Abstract
Here we review the mechanisms that determine projection neuron identity during cortical development. Pyramidal neurons in the mammalian cerebral cortex can be classified into two major classes: corticocortical projection neurons, which are concentrated in the upper layers of the cortex, and subcortical projection neurons, which are found in the deep layers. Early progenitor cells in the ventricular zone produce deep layer neurons that express transcription factors including Sox5, Fezf2, and Ctip2, which play important roles in the specification of subcortically projecting axons. Upper layer neurons are produced from progenitors in the subventricular zone, and the expression of Satb2 in these differentiating neurons is required for the formation of axonal projections that connect the two cerebral hemispheres. The Fezf2/Ctip2 and Satb2 pathways appear to be mutually repressive, thus ensuring that individual neurons adopt either a subcortical or callosal projection neuron identity at early times during development. The molecular mechanisms by which Satb2 regulates gene expression involves long-term epigenetic changes in chromatin configuration, which may enable cell fate decisions to be maintained during development.
Introduction
The nervous system is populated by an enormous variety of neurons that show distinct dendritic morphologies, local and long-distance axonal connections, neurotransmitter phenotypes, and patterns of gene expression. The generation of these diverse phenotypes from mitotically active progenitor cells utilizes a range of cellular and molecular strategies. In general, cues derived from the early regionalization of the neural tube act in conjunction with intercellular signals, temporally regulated factors, and cell-intrinsic cues to progressively determine the fates and identities of specific classes of neurons. Recent studies of the developing cerebral cortex have elucidated some of the mechanisms that underlie the production of discrete types of projection neurons. Here we review progress in understanding the strategies by which cortical neurons are assigned layer-specific fates and elaborate their long-distance projections.
The cerebral cortex is organized into layers that are generated sequentially over developmental time
Ever since the time of Cajal, scientists have appreciated that the cerebral cortex is organized in layers that are defined by the densities and morphologies of their constituent neurons. The advent of retrograde tracing techniques and intracellular dye injections revealed that, as a general rule, neurons in the upper layers 2 and 3 tend to form corticocortical connections, including projections to the contralateral hemisphere across the corpus callosum, whereas neurons in layers 5, 6, and the subplate are the source of subcortical projections to targets that include the spinal cord, pons, midbrain, and thalamus. Neurons in layers 1 and 4 extend axons locally within the cortex. Although it was long assumed that the interneurons of the cortex arise from the same progenitor population as those that generate projection neurons, cell labeling and genetic studies revealed that the bulk of these neurons derive from the ganglionic eminence, which serves as the progenitor pool for the striatum and basal ganglia as well. The regulation of interneuron fates has been reviewed recently and will not be discussed here [1,2].
Cortical projection neurons are derived from progenitor cells that line the dorsal aspect of the lateral ventricles in the forebrain. Mitotically active cells are found in the ventricular zone (VZ), immediately adjacent to the ventricles, and, at later stages, in the subventricular zone (SVZ), which forms between the VZ and the overlying intermediate zone (IZ). Classic 3H-thymidine “birthdating” studies have revealed that neurons of different layers are generated in a stereotypic temporal sequence during development [3]. The first neurons to exit the cell cycle and migrate out of the VZ occupy the preplate, which is subsequently split into two zones by the arrival of neurons that form the cortical plate. The upper preplate, which is known during development as the marginal zone, is populated by Cajal-Retzius neurons, of which many or most are derived from a region known as the cortical hem (located near the hippocampal anlage) and then migrate tangentially to populated the neocortex [4,5]. The deeper domain of the preplate becomes the subplate, a largely transient zone of neurons that plays important roles in axon targeting during development [6]. Within the cortical plate, neurons of the deepest layers (6 and 5) are generated at the earliest stages, followed by neurons of layers 4, 3 and 2.
Cellular studies of cell fate determination suggest a progressive restriction in developmental potential
Transplantation experiments have probed the process by which neurons become committed to the laminar fate that is typical of their time of origin. These studies have demonstrated that by the time a young neuron has progressed through its final mitotic division and is ready to initiate migration, the cell has acquired the information needed to migrate to the layer typical of its birthday, even in an environment in which host neurons are destined for other layers [7–9]. However, at earlier stages of differentiation, cells can show a broader developmental potential and adopt alternative fates. Transplantation experiments revealed that early progenitors, which normally produce deep layer neurons, are multipotent: these cells can directly produce upper layer neurons when transplanted into an older brain environment [7]. Interestingly, the competence of progenitors to respond to fate-inducing cues changes over developmental time. The progenitors of layer 4 neurons retain the ability to differentiate into later-generated types when transplanted into older hosts, but have lost the ability to form layer 6 neurons if transplanted into younger brains [9]. The progenitors of layer 2/3 neurons are even more restricted; when transplanted into younger hosts, these cells generate only upper layer neurons, even if they divide again in the new environment [8]. These transplantation experiments addressed the laminar positions and connections of cortical neurons, but did not track the expression of layer-specific molecular markers. However, clonal analyses of cortical progenitor cells in culture have relied on such markers, and suggest that both the sequential production of distinct laminar phenotypes and the progressive loss of competence to produce early-generated deep layer neurons can occur in isolation [10]. This suggests that cortical progenitor cells may employ a cell-intrinsic clock as part of the mechanism by which to time the production of distinct laminar phenotypes, or that feedback is required only within an individual lineage in order to produce an appropriate sequence of cell types over time.
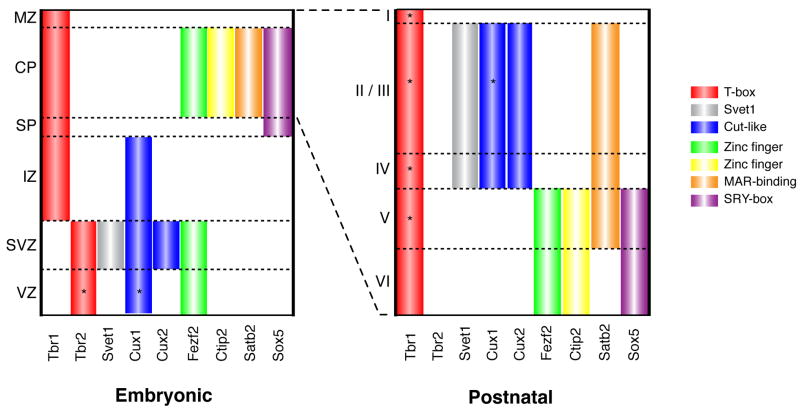
Specific patterns of gene expression and cell behavior correlate with the changes in cortical progenitor cells, and in their progeny, over time (Figure 1). For example, progenitors in the VZ at early stages of corticogenesis express a number of transcription factors (such as Fezf2, Otx1, Sox2, and Emx2) that are maintained by their progeny as young neurons populate the deep layers [11–14]. However, at middle stages of neurogenesis, as progenitors begin to produce the neurons of layers 4 through 2, the cells undergo a series of interesting cellular and molecular alterations. The traditional view of cortical neurogenesis held that all neurons are derived from the VZ, and that the SVZ was the source of glial cells [15]. However, this view has been challenged first based on histological studies [16], and subsequently by the observation that SVZ cells show patterns of gene expression that are echoed by those in upper layer neurons. For example, the noncoding RNA Svet1 and several homodomain genes, including Cux1 and Cux2 are expressed both in the SVZ and in layers 2–4 [17,18]. These corresponding patterns of gene expression suggested that upper layer neurons might be derived from the SVZ. Indeed, in the Pax6/small eye mutant, Svet1 expression is lost in both the SVZ and the upper layers, providing indirect evidence that the SVZ is necessary for the generation of upper layer neurons [18].
Figure 1. Gene expression patterns in the developing cerebral cortex during mid-neurogenesis and early postnatal life.
Summary of the expression patterns of the T-box transcription factor Tbr1, the non-coding RNA Svet1, the cut-like transcription factors Cux-1 and Cux-2, the zinc-finger transcription factors Fezf2 and Ctip2, the chromatin remodeling protein Satb2, and the SRY-box transcription factor Sox5 during embryonic and early postnatal development in the mouse. Note that many of these genes show dynamic expression patterns (particularly in postnatal life) that are not fully summarized here, and that the protein expression patterns of some are more restricted compared to those of the corresponding mRNAs. Abbreviations: MZ, marginal zone; CP, cortical plate; SP, subplate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone.
Direct evidence that the SVZ is a neurogenic zone has been derived from a recent series of elegant imaging experiments [19,20] in conjunction with related work from other labs [21–23]. Noctor and colleagues used time-lapse confocal time-lapse microscopy to track the behavior of individual GFP-labeled mitotic progenitors in the VZ and SVZ, and then to follow the behavior and fates of their daughter cells [19,24,25]. These studies revealed that cell divisions within the VZ are primarily symmetric during early development before the onset of neurogenesis, when the progenitor pool is expanding. At the onset of neurogenesis, most cell divisions in the VZ are asymmetric, with one daughter cell remaining in the VZ and the other cell exiting this zone to differentiate. Several imaging studies suggest that daughter cell fates may be regulated by the asymmetric inheritance of determinants associated with the apical membrane of the progenitor cell, such that the daughter cell that inherits this domain retains a progenitor cell fate [24–27]. At mid- to late stages of neurogenesis, the outcomes of VZ cell divisions undergo a fundamental alteration. Although the cell divisions remain asymmetric, the daughter cell that exits the VZ does not differentiate directly into a neuron, but rather moves into the SVZ and undergoes one or more symmetric neurogenic divisions, thus serving as a transit amplifying cell [23–25,28]. It is these divisions that appear to be primarily responsible for generating the neurons that migrate into the upper layers.
Collectively these studies suggest that the early-generated neurons of the deep layers are derived from progenitors in the VZ, whereas late-generated upper layer neurons are produced primarily from SVZ cells, which serve as intermediate progenitors. The corresponding patterns of gene expression between early VZ cells and the deep layers, and SVZ cells and upper layer neurons, suggest that the fates of young neurons are determined at the time of their generation. Below we review the roles of specific genes in the progressive establishment of laminar identity and patterns of connectivity among subtypes of cortical projection neurons.
Fezf2 and Ctip2 define the fates of subcortical projection neurons
VZ cells at early stages of cortical neurogenesis express a number of transcription factor genes that have the potential to determine or influence the fates of their daughter cells. Some of these (e.g., Otx1) show a clear correlation in expression between early progenitors and deep layer neurons, but have no obvious functional role in the establishment of neuronal fates or identities [13,29]. However, others do appear to play important roles in fate determination. For example, the zinc-finger transcription factor Fezf2 is expressed in telencephalic progenitor cells starting at E8.5, and its expression is retained by deep layer neurons during corticogenesis [12,30–33]. Layer 5 and 6 neurons subsequently acquire the expression of a second zinc finger transcription factor, Ctip2, during postmitotic differentiation [12,32,34]. The expression domain of Fezf2 (which is most prominent in cortex and hippocampus, but is also found in a subset of hypothalamic cells) is much more limited than that of Ctip2, which is broadly expressed in the striatum, olfactory bulb and other regions. However, within the cortex, both Fezf2 and Ctip2 are expressed by subcortically projecting neurons in layer 5 and in the corticothalamic projection neurons of layer 6 [12,32,34]. Importantly, Ctip2 expression is lost in the cortices of Fezf2 mutant mice (but not in other brain regions) [12,32], whereas the converse is not the case: Ctip2 mutants continue to express Fezf2 (J.D. Macklis, personal communication). Thus, the timing of Fezf2 and Ctip2 expression are consistent with the suggestion that Fezf2 acts upstream of Ctip2 during cortical development.
Genetic knockouts targeting either the Fezf2 or Ctip2 locus in mice have revealed striking similarities in the phenotypes of layer 5 neurons. In both cases, the development of subcortically projecting axons was highly abnormal, with the axons of corticospinal motor neurons (CSMNs) failing to extend into the corticospinal tract (CST) [12,32,33]. In Fezf2 mutant mice, defects have also been noted in projections to other subcortical targets, including the midbrain and pons [12]. Because mutation of Ctip2 causes lethality at about the time of birth [32], it has been difficult to conduct extensive studies of the fates of CSMNs in these mutants. Such experiments have been feasible in Fezf2 mutant mice, which survive into adulthood, and particularly in a mouse line in which the Fezf2 coding sequence was replaced with placental alkaline phosphatase (PLAP), which marks the axonal projections of neurons that normally express Fezf2 [12]. Unpublished evidence suggests that Fezf2 regulates a fate switch: in its absence, many layer 5 and 6 neurons acquire the phenotypes of callosal projection neurons (B. Chen, S.S. Wang, A.M. Hattox, H. Rayburn, S.B. Nelson, and S.K. McConnell, submitted). Conversely, the ectopic expression of Fezf2 in neurons that normally form corticocortical and callosal projections is sufficient to redirect their axons to subcortical targets [32,33].
Fezf2 encodes a zinc-finger protein that contains a putative repressor domain similar to that found in the Engrailed protein [35,36], and Ctip2 is known to repress transcription of the p57KIP2 locus [37]. Thus it seems plausible that Fezf2 and Ctip2 function as transcriptional repressors in the regulation of deep layer neuron fates. However, some zinc finger transcription factors can confer both activator and repressor activities [36], and in addition, Ctip2 has been implicated in chromatin remodeling at the HIV1 locus, where it recruits histone deacetylases and methylases to repress transcription [38]. The latter result suggests that the Fezf2-Ctip2 pathway has the potential to modify chromatin configuration; however, the detailed biochemical mechanisms by which these two proteins function in cortical neurons remain to be explored.
A role for Sox5 in the specification of distinct subtypes of deep layer neurons
While substantial progress has been made in unravelling the genetic and molecular pathways that control deep layer neuronal identity, little is yet known about the pathways that act upstream of Fezf2 and Ctip2. Recently, however, the SRY-box gene Sox5 has been implicated in regulating the timing of deep layer differentiation [39]. Sox5 is normally expressed by subcortically-projecting neurons in layers 5, 6, and the subplate, and its expression is largely excluded from callosal projection neurons [39]. In Sox5−/− mutants, neurons of layers 5 and 6 show aberrant migration patterns and fail to segregate into distinct layers, with cells expressing high levels of Fezf2 or Ctip2 occupying the deepest region of the cortical plate rather than their normal positions in layer 5. Cells expressing Ctip2 were also scattered throughout the upper layers, and many of these extended axons subcortically since they could be labeled retrogradely from the cerebral peduncle. Some of these neurons were likely to be mispositioned subplate cells; although mutant brains showed reduced expression of subplate-specific markers, BrdU labeling studies demonstrated that the earliest generated neurons failed to form a subplate layer and instead were distributed throughout the cortical plate. Lai et al. (2008) argued that these subplate neurons acquired a novel fate, since many earliest-generated neurons acquired Ctip2 expression, which is normally absent from the subplate. Interestingly, double mutant mice lacking both Sox5 and Ctip2 function showed a partial rescue of the migration defects seen in Sox5 mutants. These data suggest that Ctip2 expression by subplate neurons may prevent normal preplate splitting as the neurons of the cortical plate migrate into position, and thus lead to defects in lamination similar to those seen in reeler mice. Finally, Sox5 mutants displayed striking defects in the formation of subcortical projections, with axons extending along novel trajectories and apparently failing to form a coherent CST in caudal regions [39].
The ectopic expression of Sox5 in upper layer neurons prevented them from extending axons across the corpus callosum and stimulated the extension of corticofugal axons, suggesting that Sox5 actively promotes the differentiation of subcortical projection neurons. In light of the normal expression of this gene in three distinct types of deep layer neurons (layer 5 subcortical, layer 6 corticothalamic, and subplate neurons), Lai and colleagues hypothesized that varying levels of Sox5 act in combination with distinct levels of Tbr1 and Ctip2 to control the specific identities of subplate and deep layer neurons. For example, subplate neurons are characterized by high levels of Tbr1 expression, little or no Ctip2, and an intermediate level of Sox5, whereas layer 6 neurons normally express high levels of both Sox5 and Tbr1 and an intermediate level of Ctip2. As a known repressor of transcription, it is conceivable that Sox5 may directly repress Ctip2 expression in subplate neurons.
Finally, it is clear that Tbr1 plays an important role in cortical development, particularly in regulating the differentiation of subplate neurons. Tbr1 is expressed in the preplate and layer 6 during early corticogenesis [40], and Tbr1-deficient mice show defects in preplate splitting and in the positioning of early-born neurons (similar to the migration defects observed in reeler mice) [41]. Indeed, in the absence of Tbr1, the expression of reelin by Cajal-Retzius cells in the marginal zone is reduced [41]. Tbr1 knockouts also exhibit a variety of axon projection defects, with corticothalamic projections growing only as far as the internal capsule, callosal projections mostly terminating in Probst bundle without crossing the midline, and thalamocortical projections reaching the internal capsule, but then turning away from the cortex and extending into the external capsule and amygdala. These complex phenotypes highlight the difficulty in teasing apart non-autonomous gene functions in regulating processes such as migration, from possible cell-autonomous alterations in neuronal identity and connectivity. Clearly, much remains to be learned about the function of Tbr1 in cortical neurogenesis.
Mechanisms that direct upper layer neuronal identity
Although our understanding of the mechanisms that produce distinct subtypes of subcortical projection neurons in the deep layers is growing, much less is known about how the brain produces the classes of neurons that populate the upper layers. As with VZ cells and their deep layer progeny, gene expression patterns in the SVZ are correlated with those of neurons in layers 2–4. However, the functional roles of these genes remains poorly understood. For example, the T-box gene Tbr2 is expressed prominently in the SVZ [40,42], but Tbr2 knockout mice die early at the blastocyst stage [43], precluding an analysis of Tbr2 function in cortical development.
The homeodomain genes Cux1 and Cux2 are expressed in the developing brain [44], particularly in SVZ cells and their progeny in layers 2–4 [45]. The generation and analysis of Cux2 knockout animals has revealed that Cux2 promotes the exit of SVZ cells from the cell cycle, thereby limiting the numbers of upper layer neurons that are generated from this zone.. Cux2−/− mice experience an increase in the proliferation of SVZ cells and a moderate increase in the densities of cells in layers 2, 3 and 4 [46]. Birthdating experiments suggest that Cux2-deficient SVZ progenitors reenter the cell cycle at a higher frequency than in wild-type controls, leading to an increase in the size of the SVZ progenitor pool.
The POU domain transcription factors Brn-1 and Brn-2 also regulate the generation of upper layer neurons. Both genes are expressed in the VZ and SVZ starting at mid-neurogenesis [47]. Although single mutations in either Brn-1 or Brn-2 yield phenotypes in only limited areas of the brain, double mutants show severe proliferation defects in VZ and SVZ cells after E14.5, with no obvious changes in the proliferation of progenitors at earlier times [47]. Double mutant animals also show striking decreases in the numbers of layer 4 neurons, and the brains of surviving Brn-1/2 double knockouts seem to lack the upper layers as the thickness of the cortex was significantly reduced. This suggests a cell-autonomous role for Brn genes in upper layer neurogenesis. Interestingly, although the numbers of deep layer neurons did not appear to be affected in mutants, their migration was perturbed. Analysis of lamination using layer specific markers revealed that layer 5 neurons (which normally reside above layer 6) were positioned below layer 6 in the mutant cortex. The authors further provide evidence that the defects in migration could be due to a reduction of mDab1, a protein that acts downstream of reelin signaling. Although the two phenotypes (loss of upper layer cortical progenitors and defects in migration) might be independent of each other, further analysis is required to unravel the precise role of Brn genes in upper layer generation.
Satb2 and the determination of callosal projection neuron identity
Neurons that extend axons across the corpus callosum to the opposite cerebral hemisphere are a subtype of neurons that form corticocortical connections. Callosal projection neurons are particularly prominent in the upper layers, although they are present in the deep layers as well. Recent work has revealed that callosal projection neurons require the chromatin remodeling protein Satb2 for the formation of their normal projections, and that in the absence of Satb2, these cells extend axons toward subcortical targets.
Satb2 is expressed by a subset of neurons throughout the cortical layers, but its expression is most prominent in layers 2–4 [18,48–50]. Targeting of the Satb2 locus with a lacZ gene revealed that Satb2 expressing neurons normally extend axons across the corpus callosum [49]. Strikingly, these axons fail to cross the corpus callosum in Satb2 mutant brains [49,50], although some β-galactosidase− axons do cross the midline, suggesting that the corpus callosum itself is intact [49]. Instead, β-galactosidase-labeled axons assume an alternate trajectory and extend subcortically along the CST. In addition to adopting the axon trajectory of deep layer neurons, Satb2 mutants display alterations in the expression of several axon guidance molecules and a dramatic expansion of Ctip2 expression into the upper layers and within the deep layers, although the expression of Fezf2 was not obviously affected [49,50]. These data suggest that Satb2 functions to repress the expression of Ctip2 in callosal projection neurons. Indeed, the ectopic expression of Satb2 in neurons markedly reduces the fraction of cells that express Ctip2 [49,50], and alters the projections of deep layer neurons, such that the axons of electroporated cells fail to extend past the cerebral peduncle [50]. This axonal phenotype is reminiscent of that observed in Ctip2−/− brains [34], suggesting that the ectopic expression of Satb2 might alter the fate of deep layer neurons by regulating Ctip2 expression.
Collectively these data suggest that Satb2 promotes a callosal projection neuron identity in the cortex by repressing Ctip2 expression. Indeed, the repression of Ctip2 by Satb2 appears to be direct: Satb2 binds directly to matrix attachment regions (MARs) in the Ctip2 locus, where it recruits histone deacetylases [50,51] and modifies chromatin configuration to assume a less activated state [49,51]. It is not yet clear whether this repression is mutual, since Satb2 expression in Ctip2 mutant brains has not yet been reported. However, the expression of Satb2 is significantly upregulated in the deep layers of Fezf2 mutants (B. Chen, S.S. Wang, A.M. Hattox, H. Rayburn, S.B. Nelson, and S.K. McConnell, submitted), suggesting that Fezf2 can repress Satb2 expression in subcortical projection neurons. It remains to be determined whether this effect is direct or indirect, and whether Fezf2 can also directly promote the expression of Ctip2 in deep layer neurons.
Conclusions
Collectively, studies of the roles of Sox5, Fezf2, Ctip2 and Satb2 during cortical development suggest that an elegant genetic mechanism exists to control the identity of a subcortical vs callosal projection neuron (Figure 2). Early in development, when deep layer neurons are generated, Fezf2 expression in VZ cells may promote the expression of Ctip2 in young neurons, and together these genes confer a subcortical projection neuron fate during differentiation. Differences in the levels of expression of Sox5, Tbr1, and Ctip2 can further confer specific identities onto subtypes of deep-layer and subplate neurons. At the same time, Fezf2 appears to repress Satb2 (directly or indirectly) thereby repressing callosal identity. At later times during corticogenesis, when upper layer neurons are generated, Fezf2 expression is absent, which relieves the repression of Satb2. This, in turn, enables Satb2 to actively repress Ctip2 expression and promote the adoption of a callosal or corticocortical projection neuron identity. Interestingly, these two pathways also operate within a layer: for example, both subcortical and callosal projection neurons coexist within layer 5 [52], where Satb2 and Ctip2 are coexpressed by individual neurons during a brief window of time, just as layer 5 neurons are settling into their final positions within the cortical plate [49,50]. However, this period of coexpression quickly resolves, with the vast majority of layer 5 cells expressing either Satb2 or Ctip2, but not both. At this point, we do not understand what factors within the cell lead it to choose one genetic pathway over the other, but it is clear that the choice leads to fundamental differences in neuronal fate determination.
Figure 2. A working model for the specification of callosal vs. subcortical projection neuron identity during the development of the cerebral cortex.
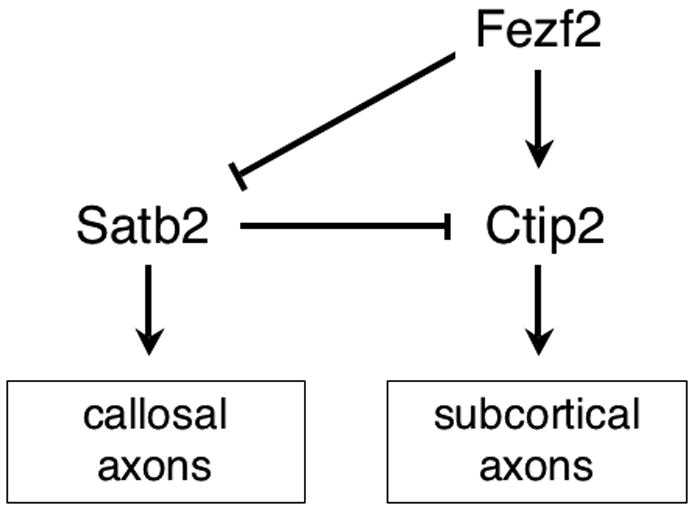
This model is based on genetic (rather than biochemical) studies, which suggest that Fezf2 acts upstream of Ctip2 and that both play essential roles in the specification of subcortical projection neuron fates. Satb2 is required for the development of callosal projection neurons and represses the expression of Ctip2 in these cells. In the absence of Satb2, callosal projection neurons extend axons subcortically. Conversely, in the absence of Fezf2, Satb2 expression is derepressed, enabling cells to take on a callosal projection neuron fate.
Acknowledgments
Supported by NIH grant EY08411 (National Eye Institute).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 3.McConnell SK. The generation of neuronal diversity in the central nervous system. Ann Rev Neurosci. 1991;14:269–300. doi: 10.1146/annurev.ne.14.030191.001413. [DOI] [PubMed] [Google Scholar]
- 4.Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C, Guan W, Pleasure SJ. A transgenic marker mouse line labels Cajal-Retzius cells from the cortical hem and thalamocortical axons. Brain Res. 2006;1077:48–53. doi: 10.1016/j.brainres.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51:627–638. doi: 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 7.McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing cerebral cortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- 8.Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17:55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 9.Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- 10**.Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. This report shows that neuronal progenitors in culture can sequentially generate neurons in the normal order in which they are normally produced in vivo. The data also suggest that progenitors become increasingly restricted in terms of their fate potential over time, and that these changes may be regulated by a cell-intrinsic clock. [DOI] [PubMed] [Google Scholar]
- 11.Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, et al. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 12**.Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. This paper describes the phenotype of Fezf2 mutant mice, with particular emphasis on the mistargeting of corticospinal axons from deep layer neurons and changes in gene expression patterns (particularly that of Ctip2) in the mutants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frantz GD, Weimann JM, Levin ME, McConnell SK. Otx1 and Otx2 define layers and regions in developing cerebral cortex and cerebellum. J Neurosci. 1994;14:5725–5740. doi: 10.1523/JNEUROSCI.14-10-05725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leingartner A, Richards LJ, Dyck RH, Akazawa C, O’Leary DD. Cloning and cortical expression of rat Emx2 and adenovirus-mediated overexpression to assess its regulation of area-specific targeting of thalamocortical axons. Cereb Cortex. 2003;13:648–660. doi: 10.1093/cercor/13.6.648. [DOI] [PubMed] [Google Scholar]
- 15.Bayer SA, Altman J. Neocortical Development. New York: Raven Press; 1991. [Google Scholar]
- 16.Smart IH, McSherry GM. Growth patterns in the lateral wall of the mouse telencephalon. II. Histological changes during and subsequent to the period of isocortical neuron production. J Anat. 1982;134:415–442. [PMC free article] [PubMed] [Google Scholar]
- 17.Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- 18*.Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. In one of the first papers to track the lineage of upper layer neurons during cortical development, the authors use Svet1 as a marker to provide evidence that upper layer neurons are generated from the SVZ of the developing cortex. [DOI] [PubMed] [Google Scholar]
- 19.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 20.Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 22.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- 24*.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004 doi: 10.1038/nn1172. This report demonstrates directly that neural progenitor cells divide in both the VZ and SVZ to give rise to neurons. A series of elegant time-lapse experiments establishes the SVZ as a region of active neurogenesis. [DOI] [PubMed] [Google Scholar]
- 25**.Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. This paper describes real-time imaging of cell divisions in the VZ and SVZ and their correlation with precursor cell types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. Embo J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 28.Ochiai W, Minobe S, Ogawa M, Miyata T. Transformation of pin-like ventricular zone cells into cortical neurons. Neurosci Res. 2007;57:326–329. doi: 10.1016/j.neures.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Panto MR, Zappala A, Tuorto F, Cicirata F. Role of the Otx1 gene in cell differentiation of mammalian cortex. Eur J Neurosci. 2004;19:2893–2902. doi: 10.1111/j.0953-816X.2004.03326.x. [DOI] [PubMed] [Google Scholar]
- 30.Hirata T, Suda Y, Nakao K, Narimatsu M, Hirano T, Hibi M. Zinc finger gene fez-like functions in the formation of subplate neurons and thalamocortical axons. Dev Dyn. 2004;230:546–556. doi: 10.1002/dvdy.20068. [DOI] [PubMed] [Google Scholar]
- 31.Inoue K, Terashima T, Nishikawa T, Takumi T. Fez1 is layer-specifically expressed in the adult mouse neocortex. Eur J Neurosci. 2004;20:2909–2916. doi: 10.1111/j.1460-9568.2004.03763.x. [DOI] [PubMed] [Google Scholar]
- 32**.Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. This paper shows that Fezl (Fezf2) is required for the specification of corticospinal motor neurons and, conversely, that overexpression of Fezl leads to overproduction of subcortical projection neurons. [DOI] [PubMed] [Google Scholar]
- 33**.Chen JG, Rasin MR, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. This paper describes both the effects of knocking down Fezf2 (Zfp312) using siRNA in deep layer neurons and the misexpression of Fezf2 in upper layer neurons by in utero electroporation. The authors present evidence that Fezf2 regulates the formation of subcortical axonal projections in deep layer neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. This paper describes a screen to identify genes enriched in deep layer neurons and the functional characterization of Ctip2 (a gene identified in the screen) through analysis of axonal connections in Ctip2 mutant mice. [DOI] [PubMed] [Google Scholar]
- 35.Brayer KJ, Kulshreshtha S, Segal DJ. The protein-binding potential of C2H2 zinc finger domains. Cell Biochem Biophys. 2008 doi: 10.1007/s12013-008-9007-6. [DOI] [PubMed] [Google Scholar]
- 36.Brayer KJ, Segal DJ. Keep your fingers off my DNA: Protein-protein interactions mediated by C2H2 zinc finger domains. Cell Biochem Biophys. 2008;50:111–131. doi: 10.1007/s12013-008-9008-5. [DOI] [PubMed] [Google Scholar]
- 37.Topark-Ngarm A, Golonzhka O, Peterson VJ, Barrett B, Jr, Martinez B, Crofoot K, Filtz TM, Leid M. CTIP2 associates with the NuRD complex on the promoter of p57KIP2, a newly identified CTIP2 target gene. J Biol Chem. 2006;281:32272–32283. doi: 10.1074/jbc.M602776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, Aunis D, Rohr O. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. Embo J. 2007;26:412–423. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. This paper reports that Sox5 controls the timing of layer 5 neuron generation by suppressing Ctip2 expression in subplate and layer 6 neurons. Misexpression of Sox5 in upper layer neurons appears to convert their to resemble those of layer 6 neurons. [DOI] [PubMed] [Google Scholar]
- 40.Bulfone A, Martinez S, Marigo V, Campanella M, Basile A, Quaderi N, Gattuso C, Rubenstein JL, Ballabio A. Expression pattern of the Tbr2 (Eomesodermin) gene during mouse and chick brain development. Mech Dev. 1999;84:133–138. doi: 10.1016/s0925-4773(99)00053-2. [DOI] [PubMed] [Google Scholar]
- 41.Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 42.Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russ AP, Wattler S, Colledge WH, Aparicio SA, Carlton MB, Pearce JJ, Barton SC, Surani MA, Ryan K, Nehls MC, et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- 44.Iulianella A, Vanden Heuvel G, Trainor P. Dynamic expression of murine Cux2 in craniofacial, limb, urogenital and neuronal primordia. Gene Expr Patterns. 2003;3:571–577. doi: 10.1016/s1567-133x(03)00123-6. [DOI] [PubMed] [Google Scholar]
- 45.Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–1420. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
- 46**.Cubelos B, Sebastian-Serrano A, Kim S, Moreno-Ortiz C, Redondo JM, Walsh CA, Nieto M. Cux-2 controls the proliferation of neuronal intermediate precursors of the cortical subventricular zone. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm199. (E-pub ahead of print). This paper shows that Cux-2 positively regulates cell cycle exit in SVZ progenitors, leading to an increased number of upper layer neurons in Cux-2 knockout animals. [DOI] [PubMed] [Google Scholar]
- 47.Sugitani Y, Nakai S, Minowa O, Nishi M, Jishage K, Kawano H, Mori K, Ogawa M, Noda T. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 2002;16:1760–1765. doi: 10.1101/gad.978002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szemes M, Gyorgy A, Paweletz C, Dobi A, Agoston DV. Isolation and characterization of SATB2, a novel AT-rich DNA binding protein expressed in development- and cell-specific manner in the rat brain. Neurochem Res. 2006;31:237–246. doi: 10.1007/s11064-005-9012-8. [DOI] [PubMed] [Google Scholar]
- 49**.Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. This paper describes the phenotype of Satb2 mutant mice using a LacZ knock-in construct to analyze the axonal projections of neurons that normally express Satb2. In the absence of Satb2, callosal projections neurons extend axons toward subcortical targets. Satb2 binds to and modifies chromatin configuration at the Ctip2 locus. [DOI] [PubMed] [Google Scholar]
- 50**.Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. This paper describes the phenotype of Satb2 mutant mice and the effect of ectopic expression of Satb2 in deep layer neurons by in utero electroporation. The authors provide evidence that Satb2 interacts with the Ctip2 locus, where is controls chromatin remodeling. [DOI] [PubMed] [Google Scholar]
- 51*.Gyorgy AB, Szemes M, de Juan Romero C, Tarabykin V, Agoston DV. SATB2 interacts with chromatin-remodeling molecules in differentiating cortical neurons. Eur J Neurosci. 2008;27:865–873. doi: 10.1111/j.1460-9568.2008.06061.x. The authors demonstrate that SATB2 binds to histone deacetylase 1 and metastasis-associated protein 2, members of the nucleosome-remodeling and histone deacetylase complex. [DOI] [PubMed] [Google Scholar]
- 52.Koester SE, O’Leary DD. Connectional distinction between callosal and subcortically projecting cortical neurons is determined prior to axon extension. Devel Biol. 1993;160:1–14. doi: 10.1006/dbio.1993.1281. [DOI] [PubMed] [Google Scholar]