF. RNA prepared on template 11 exhibits reduced cross linking to both Rap94 and D1. However, addition of VTF results in enhanced cross linking to the VTF-D1 subunit confirming that although the U9 motif may be partially occluded by the RNA polymerase VTF is still able to form close, stable, yet non-functional contacts with the nascent transcript. No crosslinking was observed to the VTF D12 subunit on template 11. Template 21 derived RNA exhibits strong cross linking both in the absence and presence of added VTF in spite of the fact that template 21 exhibits reduced transcript release.
Figure 9. Immunoprecipitation of ternary complex proteins radiolabeled by UV photocrosslinking to Step 2 labeled RNA.

A to G. Ternary complexes were formed on a set of bead-bound templates as described in the legend to Fig. 7B. UV photocrosslinking was done as described in the legend to Fig. 8. After nuclease treatment and denaturation with SDS, individual proteins were immunoprecipitated by the addition of polyclonal antibodies and collected using protein A coated beads. The precipitated samples were washed, denatured in SDS and separated by denaturing gel electrphoresis. Ternary complexes were treated with UV light in the absence or presence of 2 pmoles of VTF. P, 146 kDa RNA polymerase subunit; R, Rap94 RNA polymerase subunit; D1, large subunit of VTF, D12, small subunit of VTF.
NPH I is active during transcript elongation
Prior studies demonstrated that NPH I stimulates transcription elongation through the A9 stretch present in the template strand of Ter29, at low UTP levels (Deng and Shuman, 1998). The accumulation of pause products when transcription was conducted in presence of AMPPnP indicated that an ATPase may be involved in reducing transcription pausing (Hagler et al 1994). Heparin treatment of ternary complexes increased U9P levels pointing to NPH I as an elongation factor (Deng and Shuman, 1996) . NPH I is known to interact with the N-terminal region of the Rap94 subunit of the RNA polymerase through its C-terminal end (Mohamed and Niles, 2000). This interaction was shown to be required for transcript release and transcription termination, in vitro. The body of data indicates that NPH I is a component of the transcription elongation complex that is able to reduce transcription pausing as well as participate in transcript release. To directly evaluate the requirement for NPH I’s ATPase activity and Rap94 binding ability for stimulation of transcription elongation, the following experiments were conducted. In an initial study, the ability of NPH I to stimulate transcription elongation through the T9 sequence was retested. For these studies, an extract of Cts50 virus infected cells, which are transcription competent but lack NPH I activity, were employed (Christen et al, 1998). Step 2 transcription was conducted at 1 mM ATP, GTP, CTP and different levels of UTP, in the presence and absence of NPH I, Fig 10, A, B. In the absence of UTP, elongation of G21 RNA in Step 2 yields U9 pause products, Fig. 10A, lane 3. As the amount of UTP is raised, read through transcript is increased along with reduced U9P RNA, Fig. 10A, lanes 4 to 9. When NPH I is present during Step 2 transcription, Fig. 10B, two facts become apparent. First, at low UTP, RNA polymerase elongates through the T9 sequence producing longer U9 pause products not readily observed in the absence of NPH I. Second, the RT transcript appears at 0.5 µM UTP, Fig. 10B, lane 4, 5 fold lower than in the absence of NPH I, Fig. 10A, lane 6. This is a modest stimulation but fully consistent with prior observations of Deng and Shuman (1997b). The activity of NPH I mutant proteins that lack either ATPase activity or Rap94 binding were compared to the wild type NPH I, at 2 µM UTP, in the presence of VTF, Fig. 10 C, D, E. With wild type NPH I, efficient termination, rather than RT synthesis is seen as the level of NPH I is elevated. Addition of wild type NPH I also stimulates elongation of the U9P RNA. However, for NPH I mutants that lack either ATPase activity or the ability to bind to the Rap94 subunit of the RNA polymerase, not only is termination activity lost but the ability to stimulate transcription through the T9 sequence in the template strand is lost, Fig. 10 D, E. These results were quantified and presented graphically in Fig. 11 where the percentage of the U9 pause RNA is plotted verses the concentration of NPH I tested. For wild type NPH I there is a two fold reduction in the amount of the U9 pause RNA synthesized at 2 µM UTP. For the NPH I mutant that is truncated by 5 amino acids from the C-terminus there is no change in the amount of the U9 pause RNA demonstrating that the interaction between NPH I and Rap94 is required to stimulate elongation. Interestingly, for the ATPase− mutation, there is an increase in the U9 pause RNA indicating that this mutant is able to interfere with elongation, acting as a dominant negative mutation. Therefore, we can conclude that both the ability of NPH I to bind to the RNA polymerase and to catalyze ATP hydrolysis are necessary to stimulate transcript elongation. Importantly, we can also conclude that NPH I is active during transcription elongation, in vitro, even though this activity is only observed when the elongation rate is reduced. It is likely that the full value of NPH I in transcription elongation will be observed only in vivo, where early gene transcription takes place in the confines of the virus core and not on a short linear DNA template.
Figure 10. NPH I stimulates transcription elongation.

A to E. A, B. Step 2 transcription was conducted at 1 mM CTP, GTP, ATP and different UTP concentrations in the absence or presence of 1 pmole of NPH I. Lane 1, Step 1 G21 RNA; lane 2, Step 2 elongation in the presence of 1 mM NTPs, 2 pmoles of VTF; RT, read through transcript; Term, termination product; lane 3, transcription in the presence of 1 mM ATP, CTP and GTP. U9P, pause transcripts that map to the A9 sequence in the template strand; lanes 4 to 9, RNA synthesis in Step 2 at 1 mM CTP, GTP, ATP and different UTP concentrations. C to E. Step 2 transcription was carried out at 2 µM UTP, 1 mM ATP, CTP, GTP, 2 pmoles VTF and different concentrations of wild type NPH I (C) or the m2 mutation that lacks ATPase activity but binds to Rap94 (D), or a C-terminal deletion of 5 amino acids that retains ATPase activity but fails to bind to Rap94 (E) (Piacente et al, 2003).
Figure 11.

The results presented in Fig. 10C to E were quantified and the percentage of the U9P RNA was plotted versus the concentration of wild type or mutant NPH I. The results represent the average of three determinations. NPH I(◆); C-term △ NPH I (▲); ATPase - NPH I m2 (■)
Discussion
Prior studies demonstrated a link between the NTP concentration in an elongation reaction and the site of transcription termination (Hagler et al, 1994; Deng and Shuman, 1997b). As the concentration of NTPs was increased the length of the terminated transcript was also extended, thus linking the kinetics of elongation to the termination event. This observation was extended and refined through both a direct measurement of the in vitro elongation rate and an evaluation of the effect of the elongation rate on transcription termination efficiency and specificity. The maximum elongation rate observed in Step 2 transcription, at 1 mM NTPs, is 500 nucleotides per minute or 8.3 nucleotides per second. This compares favorably to that observed for the E. coli RNA polymerase (Schafer et al, 1991) and is one quarter to one half the rate observed by others for the vaccinia virus RNA polymerase (Hagler and Shuman, 1992; Prins et al, 2004), in vitro. The difference in the observed transcription elongation rate may be due to the specific assay conditions or to the template employed. Transcription termination efficiency was shown to be inversely correlated to the elongation rate at low NTP levels. As the elongation rate approached a maximum, the termination efficiency reached a minimum plateau. One interpretation relates the elongation rate to the physical constraints placed on the position of the UUUUUNU termination motif in the elongating transcription complex. UUUUUNU must emerge from the product channel in the RNA polymerase to be available for interaction with both VTF and the Rap94 subunit of the RNA polymerase. However, the termination motif in the nascent RNA must remain close enough to the RNA polymerase to remain capable of participating in the transcription termination sequence of events. The location of the motif in mRNA in vivo is limited to a range of 30 to 50 bases from the poly A addition site (Yuen and Moss, 1986), indicating that the permissible positions are limited. On the Ter 29 kas template, during Step 2 transcription, at the maximum elongation rate it would take about 3 seconds for the UUUUUNU motif to appear outside of the RNA polymerase and an additional 3 seconds for it to extend beyond the 50 nucleotide distance from the 3’ end of the RNA. Therefore, there is about a 2 to 3 second window for a transcription termination competent complex to form and to function, in vitro. Thus, if the elongation rate is reduced, the movement of the U9 motif away from the RNA polymerase is slowed providing more time to construct a capable termination complex and the termination efficiency is enhanced. As the maximal elongation rate is approached, the termination efficiency is reduced. This reduction is reflected by reaching a plateau in termination efficiency and also by generating extended terminated transcripts, Fig. 1 (Hagler et al, 1994; Deng and Shuman, 1997b).
Importantly, not only is termination efficiency affected by the elongation rate but termination specificity is altered. Base substitutions and chemical modifications are known to impede the function of the UUUUUNU termination motif (Yuen and Moss, 1987; Shuman and Moss, 1988; Deng and Shuman, 1997a, Mohamed et al, 2006). At the standard 1 mM NTP concentrations employed, most mutations reduce termination to background levels, in vitro. However, when the elongation rate is reduced, termination is observed for several variations of the basic UUUUUNU motif, Fig. 1, Fig. 2. At the lowest elongation rate measured, 50% termination was observed for three variant sequences. This is a remarkable decrease in sequence specificity. As the elongation rate was increased, termination efficiency falls dramatically and the expected specificity is restored. At a low elongation rate, the length of time available is increased either to recognize the termination motif or for the VTF/Rap94/U9 motif function to take place. As the elongation rate is increased time is reduced concomitant with a reduction in termination efficiency and the increase in motif sequence specificity. Thus, the UUUUUNU termination motif does not have to be stringently maintained if there is enough time for the proper termination complex to form and to act.
The physical relationship between the position of the U9 termination motif in the nascent transcript and the RNA polymerase was varied through the construction of a set of alternative templates. Measurement of transcript release efficiency was done in order to determine the boundaries of acceptable positioning of the UUUUUNU motif in the ternary complex. Efficient VTF dependent release was observed over a range extending from 28 bases to 91 bases from the 3’ end of the U9 motif to the 3’ end of the transcript. This is well outside of the anticipated 30 to 50 nucleotide location of the termination motif observed in mRNA. The difference may well reflect the static nature of the transcript release assay as opposed to the dynamic state of an elongating transcription complex. Alternatively, in virus cores, other viral proteins may interact with the nascent RNA thus impeding termination once the termination signal moves beyond the observed 50 bases. UV photo cross linking demonstrated that the efficiency of interaction among the UUUUUNU motif, VTF and Rap94 is similar for all locations that exhibit substantial transcript release, in vitro.
When the termination motif is positioned within 21 bases from the 3’ end of the RNA in the ternary complex, release is impeded. Cross linking studies demonstrate that the relative efficiency of cross linking of the nascent RNA to RNA polymerase subunits is elevated, which is likely to be due to increased proximity of the U9 signal to the RNA polymerase. However, both VTF and Rap94 remain able to interact with the nascent RNA even though the U9 signal may be partially occluded by the RNA polymerase. This may mean that beyond a simple interaction, the VTF/UUUUUNU/Rap94 must carry out a function in transcript release that is impaired when the termination signal lies too close to the RNA polymerase.
The relationship between elongation rate and transcript release during on going transcription was evaluated. Surprisingly, a high rate of release of paused transcripts or terminal transcripts was observed over the entire range of elongation rates tested. Furthermore, transcription-related release was found to be largely independent of either VTF or the presence of a wild type termination motif in the nascent RNA. However, release required both NPH I and an energy source. This observation lies in marked contrast to the widely recognized requirement for both VTF and the termination motif for release of nascent transcripts from bead-bound ternary complexes, Fig. 1F, Fig. 7B, (Shuman et al, 1987; Deng et al, 1996). The requirement for NPH I and ATP or dATP demonstrates that transcript release reflects a true termination event and is not simply due to random dissolution of ternary complexes.
Key to understanding this phenomenon is to differentiate elongating ternary complexes from static bead-bound ternary complexes. The latter are constructed by elongation through an A-less cassette in the absence of ATP. The paused complex that is formed can not release RNA since ATP or dATP is absent. These data demonstrate that if dATP is present in the elongation reaction when the equivalent ternary complex is formed, there is rapid and efficient transcript release, Fig. 3 to Fig. 6. Importantly, NPH I is also required for release when a pause occurs during transcription. The ability of NPH I to stimulate elongation requires both ATPase catalytic activity and the ability to interact with the Rap94 subunit of the RNA polymerase. Therefore, the most parsimonious conclusion is that NPH I is active normally during transcription elongation.
NPH I exists in two forms, the quiescent inactive conformation and the active form, which requires single stranded DNA binding for activity (Paoletti and Moss, 1974; Christen et al, 1999). NPH I is required for transcript release both from an elongating complex that encounters a pause in transcription and from an isolated bead bound complex. However, in the latter case a productive interaction between VTF and the U9 termination motif in the nascent transcript is also required. It is tempting to speculate that in the isolated ternary complex, NPH I activity is impaired and one function of VTF is to mediate its transition into its active form. The C-terminal region of NPH I binds to the N-terminal domain of Rap94 (Mohamed and Niles, 2000). Perhaps the interaction between VTF and Rap94 with UUUUUNU transmits an activating signal to NPH I.
These data argue for a two step termination pathway. One step requires only the presence of a pause in ongoing transcription, an energy source e.g. dATP or ATP and active NPH I. This reflects the second step in termination and is observed only when NPH I is in its active conformation. The first step employs the termination factors, VTF, Rap94 and the termination motif UUUUUNU, either in the nascent RNA or added in trans (Mohamed and Niles, 2003a,b), which is responsible for initiating the termination sequence of events.
Materials and Methods
Transcription assays
Ter29 is a chimeric gene that was constructed as a template for in vitro transcription termination and transcript release studies, Fig. 1A (Deng et al, 1996). A plasmid containing the Ter29 sequence was generously provided by Dr. Stewart Shuman of Memorial Sloan Kettering Cancer Center. Ter29 contains a strong early promoter that is followed by a 20 base pair G-less cassette, Fig. 1A. A 57 base pair A-less cassette containing a T9 stretch that starts at nucleotide 29 follows. An adjacent 112 base pair sequence terminates the linear DNA template. A GC rich sequence to the left of the T9 motif in Ter29 was changed into a Kas I recognition site GGCGCC in order to construct various derivatives of Ter29 for these studies. This is now referred to as Ter29 kas. Changes in the A-less cassette region of Ter29 kas were made by purchasing synthetic oligonucleotides that, when annealed, formed double stranded DNA fragments that possessed a Kas I overhang on the left end and a Xba I overhang on the right end. This fragment was ligated to a synthetic double stranded DNA fragment that contained the sequence derived from the Ter29 promoter and G-less cassette. The promoter containing fragment was flanked by a Sal I site on the left end and a Kas I site on the right. A three way ligation was conducted which joined the two DNA fragments to pGEM3Zf+ cut with Sal I and Xba I. The base sequence of all constructs was determined. A linear double stranded DNA fragment containing the Ter29 kas sequence was amplified by PCR using the M13 forward and reverse primers. One primer contained a 5’ biotin and the other primer was phosphorylated by T4 polynucleotide kinase using γ32PATP of known specific activity. After agarose gel purification of the PCR product and binding to strepavidin coated magnetic beads, the molar concentration of DNA was calculated. Transcription termination was assayed in the standard two step procedure as described (Hagler et al, 1994; Deng et al, 1996). Step 1 transcription was conducted in a 20 µl reaction at 30° C in a mixture containing: 20 mM Tris HCl, pH 8.0, 6 mM MgCl2, 2 mM dithiolthreitol, 8% glycerol, 1 mM ATP, 1 mM CTP 0.25 mM 3’ O methyl GTP, 5 µCi α32P UTP, 0.25 µl of virus extract and 100 fmoles of bead-bound template. Step 1 transcription through the 20 base pair G-less cassette yields a 21 base radiolabeled G21 RNA. Beads were collected with a magnet washed with 0.25 M potassium acetate followed by three washing steps with assay salts and resuspended in assay salts. In Step 2, elongation of the G21 RNA containing ternary complexes was done in 20 µl, in the presence of four NTPs, at 1 mM, and the presence or absence of 2 to 3 pmoles of VTF, for 10 minutes at 30° C. Step 2 synthesis yields a 189 nucleotide read through transcript and a termination product of about 75 nucleotides in length. RNA was extracted and separated by electrophoresis through a 11% polyacrylamide 8 M urea gel. The migration positions of the RNA were observed by autoradiography and the amount of the termination product and the read through RNA were determined by densitometry. The percentage termination was calculated by dividing the density of the terminated RNA by the sum of the densities of the terminated RNA and the read through RNA.
Measurement of the transcription elongation rate
The transcription elongation rate during Step 2 transcription was measured at different concentrations of the CTP, GTP, UTP and 5 mM ATP, in the absence of VTF. The ATP concentration was maintained in order to permit transcription termination (Hagler et al, 1994). The M29 mutant template was employed because it lacks the T9 sequence found in Ter29 kas and does not form the prominent U9 pause transcripts at low NTP levels. Step 1 transcription was done in the absence of 3’ O methyl GTP, which yields stable ternary complexes that possess a 20 nucleotide RNA with a 3’ OH group. This eliminates the need to excise the 3’ O methyl GMP present in the normal G21 RNA (Hagler and Shuman, 1993). Step 2 elongation reactions were stopped at times post initiation, the RNA was extracted and the migration positions evaluated as described. The rate was calculated by dividing the size of the longest transcript by the time of incubation.
NPH I stimulation of transcription elongation
The ability of NPH I to stimulate transcription elongation was measured as described by Deng and Shuman (1998), using the Ter29 kas template. An extract of C50 virus infected cells was employed, since this extract exhibits reduced NPH I activity (Christen et al, 1998). Initially, Step 2 transcription elongation through the T9 sequence in the Ter29 kas template was measured in the presence or absence of NPH I at 1 mM ATP, GTP, CTP and different UTP concentrations. At 2 µM UTP, which exhibits the NPH I stimulation of elongation, wild type NPH I and NPH I mutants that lack either ATPase activity or the ability to interact with Rap94 were compared for their ability to stimulate transcription elongation.
Measurement of transcript release
To measure transcript release from isolated bead-bound ternary complexes, ternary complexes located at the end of the A-less cassette were prepared by Step 2 transcription in presence of 1 mM CTP, GTP and UTP, and the absence of ATP and VTF. After isolating and washing, beads were resuspended in 20 µl transcription assay mix and incubated further for a 10 minutes at 30° or 37° C, in the presence or absence of 5 mM ATP and 2 pmoles of VTF. Bound and free RNA were extracted, separated by gel electrophoresis and quantified as described above. The percentage of free RNA was calculated by dividing the free RNA by the sum of bound and free RNA.
UV photo crosslinking of proteins to the nascent RNA in isolated ternary complexes
Protocols for preparation of Step 2 labeled ternary complexes and UV photo crosslinking of RNA to protein were described in detail previously (Christen et al, 2008). A set of templates were constructed that varied in the position of the T9 sequence in relation to the 3’ end of the A-less cassette, as described above. Construction of ternary complexes at the 3’ end of the A-less cassette yields a set of bead bound ternary complexes that differs in the position of the U9 termination in relation to the RNA polymerase in the isolated ternary complex. After irradiation, samples were treated with ribonuclease T1 and DNAse I, heat denatured in SDS sample buffer and analyzed after electrophoresis through a 10% polyacrylamide gel. Alternatively, for immunoprecipitation, samples were treated with ribonuclease T1 and denatured in 0.5% SDS and 10 mM β-mercaptoethanol prior to incubation with polyclonal antisera. Immunoprecipitates were separated by SDS gel electrophoresis and the migration positions of the radiolabeled proteins were observed by autoradiography (Niles and Seto, 1988; Christen et al, 1992).
Preparation of virus infected cell extracts or virion RNA polymerase
Transcription competent Cts50 virus infected cell extracts that lack NPH I activity were prepared as described (Christen et al, 1998). Vaccinia virus was grown and stored as described (Condit and Motyczka, 1981). Early gene transcription competent RNA polymerase was prepared from gradient purified virus through two DEAE cellulose column chromatography steps as described (Baroudy and Moss, 1980; Shuman et al, 1987). Virus infected cell extracts and virion RNA polymerase were aliquoted and stored at −80° C without loss of activity. VTF (Higman et al, 1992) and NPH I (Christen et al, 1999) were prepared from E. coli engineered to over express each enzyme, as described.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn BY, Gershon PD, Moss B. RNA polymerase-associated protein Rap94 confers promoter specificity for initiating transcription of vaccinia virus early stage genes. Journal of Biological Chemistry. 1994;269:7552–7557. [PubMed] [Google Scholar]
- Ahn BY, Moss B. RNA polymerase-associated transcription specificity factor encoded by vaccinia virus. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:3536–3540. doi: 10.1073/pnas.89.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroudy BM, Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. Journal of Biological Chemistry. 1980;255:4372–4380. [PubMed] [Google Scholar]
- Broyles SS, Moss B. Identification of the vaccinia virus gene encoding nucleoside triphosphate phosphohydrolase I, a DNA-dependent ATPase. Journal of Virology. 1987;61:1738–1742. doi: 10.1128/jvi.61.5.1738-1742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles SS. Vaccinia virus transcription. J. Gen Virol. 2003;84:2293–2303. doi: 10.1099/vir.0.18942-0. [DOI] [PubMed] [Google Scholar]
- Christen L, Higman MA, Niles EG. Phenotypic characterization of three temperature sensitive mutations in the vaccinia virus early gene transcription initiation factor. J. Gen. Virol. 1992;73:3155–3167. doi: 10.1099/0022-1317-73-12-3155. [DOI] [PubMed] [Google Scholar]
- Christen LM, Sanders M, Wiler C, Niles EG. Vaccinia virus nucleoside triphosphate phosphohydrolase I is an essential viral early gene transcription termination factor. Virology. 1998;245:360–371. doi: 10.1006/viro.1998.9177. [DOI] [PubMed] [Google Scholar]
- Christen L, Sanders M, Niles EG. Interaction of the vaccinia virus nucleoside triphosphate phosphohydrolase I with linear oligonucleotides. Biochemistry. 1999;36:8072–8089. doi: 10.1021/bi9903749. [DOI] [PubMed] [Google Scholar]
- Christen LM, Piacente S, Mohamed MR, Niles EG. Vaccinia virus early gene transcription termination factors, VTF and Rap94 interact with the UUUUUNU transcription termination motif in the nascent RNA in the transcription ternary complex. Virology. 2008 doi: 10.1016/j.virol.2008.03.031. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit RC, Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981;113:224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit RC, Motyczka A, Spizz G. Isolation, characterization and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983;128:429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Condit RC, Lewis JI, Quinn M, Christen LM, Niles EG. Use of lysolecithin-permeabilized infected-cell extracts to investigate the in vitro biochemical phenotypes of poxvirus ts mutations altered in viral transcription activity. Virology. 1996;218:169–180. doi: 10.1006/viro.1996.0177. [DOI] [PubMed] [Google Scholar]
- Deng L, Shuman S. A role for the H4 subunit of vaccinia RNA polymerase in transcription initiation at a viral early promoter. Journal of Biological Chemistry. 1994;269:14323–14328. [PubMed] [Google Scholar]
- Deng L, Shuman S. An ATPase component of the transcription elongation complex is required for factor-dependent transcription termination by vaccinia RNA polymerase. J. Biol. Chem. 1996;271:29386–29392. doi: 10.1074/jbc.271.46.29386. [DOI] [PubMed] [Google Scholar]
- Deng L, Hagler J, Shuman S. Factor-dependent release of nascent RNA by ternary complexes of vaccinia RNA polymerase. Journal of Biological Chemistry. 1996;271:19556–19562. doi: 10.1074/jbc.271.32.19556. [DOI] [PubMed] [Google Scholar]
- Deng L, Shuman S. Transcription termination by vaccinia RNA polymerase entails recognition of specific phosphates in the nascent RNA. J. Biol. Chem. 1997a;272:695–698. doi: 10.1074/jbc.272.1.695. [DOI] [PubMed] [Google Scholar]
- Deng L, Shuman S. Elongation properties of vaccinia virus RNA polymerase: pausing, slippage, 3’ end addition and termination site choice. Biochemistry. 1997b;36:15892–15899. doi: 10.1021/bi972037a. [DOI] [PubMed] [Google Scholar]
- Deng L, Shuman S. Vaccinia NPH-I, a DExH-box ATPase, is the energy coupling factor for mRNA transcription termination. Genes and Development. 1998;12:538–546. doi: 10.1101/gad.12.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger MJ, Martin SA, Paoletti E, Moss B. Modification of the 5'-terminus of mRNA by soluble guanylyl and methyl transferases from vaccinia virus. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:2525–2529. doi: 10.1073/pnas.72.7.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler J, Shuman S. Stability of the ternary transcription complexes of vaccinia virus RNA polymerase at promoter-proximal positions. J. Biol. Chem. 1992;267:7644–7654. [PubMed] [Google Scholar]
- Hagler J, Shuman S. Nascent RNA cleavage by purified ternary complexes of vaccinia RNA polymerase. Journal of Biological Chemistry. 1993;268:2166–2173. [PubMed] [Google Scholar]
- Hagler J, Luo Y, Shuman S. Factor-dependent transcription termination by vaccinia RNA polymerase. Kinetic coupling and requirement for ATP hydrolysis. Journal of Biological Chemistry. 1994;269:10050–10060. [PubMed] [Google Scholar]
- Higman MA, Bourgeois N, Niles EG. The vaccinia virus mRNA (guanine-N7-)-methyltransferase requires both subunits of the mRNA capping enzyme for activity. Journal of Biological Chemistry. 1992;267:16430–16437. [PubMed] [Google Scholar]
- Kane E, Shuman S. Temperature-sensitive mutations in the vaccinia virus H4 gene encoding a component of the virion RNA polymerase. J. Virol. 1992;66:5752–5762. doi: 10.1128/jvi.66.10.5752-5762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MR, Niles EG. Interaction between nucleoside triphosphate phosphohydrolase I and the H4L subunit of the viral RNA polymerase is required for vaccinia virus early gene transcript release. J. Biol. Chem. 2000;275:25798–25804. doi: 10.1074/jbc.M002250200. [DOI] [PubMed] [Google Scholar]
- Mohamed MR, Niles EG. The viral RNA polymerase H4L subunit is required for vaccinia virus early gene transcription termination. J. Biol. Chem. 2001;276:20758–20765. doi: 10.1074/jbc.M101641200. [DOI] [PubMed] [Google Scholar]
- Mohamed MR, Christen L, Niles EG. Antibodies directed to an epitope in the H4L subunit of the vaccinia virus RNA polymerase inhibit both active preinitiation complex formation and transcription termination activity. Virology. 2002;299:142–153. doi: 10.1006/viro.2002.1498. [DOI] [PubMed] [Google Scholar]
- Mohamed MR, Niles EG. UUUUUNU oligonucleotide stimulation of vaccinia virus early gene transcription termination, in trans. J. Biol. Chem. 2003a;278:11794–11801. doi: 10.1074/jbc.M213263200. [DOI] [PubMed] [Google Scholar]
- Mohamed MR, Niles EG. UUUUUNU stimulation of vaccinia virus early gene transcription termination: oligonucleotide sequence and structural requirements for stimulation of premature termination, in vitro. J. Biol. Chem. 2003b;278:39534–39541. doi: 10.1074/jbc.M306048200. [DOI] [PubMed] [Google Scholar]
- Mohamed MR, Piacente SC, Dickerman B, Niles EG. Effect of UTP sugar and base modifications on vaccinia virus early gene transcription. Virology. 2006;349:359–370. doi: 10.1016/j.virol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Moss BEd. In: Poxviridae: the viruses and their replication, vol. 2. Knipe DMl, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Strauss SE., editors. 2 vols. Philadelphia: Lippincott-Raven; 2007. [Google Scholar]
- Niles EG, Seto J. Vaccinia virus gene D8 encodes a virion trans-membrane protein. J. Virol. 1988;62:3772–3778. doi: 10.1128/jvi.62.10.3772-3778.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E, Moss B. Two nucleic acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Nucleotide substrate and polynucleotide cofactor specificities. Journal of Biological Chemistry. 1974;249:3281–3286. [PubMed] [Google Scholar]
- Piacente SC, Christen LM, Mohamed MR, Niles EG. Effect of selected mutations in the C-terminal region of the vaccinia virus nucleoside triphosphate phosphohydrolase I (NPH I) on binding to the H4L subunit of the viral RNA polymerase and early gene transcription termination. Virology. 2003;310:109–117. doi: 10.1016/s0042-6822(03)00092-8. [DOI] [PubMed] [Google Scholar]
- Prins C, Cresawn SG, Condit RC. An isatin-β-thiosemicarbazone-resistant vaccinia virus containing a mutation in the second largest subunit of the viral RNA polymerase is defective in transcription elongation. J. Biol. Chem. 200;279:44858–44871. doi: 10.1074/jbc.M408167200. [DOI] [PubMed] [Google Scholar]
- Rodriguez JF, Kahn JS, Esteban M. Molecular cloning, encoding sequence, and expression of vaccinia virus nucleic acid-dependent nucleoside triphosphatase gene. Proceeding of the National Academy of Sciences of the United States of America. 1986;83:9566–9570. doi: 10.1073/pnas.83.24.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G, Yuen L, Moss B. Transcription of vaccinia virus early genes by enzymes isolated from vaccinia virions terminates downstream of a regulatory signal. Cell. 1986;46:1029–1035. doi: 10.1016/0092-8674(86)90702-6. [DOI] [PubMed] [Google Scholar]
- Seto J, Celenza LM, Condit RC, Niles EG. Genetic map of the vaccinia virus HindIII D Fragment. Virology. 1987;160:110–119. doi: 10.1016/0042-6822(87)90051-1. [DOI] [PubMed] [Google Scholar]
- Schafer DA, Gelles J, Sheetz MP, Landick R. Transcription by single molecules of RNA polymerase observed by light microscopy. Nature. 1991;352:444–448. doi: 10.1038/352444a0. [DOI] [PubMed] [Google Scholar]
- Shuman S, Broyles SS, Moss B. Purification and characterization of a transcription termination factor from vaccinia virions. Journal of Biological Chemistry. 1987;262:12372–12380. [PubMed] [Google Scholar]
- Shuman S, Moss B. Factor-dependent transcription termination by vaccinia RNA polymerase: Evidence for a cis-acting termination signal in nascent RNA. J. Biol. Chem. 198;263:6220–6225. [PubMed] [Google Scholar]
- Shuman S, Moss B. Bromouridine triphosphate inhibits transcription termination and mRNA release by vaccinia virions. Journal of Biological Chemistry. 1989;264:21356–21360. [PubMed] [Google Scholar]
- Vos JC, Sasker M, Stunnenberg HG. Vaccinia virus capping enzyme is a transcription initiation factor. EMBO J. 199;10:2553–2558. doi: 10.1002/j.1460-2075.1991.tb07795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S, Gershowitz A, Moss B. Modification of the 5’ end of mRNA. Association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7-) methyltransferase complex from vaccinia virus. J. Biol. Chem. 1980;255:903–908. [PubMed] [Google Scholar]
- Yuen L, Moss B. Multiple 3' ends of mRNA encoding vaccinia virus growth factor occur within a series of repeated sequences downstream of T clusters. Journal of Virology. 1986;60:320–323. doi: 10.1128/jvi.60.1.320-323.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L, Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]


