Abstract
Background
Induced external hypothermia during ventricular fibrillation (VF) improves resuscitation outcomes. Our objectives were twofold (1) to determine if very rapid hypothermia could be achieved by intrapulmonary administration of cold perfluorocarbons, thereby using the lungs as a vehicle for targeted cardiopulmonary hypothermia, and (2) to determine if this improved resuscitation success.
Methods
Part 1: Nine female swine underwent static intrapulmonary instillation of cold perfluorocarbons (PFC) during electrically induced VF. Part 2: Thirty-three female swine in VF were immediately ventilated via total liquid ventilation (TLV) with pre-oxygenated cold PFC (−15°C) or warm PFC (33°C), while control swine received no ventilation during VF. All swine in both part 1 and 2 underwent VF arrest for 11 minutes, then defibrillation, ventilation and closed chest massage until resumption of spontaneous circulation (ROSC). The endpoint was continued spontaneous circulation for one hour without pharmacologic support.
Results
Static intrapulmonary instillation of cold PFC achieved rapid cardiopulmonary hypothermia; pulmonary artery (PA) temperature of 33.5ºC±0.2 was achieved by 10 minutes. Nine of 9 achieved ROSC. Hypothermia was achieved faster using TLV: at 6 minutes VF, cold TLV temperature was 32.9 ±0.4°C vs. cold static instillation temperature 34.3±0.2°C. Nine of 11 cold TLV swine achieved ROSC for 1 hour vs. 3 of 11 control swine (p=0.03). Warm PFC also appeared to be beneficial, with a trend toward greater achievement of ROSC than control (ROSC; warm PFC 8 of 11 vs. control 3 of 11, p=0.09).
Conclusion
Targeted cardiopulmonary intra-arrest moderate hypothermia was achieved rapidly by static intrapulmonary administration of cold PFC and more rapidly by total liquid ventilation with cold PFC; resumption of spontaneous circulation was facilitated. Warm PFC showed a trend toward facilitating ROSC.
Keywords: Hypothermia, Cardiopulmonary Resuscitation, Ventricular Fibrillation, Perfluorocarbons
Introduction
The neuroprotective benefit of induced hypothermia after cardiac arrest is well established. Two recent prospective randomized trials demonstrated improved neurological outcomes in comatose patients treated with external cooling to achieve hypothermia (32°C–34°C) after being resuscitated from an out-of -hospital cardiac arrest. 1–3
Hypothermia induced by external cooling prior to VF improved resuscitation outcome in swine cardiac arrest models.4,5 Prior investigations from our laboratory have looked at the ability of pre-arrest hypothermia to improve defibrillation of short-duration VF. Rhee et al.4 used a swine model and induced hypothermia (33°C or 30°C) via external cooling with ice to the head, thorax, and abdomen. Their study concluded that moderate hypothermia facilitated transthoracic defibrillation in this swine model compared to normothermia.
Boddicker et al.5 used a swine model to study the effect of hypothermia induced pre-arrest on defibrillation and resuscitation. Cooling was achieved via external ice application to the head, thorax, and abdomen prior to electrically induced VF. The swine underwent 8-minutes of unsupported VF. Their study showed that when VF (cardiac arrest) occurred in the setting of hypothermia, defibrillation and resuscitation were facilitated compared to normothermia. ROSC was improved; less initial defibrillation shock energy was required and there was less late refibrillation.
Based on these earlier studies, we hypothesized that very rapid targeted cardiopulmonary hypothermia induced during VF cardiac arrest (i.e., “intra-arrest” hypothermia) would facilitate resuscitation. Rather than attempting to achieve systemic hypothermia, we utilized the pulmonary bed as the avenue for targeted hypothermia of the cardiac structures while maintaining a near normal core temperature. To accomplish this we used both static intrapulmonary instillation of cold PFC and cold total liquid ventilation which refers to a complete liquid system; the lungs and ventilatory system are ventilated with PFC and there is no air exchange.
Our objectives were to determine if static instillation or total liquid ventilation (TLV) using cold preoxygenated PFC could rapidly achieve moderate targeted cardiopulmonary hypothermia (~33ºC) and facilitate resuscitation in a large closed-chest animal model of VF cardiac arrest. We studied the effect of cold PFC, warm PFC and no PFC (control).
Methods
Animal Preparation
Approval was obtained from the University of Iowa Animal Care and Use Committee. Anesthesia was induced with Ketamine 20mg/kg and Acepromazine 0.2mg/kg administered intramuscularly followed by inhaled Halothane. Pentobarbital injections (2–6mg/kg/hr) along with supplemental Halothane (0.5–4%) were given throughout the study to maintain anesthesia. The animals underwent endotracheal (ET) intubation and were ventilated with O2 (100% O2) at 7 L/min to maintain physiologic arterial pH and pO2 greater than 100 Torr. Pulmonary artery (PA) temperature was measured using a Swan-Ganz thermodilution catheter. Core body temperature was measured by a temperature thermistor placed in the inferior vena cava (IVC).
General Protocol
After baseline hemodynamic parameters were recorded, VF was induced by delivery of 60Hz AC current applied to a right ventricular pacemaker catheter. VF was allowed to persist for 11 minutes, during which the experimental protocol was performed (see below). The swine were then defibrillated using a commercially available truncated exponential biphasic waveform defibrillator (Philips Medical Systems, Andover, MA) with anterior/posterior self-adhesive electrode pads and successive shocks at 50J/100J/150J/200J as needed. A successful shock terminated VF for at least 5 seconds. Cardiopulmonary resuscitation (CPR) utilized metronome-guided manual chest compressions at 100/minute and mechanical ventilation at 15 breaths per minute with 100% O2. Chest compression effectiveness and force was guided via an arterial catheter in the femoral artery; compression force sufficient to achieve an arterial systolic pressure of at least 60 mmHg was applied.
At the beginning of CPR, intra-abdominal pressure was increased by an abdominal cuff, inflated to 20 mmHg, which was maintained throughout the CPR period. Intraabdominal pressure was used in both Part 1 and Part 2 and used in all animals. Sustained abdominal compression improves coronary perfusion pressure during CPR.6 We used the method in all swine in our study during CPR. Manual chest compressions were interrupted for no more than 5 seconds every minute to observe the ECG and arterial pressure. The end point was resumption of spontaneous circulation (ROSC), defined as SAP >60 mmHg maintained for 1 hour without pharmacologic support or mechanical compression. Coronary perfusion pressure (CPP) was calculated. ABG were determined at 2.5 minutes and 6.5 minutes of CPR. Ventilation of the control animals was performed using 100% O2 at 7 L/min during CPR.
If ROSC occurred, hemodynamic measurements were monitored during the 60 minutes post-ROSC and adjustments in ventilation were made as necessary, guided by ABG. If ROSC did not occur, CPR was continued for at least 11 minutes, until resuscitation appeared highly improbable.
The experimental protocols described below are summarized in Figure 1.
Figure 1.
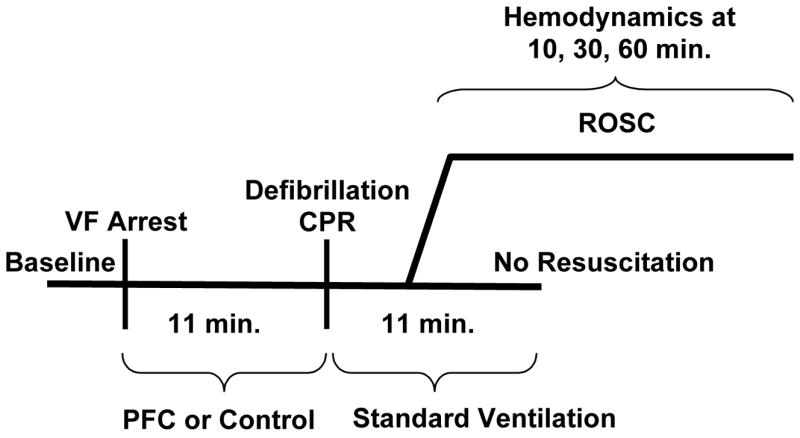
Protocols. ROSC = resumption of spontaneous circulation. PFC = perfluorocarbons. VF = ventricular fibrillation. Min = minutes.
Part 1
The purpose of these experiments was to determine the feasibility of static induction of chilled PFC for the induction of cardiac hypothermia. We studied a total of 9 female swine (19.5–25.9kg). Immediately after VF was initiated the pre-cooled (−12ºC), pre-oxygenated (100% O2, 2 minutes) PFC were instilled into the lungs. The endotracheal tube was disconnected from the ventilator and PFC (Fluorinert™ FC-77, 3M Company, St. Paul, MN), a 1:1 mixture of two isomers of C8F16O, 40ml/kg, was instilled into the lungs via the endotracheal tube. The tube was then plugged with a rubber stopper. After 11 minutes of unsupported VF, the endotracheal tube was unplugged and reconnected to the ventilator with the expiratory circuit connected to a reservoir to collect the PFC. Closed chest compression was begun; the PFC were expelled from the lungs by the force of chest compressions. The majority of the PFC fluid was expelled in the first minute of CPR.
CPR was begun immediately after the initial defibrillation shock(s), and included manual chest compressions and mechanical ventilation with 100% O2 at 7L/minutes. Epinephrine, 1mg IV, was given every minute after the first minute of CPR. Atropine, 0.4 mg IV, was given if atrioventricular block or severe sinus bradycardia were noted.
Part 2
The purpose of these experiments were to determine the feasibility and effectiveness of TLV with cold PFC for the induction of hypothermia compared to static instillation and to determine whether TLV with cold PFC has a different effect on ROSC compared to TLV with warm PFC or no intervention. Our goal was to achieve a faster cooling time and more controlled cooling. We studied a total of 33 female swine (weight 19.6–27.3 kg). The animals were randomly assigned to three groups (n=11 in each group) (1) cold PFC TLV (−15ºC); (2) warm PFC TLV (33ºC); (3) control (no PFC). In the two groups receiving PFC TLV, immediately after VF was initiated the ventilator system containing the PFC (Fluorinert™ FC-77, 3M Company, St. Paul, MN) was connected to the endotracheal tube and TLV with cold or warm PFC was initiated at 6 breaths/minute with a tidal volume of 200–220 cc and maintained for 10.5 minutes. Prior to installation the cold PFC were chilled to −15°C while the warm PFC were warmed to 33°C. The PFC were chilled prior to instillation to −15°C in order to cool the heart as quickly as possible. We anticipated rapid PFC warming once instilled into the lungs; indeed, the PFC temperature in the reservoir used for the liquid PFC reached 0°C in approximately one minute after instillation. Prior to installation both cold and warm PFC were pre-oxygenated (100% O2, 2 minutes). The liquid ventilation system consisted of a Harvard large animal respirator connected to a PFC reservoir which could be cooled or warmed (Figure 2). The system was a closed circuit in which PFC fluid was continuously circulated through the animal and reservoir system. Suction could be applied to the reservoir to increase recovery of PFC from lungs during exhalation in order to control ventilation pressure by modifying the amount of PFC in the lungs. The lungs were not allowed to fill with PFC above the filling volume of 40ml/kg. To minimize barotrauma during liquid ventilation, the ventilation rate was low, 6 breaths/minute, average maximum intra-tracheal pressure (16.2 mmHg±SE0.9) was kept close to normal air ventilation pressures by controlling intrapulmonary PFC volume via changes in ventilation rate and suction during exhalation. The highest average ventilation pressures were seen during minute 2 of liquid ventilation (20.9 mmHg±SE0.7). The highest ventilation pressure during one stroke was 26 mmHg. After 10 minutes, 30 seconds of arrest and liquid ventilation, the TLV ventilator was stopped in the exhalation phase, the ET tube was unplugged and the liquid PFC allowed to drain with the help of suction. The ET tube was reconnected to the air ventilator with the expiratory circuit connected to a reservoir to collect the residual PFC during the closed chest compression. As in part one, defibrillation was performed, chest compression begun and 100% O2 at 7L/minute ventilation was resumed at 11 minutes of VF. The majority of the PFC fluid was expelled by the end of the first minute of CPR.
Figure 2.
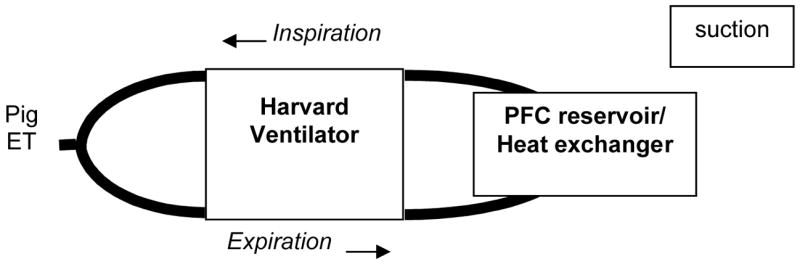
Total Liquid Ventilation System - During liquid ventilation, the primed liquid ventilation system was connected to the swine’s endotracheal tube. The pre-oxygenated warm or cold PFC was circulated through the reservoir and lungs via the ventilator during inhalation and exhalation. ET = endotracheal tube.
A third group of swine, serving as controls, received no air or PFC ventilation during the 11-minute arrest period. This group was designed as an animal model of the typical clinical situation of out-of-hospital cardiac arrest, where no or ineffective CPR is administered until EMS personal arrive.
After the arrest period, all swine were reconnected to the standard ventilator for respiratory support during resuscitation. CPR was begun after the initial defibrillation shock(s), As in Part 1, Epinephrine, 1mg IV, was given every three minutes after the first minute of CPR as needed for continuing hypotension (arterial pressure <60mmHg). Atropine, 0.4 mg IV, up to three doses was given if atrioventricular block or severe sinus bradycardia were noted.
Statistical Analysis
Data are reported as the mean ± standard error (SE) unless noted.
The baseline hemodynamic levels of the animals in the cold, warm, and control groups were compared using either the two-sample t-test or the Wilcoxon rank-sum test. The statistical program Proc Mixed 7 used for analysis of repeated measure experiments, mixed effect models and for the measurements that are carried over from one time point to another time point. In order to explore the time when the temperature difference begins to be evident between the groups, we defined a contrast. A repeated measures analysis of variance was utilized with PA temperature as a response, with the goal to assess the effect of the type of ventilation (cold, warm, and control). If statistical significance was reached, we used Bonferroni adjustment for pairwise comparison. We investigated the effect of CPR (CPR 2.5 and CPR 6.5) on the hemodynamics, pH, pCO2 and pO2. This analysis was conducted using a generalized linear model approach adjusted for the 3 three treatments (cold, warm and control) and their interaction with CPR. In order to investigate the effect of treatment (cold, warm, and control) on CPR chest compression rate (CR) and coronary perfusion pressure (CPP), we use a repeated measures analysis. To analyze the difference in defibrillation shocks between the cold, warm, and control groups we utilized a non-parametric analysis of variance procedure to test the equality of medians. We evaluated a Monte Carlo estimate of exact p-value through the Savage method. Fisher’s exact test was used to compare the percentage of ROSC between the groups.
Results
Part I
Baseline hemodynamics and hemodynamics during CPR are illustrated in Table 1. Table 2 shows ABG data at two time points during CPR. After induction of VF, cold PFC was instilled into the lungs via the ET tube. Both PA and IVC temperatures were recorded every minute from 2 minutes until 10 minutes to emphasize that cold PFC in the lungs cool only the cardiopulmonary structures and not the whole body. By 2 minutes after instillation the PA temperature had fallen significantly compared to baseline; by 10 minutes the PA temperature had fallen from baseline 37.1±0.2°C to 33.5±0.2°C, (p<0.0001) (Figure 3). There was only a minimal decrement in the IVC temperature: 37.0±0.1°C to 36.1±0.2°C (p<0.0001) between 2 and 10 min during VF arrest.
Table 1.
Hemodynamics, Part 1
Part 1: 11 min. arrest, cold static PFC installation
| Time during CPR
|
||||
|---|---|---|---|---|
| Baseline | I min | 5 min | 10 min | |
| MAP (mmHg) | 64±4 | 33±2 | 49±3 | 46±1 |
| HR/CR (bpm) | 120±3 | 97±2 | 92±1 | 93±1 |
| CVP (mmHg) | 8±0 | 13±2 (n=7) | 21±2 | 21±3 |
| CO (L/min) | 4.18±0.22 | - | - | - |
| CPP (mmHg) | 42±2 | 8±2 | 14±2 | 11±2 |
| MPAP (mmHg) | 17±0 | 39±3 | 60±6 | 66±8 |
| n=9 | n=9 | n=7 | n=2 | |
Abbreviations: MAP = mean arterial pressure, HR = heart rate (baseline), CR = compression rate (during CPR), CVP = central venous pressure, CO = cardiac output, CPP = coronary perfusion pressure, MPAP = mean pulmonary arterial pressure
n = animals not yet achieving ROSC
Table 2.
Arterial Blood Gases, Part 1
| 2.5 min. CPR n = 8
|
6.5 min. CPR n = 4
|
||||
|---|---|---|---|---|---|
| pH | pCO2
mmHg |
pO2
mmHg |
pH | pCO2
mmHg |
pO2
mmHg |
| 7.30±0.03 | 47±4 | 269±67 | 7.15±0.04 | 60±6 | 273±79 |
n = animals not yet achieving ROSC
Figure 3.
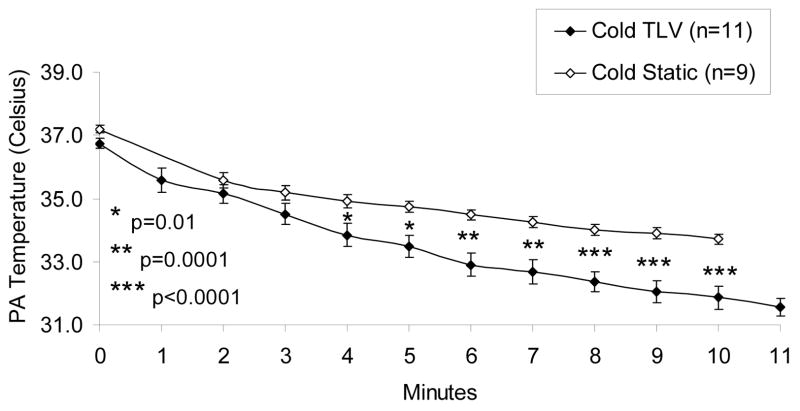
Temperature Comparison of Cold Static Instillation PFC Group vs. Cold Total Liquid Ventilation PFC Group. PA = pulmonary artery.
The PFC liquid was expelled via the ET tube into the reservoir upon initiation of CPR; the mean recovery of the PFC was 28.3±0.9 ml/kg, about 70% of the instillation volume, almost all in the first minute of closed-chest massage. ROSC was achieved in 100% (9/9) of the swine.
Part 2
There were no significant differences at baseline in weight, mean arterial pressure (MAP), heart rate (HR), central venous pressure (CVP), coronary perfusion pressure (CPP), and cardiac output (CO) between the three groups (Table 3). There were no significant differences in MAP or CVP between the three groups at 1, 5, or 10 minutes of VF (Table 3). Compression rate (during CPR) was significantly different when comparing the three groups at different time points. The compression rate was significantly less when comparing the cold TLV group and control group at 1, 5 and 10 minutes VF. The compression rate was significantly less at 1 and 5 minutes when comparing cold TLV to warm TLV. While a statistically significant difference in compression rate does exist between the groups, the physiologic significance of this is likely minimal (Table 3).
Table 3.
Hemodynamics, Part 2
Part 2: 11-minute arrest, TLV
| Group | Time | MAP (mmHg) | HR/CR (bpm) | CVP (mmHg) | CO (L/min) | CPP (mmHg) | n = |
|---|---|---|---|---|---|---|---|
| Cold TLV | Baseline | 63±2 | 123±9 | 8±0 | 4.11±0.32 | 44±2 | 11 |
| Warm TLV | Baseline | 60±2 | 122±5 | 8±1 | 3.76±0.30 | 41±2 | 11 |
| Control | Baseline | 63±1 | 125±7 | 8±1 | 3.90±0.21 | 43±2 | 11 |
|
| |||||||
| Cold TLV | 1 minute | 32±1 | 88±1* | 8±1 | - | 7±1 | 11 |
| Warm TLV | 1 minute | 37±2 | 93±0≠ | 10±1 | - | 9±2 | 11 |
| Control | 1 minute | 32±3 | 92±0 | 9±1 | - | 9±2 | 11 |
|
| |||||||
| Cold TLV | 5 minutes | 40±1 | 87±1** | 11±1 | - | 12±1 | 8 |
| Warm TLV | 5 minutes | 43±1 | 93±0σ | 15±1 | - | 9±1 | 10 |
| Control | 5 minutes | 42±2 | 92±0 | 14±1 | - | 12±1 | 11 |
|
| |||||||
| Cold TLV | 10 minutes | 38±1 | 84±3*** | 12±1 | - | 10±1 | 4 |
| Warm TLV | 10 minutes | 51±8 | 91±1 | 15±1 | - | 10±1 | 6 |
| Control | 10 minutes | 37±3 | 92±0 | 12±1 | - | 10±1 | 8 |
Abbreviations as in Table 1
Baseline variables show no significant differences between the three groups. No significant differences in MAP or CVP between the three groups at 1, 5, or 10 minutes of VF.
p=0.003 cold TLV vs. control at 1 minute VF
p=0.005 cold TLV vs. control at 5 minutes VF
p=0.005 cold TLV vs. control at 10 minutes VF
p=0.0009 cold TLV vs. warm TLV at 1 minute VF
p=0.002 cold TLV vs. warm TLV at 5 minutes VF
Compared to the experiments of part 1 using static endotracheal instillation of cold PFC, hypothermia (target 33ºC) was achieved faster using cold TLV: temperature differences were significant by 4 minutes of VF (Figure 3).
In the warm and control groups baseline PA temperatures were 37.6±0.4°C and 37.5±0.3°C, and did not change during VF (Figure 4). In all three groups there was no significant difference in the IVC temperatures when comparing time points.
Figure 4.
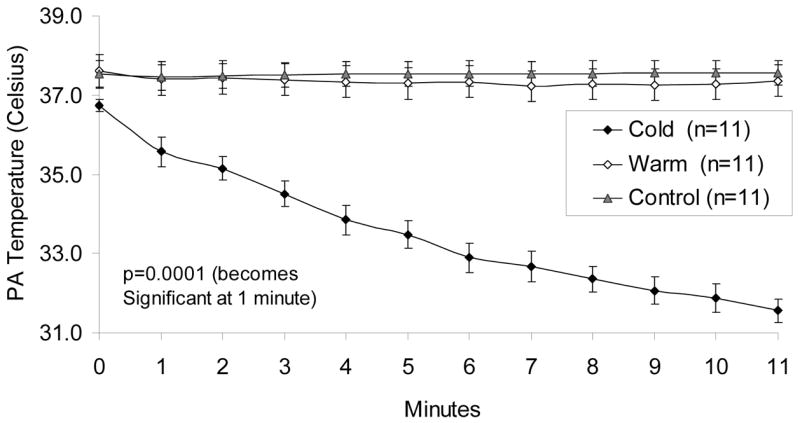
Total Liquid Ventilation: Pulmonary Artery Temperatures
ABG were determined at baseline (0), 2.5, 6.5 minutes of CPR and 60 minutes ROSC. Our ABG data obtained during CPR at 2.5 and 6.5 minutes showed no significant difference in pH and pCO2 between our three groups (Table 4). However, pO2 was significantly higher in the cold TLV group compared to warm PFC and control groups at 2.5 and 6.5 minutes of CPR (Table 4).
Table 4.
Arterial Blood Gases, Part 2
| Baseline | Arrest | ROSC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 min. | 2.5 min. | 6.5 min. | 60 min. | |||||||||
| pH | pCO2 | pO2 | PH | pCO2 | pO2 | pH | pCO2 | pO2 | pH | pCO2 | pO2 | |
|
| ||||||||||||
| Cold TLV | 7.41±0.01 | 41±1 | 153±5 | 7.29±0.02 | 49±3 | 157*±35 | 7.22±0.03 | 51±5 | 180**±36 | 7.30±0.02 | 45±2 | 151±20 |
| n = 11 | n = 11 | n = 7 | n = 9 | |||||||||
|
| ||||||||||||
| Warm TLV | 7.43±0.01 | 41±1 | 152±10 | 7.31±0.03 | 43±5 | 63±4 | 7.2±0.02 | 56±6 | 90±10 | 7.30±0.01 | 46±1 | 147±16 |
| n = 11 | n = 11 | n = 10 | n = 8 | |||||||||
|
| ||||||||||||
| Control | 7.41±0.01 | 44±1 | 144±9 | 7.3±0.02 | 49±2 | 71±15 | 7.22±0.03 | 57±3 | 59±6 | 7.35±0.06 | 39±2 | 176±35 |
| n = 11 | n = 11 | n = 11 | n = 3 | |||||||||
p=0.009 cold TLV vs. warm TLV and control,
p=0.002 cold TLV vs. warm TLV and control
There were no significant differences between the 3 groups in the defibrillation data (i.e. early shocks required for initial VF termination, late shocks required for refibrillation or total shocks).
Nine of 11 (82%) cold TLV swine achieved and maintained ROSC for 1 hour when compared to 3 of 11 (27%) control swine (p=0.03); eight of 11 (73%) warm TLV swine achieved and maintained ROSC vs. 3 of 11 C swine (p=0.09) (Figure 5).
Figure 5.
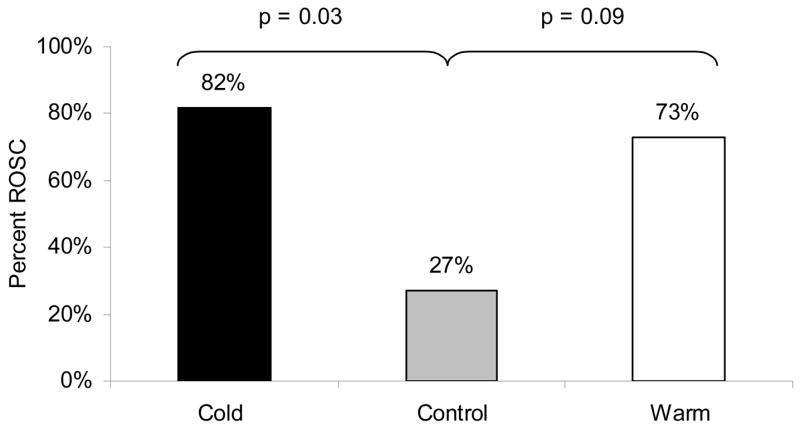
ROSC: Effect of Cold Total Liquid Ventilation, Warm Total Liquid Ventilation and Control.
Discussion
In this large animal model of long-duration VF cardiac arrest, static instillation of chilled PFC achieved rapid cardiopulmonary hypothermia. TLV with cold PFC achieved moderate hypothermia more rapidly than static instillation and achieved a higher ROSC rate compared to a control group which received no PFC. This control group was included to simulate a frequent clinical prehospital scenario of untreated VF, where bystander CPR is ineffective or not performed at all. This is the first study to report intra-arrest targeted cardiopulmonary cooling in a large closed-chest animal VF arrest/resuscitation model using static or TLV with cold oxygenated PFC.
Both PA and IVC temperatures were measured to emphasize that cold TLV cools only the cardiopulmonary structures (as measured in the pulmonary artery) and not the rest of the body (IVC measurement). The drop in the PA temperature is likely due to having very cold material in the lungs which are in close contact with the heart and pulmonary artery, during the period of circulatory arrest.
A trend (p=0.09) toward improved ROSC was evident in the warm PFC swine vs. control swine. This was not anticipated in our original hypothesis. How could warm PFC improve ROSC? The hemodynamic data, including CPP, were similar in the 3 porcine groups. PFC cold or warm may improve gas exchange by recruiting dependent lung regions. PFC may improve lung compliance and matching of ventilation and perfusion.8 Liquid ventilation has been associated with a reduction in the amounts and types of mediators released by pulmonary inflammatory cells.9
Abella et al.10 described a murine model of KCl induced cardiac arrest. Mice that received rapid intra-arrest cooling by external ice application to 30°C had greater initial and 72 hour survival following resuscitation than those mice with delayed cooling or normothermia. This result is consistent with chick cardiomyocyte ischemia models, where hypothermia preceding reperfusion yielded less cell death and better return of contractions.11
Unfortunately most methods currently available for myocardial cooling are too slow. External cooling in humans and large mammals requires several hours to achieve therapeutic hypothermia.4,5 Cold PFC offer a more rapid alternative. Harris et al.12 achieved rapid whole body cooling times in dogs using cyclic lung lavage with FC-75 PFC administered through a dual-lumen endotracheal system (gas/liquid ventilation). Tissier et. al.8 recently reported a small animal (rabbit) open-chest myocardial infarct model utilizing TLV with PFC to achieve rapid cardiac cooling. Their study focused on limiting myocardial infarct size by inducing hypothermia with TLV; VF and resuscitation were not studied.
Intravenous (IV) administration of cold saline has been shown to rapidly reduce body temperature in animals and humans, 13,14 and a recent trial of administering IV saline to just resuscitated patients during ambulance transport has demonstrated the clinical feasibility of this cooling approach. 15 The effect of administration cold IV saline on ROSC and long term survival is unknown.
PFC are clear, colorless, odorless, non-conducting, and nonflammable liquids. They are twice as dense as water and have excellent gas-carrying capacity, mainly oxygen and carbon dioxide.16 Previous clinical trials of liquid ventilation have emphasized improved oxygenation rather than cooling.17–20 Our ABG data obtained during CPR at 2.5 and 6.5 minutes showed no significant difference in pH and pCO2 between our three groups. However, pO2 was significantly higher in the cold PFC group compared to warm PFC and control groups. The higher pO2 in the cold TLV group could have contributed to improve ROSC. However, the warm TLV group had very similar pO2 levels compared with the control group and the warm TLV ROSC was near significant (p=0.09) compared with the controls. Thus, ROSC was improved in the warm TLV group even without the elevated pO2 levels. On balance, it appears that the improved oxygenation in cold TLV possibly contributed to improved ROSC, but other factors may play a role.
We utilized only female swine in our experiments because earlier intra-arrest PFC data in our laboratory revealed significantly improved ROSC in female swine compared to male swine. There were no differences at baseline or confounding variables to explain this male-female difference in these prior experiments. To our knowledge there are no data in the literature citing the differences in the protective effect of hypothermia based on gender. The endothelial protective function of physiologic amounts of estrogen is known, 21 but our swine were pre-pubertal.
TLV with PFC appeared to have lung toxicity in these experiments. We noted that after the one hour post-resuscitation ROSC period, TLV animals were becoming hypoxic, acidotic, and tachypneic when the ventilator was removed. In one animal we obtained lung tissue for histopathologic examination, which revealed pulmonary edema. What are possible mechanisms for this toxicity?
Atelectasis is a known complication of PFC use.8 Tissier et al 8 noted that pulmonary toxicity was avoided in their rabbit model by use of positive-end-expiratory pressure (PEEP), intended to avoid atelectasis. However, in preliminary experiments from our laboratory, PEEP at 2 and 5 cmH2O during the resuscitation phase did not prevent the PFC pulmonary toxicity in our resuscitation model.
The particular PFC we used is an industrial fluorinated liquid intended for use as a solvent, not for biologic purposes. At the time of this study no biologic grade PFC’s were available.
Previous investigators have demonstrated a species-dependent lung susceptibility to chemicals that appears to be directly related to pulmonary intravascular macrophages (PIMs).22–24 PIMs are a separate class of pulmonary macrophages within the mononuclear phagocyte system which are responsible for circulating particle uptake within the pulmonary capillary endothelium. There are marked species differences in the fate of circulating particles; swine have extensive pulmonary localization due to phagocytosis by PIMs.24 Swine appear to be very sensitive to foreign materials, such as Albunex (an ultrasound contrast agent) and thus, may be sensitive to PFC. Acute pulmonary hypertension, pulmonary edema and respiratory distress develop in swine when exposed to these foreign materials. The susceptibility of the lungs of swine appears mediated via arachidonic acid cascade metabolites and treatment with indomethacin or thromboxane A2 receptor antagonist appears to prevent the acute pulmonary hypertension. The same process may be occurring in the swine when exposed to PFC resulting in an inflammatory response in the microvasculature resulting in acute lung injury. 22–24
Recent preliminary experiments in our laboratory have utilized another PFC (Perflutel RM 101, Miteni, Milan, Italy). This PFC is compatible with biologic use and was utilized in small animal models (rabbits) of TLV by Tissier et al. 8 where it did not cause lung toxicity. However, it did cause similar pulmonary toxicity in our swine model. Rabbits do not have the high concentration of PIMS that swine possess. This supports the suggestion that the lung toxicity is species-specific, related to PIMS.
Harris et al 12 using a combined gas-liquid ventilation technique in dogs, reported “foam-rubber” lung lesions suggesting both barotrauma and volume trauma. We maintained peak intratracheal inspiratory pressures at 21 mmHg or less via a pressure release valve in the two TLV animal groups. The cold PFC was pre-cooled to −12ºC before their introduction into the lungs. This very cold liquid might have caused damage directly through freezing lung tissue. However, post-ROSC hypoxia and acidosis were also seen in warm TLV animals, where freezing could not occur. Further TLV studies using PFC formulated for biologic use need to be done to further understand the mechanism and prevention of pulmonary toxicity if this approach is to be clinically feasible.
Limitations
Intratracheal PFC instillation immediately after cardiac arrest requires endotracheal intubation, which is not performed in many clinical situations. Our method of targeted cardiopulmonary hypothermia will not cool the brain, and therefore may not achieve the cerebral protection accomplished by methods such as external cooling by ice which is slow, or by a single aortic flush of cold (4º C) saline, which is rapid.25 Conceivably however, the method we studied, intratracheal PFC instillation, could be combined with external cooling and/or IV cold saline to cool both heart and brain.
ROSC is a surrogate for long-term neurologically intact survival from cardiac arrest, the ultimate goal of resuscitation research. The long-term benefit to the heart and brain of either cold or warm PFC during resuscitation, if any, remains to be established.
Our mechanical ventilation rate was 15 breaths/minute which is higher than current American Heart Association resuscitation guidelines. All groups received the same ventilation rate.
Conclusion
Targeted cardiopulmonary intra-arrest moderate hypothermia was achieved very rapidly via total liquid ventilation with cold perfluorocarbons. This facilitated resumption of spontaneous circulation. A trend toward similar benefit using warm PFC was also demonstrated.
Supplementary Material
Acknowledgments
Supported in part by NHLBI grant #5 RO1 HL 71676-03. No assistance was used in the writing of the manuscript.
Abbreviations
- VF
Ventricular fibrillation
- PFC
Perfluorocarbons
- TLV
Total liquid ventilation
- ROSC
Resumption of spontaneous circulation
- ET
Endotracheal
- ABG
Arterial blood gases
- IVC
Inferior vena cava
- MAP
Mean arterial pressure
- SAP
Systemic arterial pressure
- HR
Heart rate
- PAP
Pulmonary artery pressure
- CVP
Central venous pressure
- CO
Cardiac output
- ECG
Electrocardiogram
- CPR
Cardiopulmonary resuscitation
- CPP
Coronary perfusion pressure
Footnotes
Conflict of Interest Statement None of the authors have any financial or personal relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kimberly S. Staffey, Email: Kimberly-staffey@uiowa.edu.
Raghuveer Dendi, Email: rdendi@bidmc.harvard.edu.
Leonard A. Brooks, Email: Leonard-brooks@uiowa.edu.
Andrew M. Pretorius, Email: Andrew-pretorius@uiowa.edu.
Laynez W. Ackermann, Email: Laynez-ackermann@iowa.edu.
K. D. Zamba, Email: Gideon-zamba@uiowa.edu.
Eric Dickson, Email: Eric-dickson@iowa.edu.
Richard E. Kerber, Email: Richard-kerber@iowa.edu.
References
- 1.The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 2.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the international liaison committee on resuscitation. Circulation. 2003;108:118–21. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 4.Rhee BJ, Zhang Y, Boddicker KA, Davies LR, Kerber RE. Effect of hypothermia on transthoracic defibrillation in a swine model. Resuscitation. 2005;65:79–85. doi: 10.1016/j.resuscitation.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Boddicker KA, Zhang Y, Davies R, Kerber RE. Hypothermia improves defibrillation success and resuscitation outcomes from ventricular fibrillation. Circulation. 2005;111:3195–3201. doi: 10.1161/CIRCULATIONAHA.104.492108. [DOI] [PubMed] [Google Scholar]
- 6.Lottes AE, Rundell AE, Geddes LA, Kemeny AE, Otlewski MP, Babbs CF. Sustained abdominal compression during CPR raises coronary perfusion pressures as much as vasopressor drugs. Resuscitation. 2007;75:515–524. doi: 10.1016/j.resuscitation.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 7.The SAS System for Mixed Models’, SAS version 9.1. SAS Institute Inc.; Cary, NC: [Google Scholar]
- 8.Tissier R, Hamanaka K, Kuno A, Parker J, Cohen M, Downey J. Total liquid ventilation provides ultra-fast cardioproective cooling. J Am Coll Card. 2007;49:6001–5. doi: 10.1016/j.jacc.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Wolfson M, Shaffer T. Liquid ventilation: an adjunct for respiratory management. Pediatric Anesthesia. 2004;14:15–23. doi: 10.1046/j.1460-9592.2003.01206.x. [DOI] [PubMed] [Google Scholar]
- 10.Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–91. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 11.VandenHoek T, Shao Z, Chang-Qing L, et al. Do we reperfuse or cool down first to resuscitate ischemic tissue? (abstr) Circulation. 2000;102:II-570. [Google Scholar]
- 12.Harris SB, Darwin MG, Russell SR, O’Farrell JM, Fletcher M, Wowk B. Rapid (0.5 degrees C/min) minimally invasive induction of hypothermia using cold perfluorochemical lung lavage in dogs. Resuscitation. 2001;50:189–204. doi: 10.1016/s0300-9572(01)00333-1. [DOI] [PubMed] [Google Scholar]
- 13.Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56:9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 14.Nordmark J, Rubertsson S. Induction of mild hypothermia with infusion of cold (4°C) fluid during ongoing experimental CPR. Resuscitation. 2005;66:357–365. doi: 10.1016/j.resuscitation.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Kim F, Olsufka M, Longstreth W, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4°C normal saline. Circulation. 2007;115:3064–3070. doi: 10.1161/CIRCULATIONAHA.106.655480. [DOI] [PubMed] [Google Scholar]
- 16.Kaisers U, Kelly KP, Busch T. Liquid ventilation. Br J Anaesth. 2003;91:143–51. doi: 10.1093/bja/aeg147. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan JS, Wolfson MR, Rubenstein SD, Shaffer TH. Liquid ventilation of preterm baby. Lancet. 1989;2:1095. doi: 10.1016/s0140-6736(89)91101-x. [DOI] [PubMed] [Google Scholar]
- 18.Kacmarek RM, Wiedemann HP, Lavin PT, Wedel MK, Tutuncu AS, Slutsky AS. Partial liquid ventilation in adult patients with the acute respiratory distress syndrome. Am J Respir and Crit Care Med. 2006;173:882–89. doi: 10.1164/rccm.200508-1196OC. [DOI] [PubMed] [Google Scholar]
- 19.Hirschl RB, Pranikoff T, Wise C, et al. Initial experience with partial liquid ventilation in adult patients with the acute respiratory distress syndrome. JAMA. 1996;275:383–89. [PubMed] [Google Scholar]
- 20.Leach CL, Greenspan JS, Rubenstein SD, et al. Partial liquid ventilation with perflubron in premature infants with severe respiratory distress syndrome. The LiquiVent Study Group. N Engl J Med. 1996;335:761–67. doi: 10.1056/NEJM199609123351101. [DOI] [PubMed] [Google Scholar]
- 21.Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovas Res. 2002;53:597–604. doi: 10.1016/s0008-6363(01)00473-4. [DOI] [PubMed] [Google Scholar]
- 22.Brain JD, Molina RM, DeCamp MM, Warner AE. Pulmonary intravascular macrophages: their contribution to the mononuclear phagocyte system in 13 species. Am J Physiol. 1999;276:L146–L154. doi: 10.1152/ajplung.1999.276.1.L146. [DOI] [PubMed] [Google Scholar]
- 23.Ostensen J, Hede R, Myreng Y, Ege T, Holtz E. Intravenous injection of Albunex microspheres causes thromboxane mediated pulmonary hypertension in pigs, but not in monkeys or rabbits. Acta Physiol Scand. 1992;144:307–315. doi: 10.1111/j.1748-1716.1992.tb09299.x. [DOI] [PubMed] [Google Scholar]
- 24.Winkler GC. Review of the significance of pulmonary intravascular macrophages with respect to animal species and age. Expl Cell Biol. 1989;57:281–286. doi: 10.1159/000163539. [DOI] [PubMed] [Google Scholar]
- 25.Behringer W, Prueckner S, Kentner R, et al. Rapid hypothermic aortic flush can achieve survival without brain damage after 30 minutes cardiac arrest in dogs. Anesthesiology. 2000;93:1491–99. doi: 10.1097/00000542-200012000-00022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.