Abstract
Perimesencephalic subarachnoid hemorrhage (SAH) is a relatively benign form of SAH. The cause of this condition is unknown but venous leakage has been believed to be the most common etiology. This report describes a patient with perimesencephalic SAH who presented with a concurrent acute pontine infarct demonstrated on diffusion-weighted MRI. These findings suggest that in some instances perimesencephalic SAH is caused by rupture of a perforating artery.
Introduction
Perimesencephalic subarachnoid hemorrhage (SAH) was first described by van Gijn et al.1 This entity is characterized by the presence of subarachnoid hemorrhage, predominantly located in the perimesencephalic cistern, in the absence of an identifiable underlying vascular lesion on four-vessel digital subtraction cerebral angiography. Perimesencephalic SAH is a relatively benign form of SAH. Recurrent bleeding and clinically significant vasospasm, main factors contributing to morbidity and mortality in aneurismal SAH are both very uncommon in perimesencephalic SAH.2 The etiology of perimesencephalic hemorrhage is unknown. Both venous and arterial sources of bleeding have been proposed as potential causes of perimesencephalic hemorrhage.3 The clinical and radiological findings of the patient described in this report suggest that rupture of a pontine perforator artery can lead to perimesencephalic SAH.
Case report
A 74 year-old woman with a past medical history of hypertension presented 10 hours after the acute onset of headache and neck pain to the emergency room. The headache was associated with nausea and vomiting. The patient had no significant past medical history of headaches and had not had warning signs in the days or weeks preceding symptom onset. Non-contrast CT of the head obtained 13 hours after symptom onset demonstrated substantial subarachnoid hemorrhage located predominantly in the prepontine cistern and extending into the region of the Circle of Willis. (figure A) Four-vessel digital subtraction angiography, obtained 36 hours after symptom onset demonstrated no aneurysm and no alternative vascular lesion that could account for the patient’s SAH. A diagnosis of nonaneurysmal perimesencephalic SAH was made based on the pattern of bleeding on CT and the negative angiogram. A small acute left paramedian pontine infarct was incidentally noted on diffusion-weighted MRI of the brain obtained on day 2. (figure B) FLAIR imaging demonstrated periventricular white matter changes consistent with chronic small vessel ischemic disease. The MRI findings prompted a neurological consultation. On detailed neurological testing the patient was noted to have slight slowing of fast-finger movements on the right. She also had mildly impaired handwriting, a symptom which she had not noted as she had not written anything since admission. Otherwise the patient was asymptomatic from her small pontine infarction. A retrospective review of the patient’s baseline CT scan, which was obtained 13 hours after symptom onset, demonstrated a subtle hypodensity consistent with an acute infarction in the right paramedian pons. This lesion was of the same dimension and in the same location as the acute infarct visible on DWI. The patient was discharged to home on day 5. She recovered completely from her SAH and pontine infarct. Repeat four-vessel angiography, obtained 6 weeks following her initial presentation was unremarkable.
Figure.
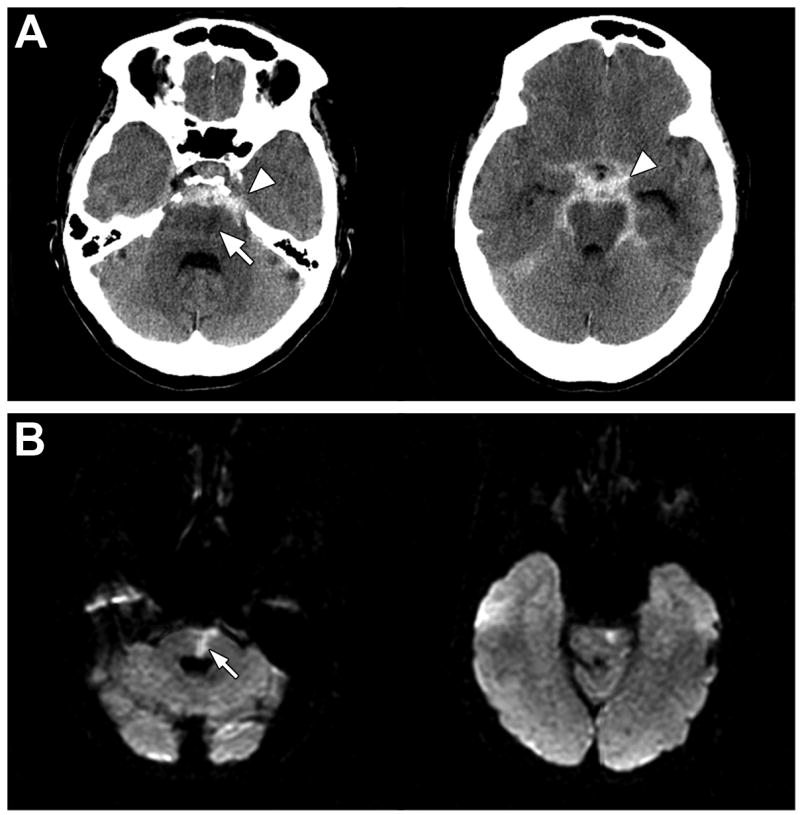
CT scan (A) obtained 13 hours after symptom onset demonstrates extensive perimesencephalic subarachnoid hemorrhage (arrowheads) and a left paramedian pontine hypodensity (arrow) consistent with an ischemic infarct. A diffusion-weighted MRI (B) obtained two days after symptom onset confirms the presence of an acute infarction of the pons (arrow).
Discussion
The development of a pontine infarct in the acute setting of perimesencephalic subarachnoid hemorrhage is rare and cannot be readily explained by venous leakage, which has been proposed as the most likely etiology of perimesencephalic SAH.2, 4 A rupture in the very proximal segment of a perforating artery within the subarachnoid space could explain the concurrent presentation of an acute pontine infarct and a perimesencephalic SAH. This has been suggested as the etiology of perimesencephalic SAH based on CT findings, 5 but has not yet been confirmed with MRI. The diffusion-weighted imaging findings of this patient provide further evidence that in some instances, perhaps those with more extensive SAH, perimesencephalic SAH is likely caused by rupture of a perforating artery.
Although lacunar infarct are generally caused by thrombosis of a perforating artery6, the presence of an extensive amount of subarachnoid blood in the setting of a lacunar infarct, as was seen in this patient, is inconsistent with a thrombotic etiology. Perforating arteries have a very short proximal segment that runs within the subarachnoid space followed by a relatively long intra-parenchymal segment. A rupture of a perforating artery is most likely to occur within the long intra-parenchymal segment. Ruptures within the intra-parenchymal segment are a common cause of intracerebral hemorrhage (ICH).7 Although ischemic injury of the brain parenchyma due to interruption of blood flow may develop concurrent with ICH, visualization of an infarct would generally be obscured by the presence of blood products in the brain parenchyma. Spontaneous rupture of the proximal segment of a perforating artery within the subarachnoid space, on the other hand, causes subarachnoid hemorrhage which would not impair visualization of the adjacent brain parenchyma on CT or MRI. A lacunar infarct caused by proximal vessel rupture could therefore be visible on imaging studies such as diffusion weighted MRI (DWI).
The simultaneous occurrence of a perimesencephalic hemorrhage and a pontine lacunar infarct is not definitive proof that both are caused by a proximal rupture of a perforating artery. Other potential explanations seem, however, less likely. Infarction occurring as a complication of DSA is excluded by the presence of early infarct signs on the baseline CT, which was obtained prior to angiography. Vasospasm is a common cause of ischemia in the setting of SAH but an unlikely cause in this patient. First, vasospasm is uncommon in the setting of perimesencephalic hemorrhage; second, it almost never occurs in the first 24 hours; thrird, ischemia in the distribution of a perforator artery is atypical for vasospasm and fourth, both angiography and TCD did not show signs of vasospasm. Venous leakage could potentially cause perimesencephalic hemorrhage with secondary compression of a basilar perforator leading to ischemic injury. This seems an unlikely explanation given that the pressure in the venous system is much lower than the arterial pressure. It is possible that the lacunar infarct and the perimesencephalic hemorrhage occurred as two independent processes. The probability of two relatively uncommon processes occurring within a very narrow time span is, however very small. A unifying diagnosis that explains both processes seems more likely. Finally, both conditions could have been caused by a dissection of the basilar artery. Based on the presence of a small bulge on the basilar artery on angiography in two patients with perimesencephalic SAH, Matsumaru et al have speculated that rupture of a basilar dissection into the subarachnoid space could cause perimesencephalic subarachnoid hemorrhage.8 If the dissection severed the take-off of a perforating artery it could simultaneously cause SAH and parenchymal ischemia. A bulge on the basilar artery was, however, not seen on either DSA or MRA in our patient. Other findings that might point to a dissection such as a narrowed lumen or intramural clot were also not seen on neuroimaging in this case.
It is not known how often non-aneurysmal SAH may be caused by perforator rupture. The paucity of reports on the concurrent presentation of a lacunar infarct and non-aneurismal SAH suggests that perforator rupture is a rare cause. It is, however, possible that perforator rupture is a more common cause as a proximal perforator rupture may lead to SAH without causing a lacunar infarct in patients with sufficient collateral flow. Furthermore, lacunar infarcts in the setting of perimesencephalic SAH may have been overlooked in previous cases. This is illustrated by the subtlety of the clinical and CT findings in this case. A systematic assessment of MRI findings in a cohort of patients with perimesencephalic SAH may provide further insight in the prevalence of acute lacunar infarcts in patients with this disorder.
Footnotes
Disclosure: The author reports no conflict of interest
Search Term: [8] Subarachnoid hemorrhage, [6] Infarction, [120] MRI, [128] DWI
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Gijn J, van Dongen KJ, Vermeulen M, Hijdra A. Perimesencephalic hemorrhage: A nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985;35:493–497. doi: 10.1212/wnl.35.4.493. [DOI] [PubMed] [Google Scholar]
- 2.Rinkel GJE, Wijdicks EFM, van Gijn J, Hasan D, Vermeulen M, Hageman LM, Kienstra GEM, Franke CL. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. The Lancet. 1991;338:964–968. doi: 10.1016/0140-6736(91)91836-j. [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI, Wijdicks EF. Origin of pretruncal nonaneurysmal subarachnoid hemorrhage: Ruptured vein, perforating artery, or intramural hematoma? Mayo Clinic proceedings. 2000;75:1169–1173. doi: 10.4065/75.11.1169. [DOI] [PubMed] [Google Scholar]
- 4.van der Schaaf IC, Velthuis BK, Gouw A, Rinkel GJE. Venous drainage in perimesencephalic hemorrhage. Stroke. 2004;35:1614–1618. doi: 10.1161/01.STR.0000131657.08655.ce. [DOI] [PubMed] [Google Scholar]
- 5.Tatter SB, Buonanno FS, Ogilvy CS. Acute lacunar stroke in association with angiogram-negative subarachnoid hemorrhage : Mechanistic implications of two cases. Stroke. 1995;26:891–895. doi: 10.1161/01.str.26.5.891. [DOI] [PubMed] [Google Scholar]
- 6.Fisher CM. Lacunar strokes and infarcts: A review. Neurology. 1982;32:871–876. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 8.Matsumaru Y, Yanaka K, Muroi A, Sato H, Kamezaki T, Nose T. Significance of a small bulge on the basilar artery in patients with perimesencephalic nonaneurysmal subarachnoid hemorrhage. Report of two cases. Journal of neurosurgery. 2003;98:426–429. doi: 10.3171/jns.2003.98.2.0426. [DOI] [PubMed] [Google Scholar]


