Summary
In diverse eukaryotes micro RNAs (miRNAs) and small interfering RNAs (siRNAs) regulate important processes that include mRNA inactivation, viral defense, chromatin modification, and transposon silencing. Recently, nucleolus-associated Cajal bodies in plants have been implicated as sites of siRNA and miRNA biogenesis, whereas in animals siRNA and miRNA dicing occurs in the cytoplasm. The plant nucleolus also contains proteins of the nonsense-mediated mRNA decay pathway that in animals are found associated with cytoplasmic processing bodies (P-bodies). P-bodies also function in the degradation of mRNAs subjected to miRNA and siRNA targeting. Collectively, these observations suggest interesting variations in the way siRNAs and miRNAs can accomplish their similar functions in plants and animals.
Introduction
siRNAs and miRNAs are ~20–25 nt RNAs involved in silencing homologous genes or their transcripts [1,2] (See the Glossary for abbreviations used in this review). miRNAs typically inactivate developmentally important mRNAs by inhibiting their translation or bringing about their cleavage and degradation. Like miRNAs, siRNAs can target homologous mRNAs for cleavage and degradation [3], but they can also cause transcriptional silencing of homologous DNA sequences [4,5]. In plants, and perhaps mammals, this involves a process known as RNA-directed DNA methylation [6]. In at least some systems siRNAs also transcriptionally silence genes by directing repressive post-translational modifications of histones [4,5,7].
BOX 1- Glossary of Abbreviations used in the article
AGO: ARGONAUTE, proteins in this family bind small RNAs including siRNAs and miRNAs
DCL1: Arabidopsis DICER-LIKE 1, involved in miRNA biogenesis
DCL3: Arabidopsis DICER-LIKE 3, involved in 24 nt siRNA biogenesis
DCR1: Drosophila DICER 1, involved in the biogenesis of miRNAs
DCR2: Drosophila DICER 2, involved in the biogenesis of siRNAs
DRD1: DEFECTIVE IN RNA-DIRECTED DNA METHYLATION 1, a putative c hromatin remodeling protein involved in RNA-directed DNA methylation
DRM2: DOMAINS REARRANGED METHYLYTRANSFERASE 2, the primary Arabidopsis de novo DNA methyltransferase
dsRNA: double-stranded RNA
EJC: Exon-joining complex; proteins that remain asociated with spliced mRNAs
GFP: GREEN FLUORESCENT PROTEIN
GW182: a glycine (G) and tryptophan (W)-rich protein enriched in P-bodies (also known as GW-bodies)
HYL1: HYPONASTIC LEAVES 1, a dsRNA-binding protein that interacts with DCL1
LOQ: Drosophila RNA-binding protein LOQUACIOUS
miRNA: microRNA
NMD: Nonsense-mediated decay pathway, functions in the elimination of aberrant mRNAs
P-body: Processing bodies involved in post-transcriptional regulation and fates of mRNAs
Pol II: RNA POLYMERASE II; mRNA and miRNA genes are transcribed by Pol II
R2D2: Drosophila protein with both RNA and DNA-binding domains
RDR2: RNA-DEPENDENT RNA POLYMERASE 2, required for the biogenesis of 24nt siRNAs in Arabidopsis
RISC: RNA-induced silencing complex, includes an ARGONAUTE protein and siRNA or miRNA
RNA: Ribonucleic acid
RNP: ribonucleoprotein, a complex of RNA and proteins
siRNA: small interfering RNA
snRNP: small nuclear ribonucleoprotein
snRNA: small nuclear RNA present in snRNPs, typically involved in mRNA processing
snoRNP: small nucleolar ribonucleoprotein
TMG cap: the 2,2,7-trimethyguanosine (TMG) cap on the 5' ends of snRNAs
miRNAs and siRNAs are generated from double-stranded RNA (dsRNA) precursors by Dicer endonucleases. Whereas miRNAs are derived from dedicated genes that produce transcripts that fold into imperfect dsRNA hairpins, siRNAs are generated from perfectly-paired dsRNAs that result from transcripts of overlapping genes, viruses that replicate via dsRNA intermediates or RNA-dependent RNA polymerases that generate complementary strands from single-stranded RNA templates [8]. Fission yeast (S. pombe), nematodes (C. elegans) and mammals have a single Dicer; Drosophila has two dicers and Arabidopsis has four. Arabidopsis Dicers, which figure prominently in our review, have partially redundant functions, but DCL1 is primarily responsible for miRNA production whereas DCL2, DCL3 and DCL4 specialize in siRNA production [9,10]. Following dicing, single-stranded siRNAs or miRNAs associate with effector complexes [e.g. RNA-induced silencing complexes (RISCs)] that mediate gene silencing. At the heart of these effector complexes is an Argonaute (AGO) family protein that binds the siRNA or miRNA [11,12]. The RISC then uses the associated small RNA to seek out homologous target sites in order to bring about the translational arrest or degradation of mRNAs or the transcriptional silencing of chromatin targets [2,4,5]. Although plant and animal miRNA and siRNAs mediate similar functions, recent findings indicate that their biogenesis may take place in different compartments of the nucleus (Figure 1) or cytoplasm, the implications of which are the focus of our review.
Figure 1.
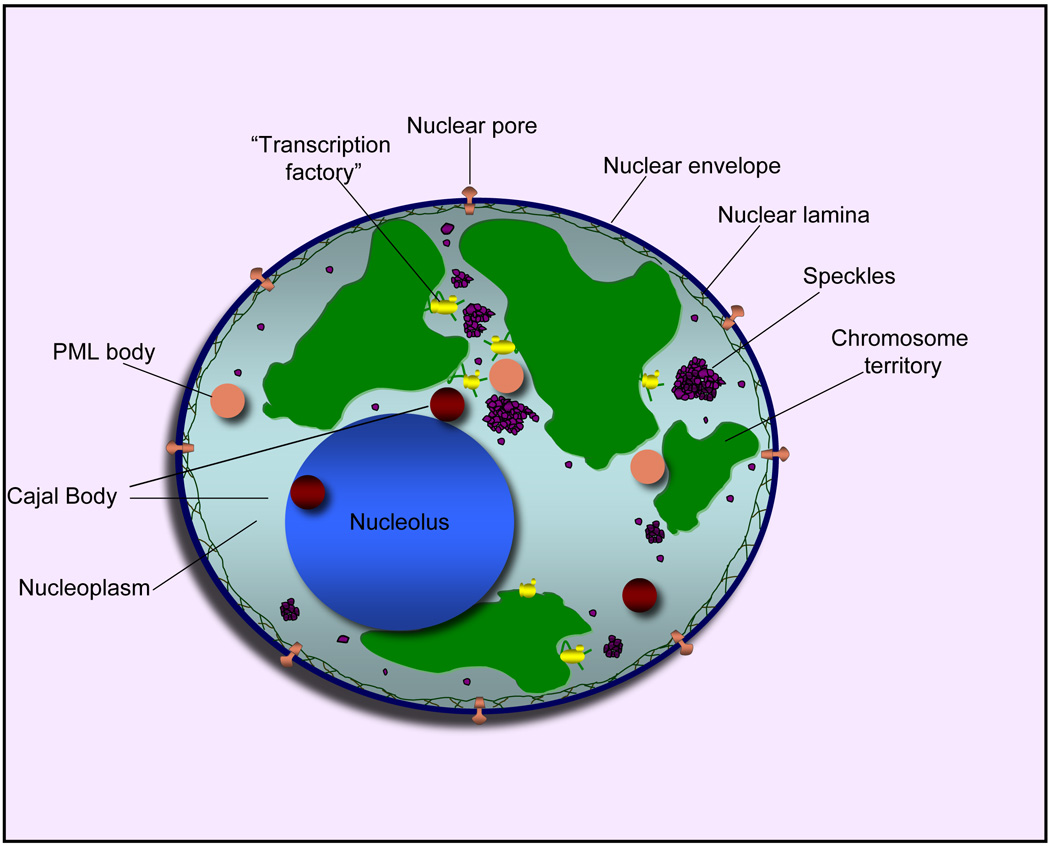
Compartments of the interphase cell nucleus. The nuclear envelope is a double membrane punctuated by nuclear pores through which molecules traffic to and from the cytoplasm. The nuclear lamina is a meshwork of proteins mediating nuclear envelope structure and chromatin attachment. In the nucleus, chromosomes occupy non-random positions known as chromosome territories [52]. Genes transcribed simultaneously tend to cluster in "transcription factories” [53]. In the vicinity of transcription factories for Pol II-transcribed protein-coding genes, speckles serve as storage, recycling or assembly sites for snRNPs and other splicing proteins. The nucleolus is the site of ribosome biogenesis and numerous RNA-related functions. Cajal bodies are spherical structures involved in the biogenesis of ribonucleoprotein complexes including siRNA and miRNA RISC complexes in plants. PML bodies are linked to various aspects of transcriptional regulation, virus accumulation, tumor suppression and DNA repair.
Dicing in animals
In Drosophila, representing the animal kingdom for our discussion, miRNA primary transcripts are first cleaved in the nucleus by the DROSHA endonuclease in a reaction aided by the double-stranded RNA-binding domain protein PASHA (Figure 2) [13,14]. The resulting pre-miRNA is exported to the cytoplasm by EXPORTIN-5, a Ran-GTP dsRNA binding protein [15]. In the cytoplasm, DCR1 cleaves the pre-miRNA into a mature miRNA duplex. The duplex is unwound and the miRNA strand becomes bound to AGO1 with the help of LOQ, a protein with three dsRNA binding domains [16]. A similar small RNA loading process involving R2D2, which has two RNA binding domains, is responsible for siRNA association with AGO2 following cytoplasmic DCR2 dicing [17]. In mammals and nematodes that have only one Dicer, miRNAs and siRNAs are necessarily produced by the same enzyme. Following assembly of the miRNA or siRNA RISC complexes, there is increasing evidence that they exert their post-transcriptional regulatory functions in the context of processing bodies, or P-bodies (see Figure 2), as we will discuss.
Figure 2.

Roles of P-bodies (processing bodies) in miRNA and siRNA functions and post-transcriptional regulation in animals. Pol II transcription gives rise to imperfectly base-paired double-stranded pri-miRNAs as well as pre-mRNAs, both of which undergo processing in the nucleus followed by export to the cytoplasm. Pre-miRNAs processed by Drosha in the nucleus undergo further cleavage by Dicer in the cytoplasm and one strand is loaded into an Argonaute-containing RNA-induced silencing complex (RISC; denoted miRISC in the figure). Similarly, perfectly base-paired double-stranded siRNA precursors are diced and one strand is loaded into a RISC complex (denoted siRISC). The miRNA or siRNA strands within the RISC complexes target homologous mRNAs for translational arrest or destruction in P-bodies (GW bodies) that are enriched for activities including the AGO-interacting GW182 protein, decapping (DCP) and exonuclease (XRN1) enzymes and proteins of the nonsense-mediated decay (NMD) pathway. mRNAs targeted to P-bodies can also be stored and released to the cytoplasm for translation.
A nucleolus associated siRNA processing center in plants
In Arabidopsis, a pathway generating 24 nt siRNAs is responsible for RNA-directed DNA methylation and transcriptional silencing of homologous loci [6,8,18]. The pathway begins with RNA POLYMERASE IVa (Pol IVa), a plant-specific nuclear enzyme that is structurally related to DNA dependent RNA polymerases including Pol I, II and III [19,20]. Acting downstream of Pol IVa is RNA-DEPENDENT RNA POLYMERASE 2 (RDR2), one of six Arabidopsis RDRs (Figure 3). RDR2 is thought to generate dsRNAs that are cleaved by DICER-LIKE 3 (DCL3) and then associate with ARGONAUTE4 (AGO4). Resulting AGO4 effector complexes mediate the methylation of siRNA-homologous loci in a process involving Pol IVb (an alternative form of Pol IV with a distinct subunit structure), the chromatin remodeler DRD1 and the de novo DNA methyltransferase, DRM2 (Figure 3) [6]. Some of these proteins colocalize with the source/target loci, including Pol IVa, Pol IVb, and DRD1 [21]. However RDR2, DCL3 and AGO4 colocalize with one another at a distance from the source/target loci within a compartment in the nucleolus [21,22]. This compartment lacks pre-ribosomal RNA, which is abundant elsewhere in the nucleolus, but contains 24 nt siRNAs detectable by RNA- fluorescence in situ hybridization [21]. Colocalization of RDR2, DCL3, AGO4 and siRNAs suggest that this compartment is a center for siRNA processing and AGO4 effector complex assembly [21,22]. The fact that the siRNA processing center is distant from the source/target loci indicates that trafficking of RNAs and AGO4 effector complexes must take place within the nucleus, by unknown mechanisms (Figure 3).
Figure 3.
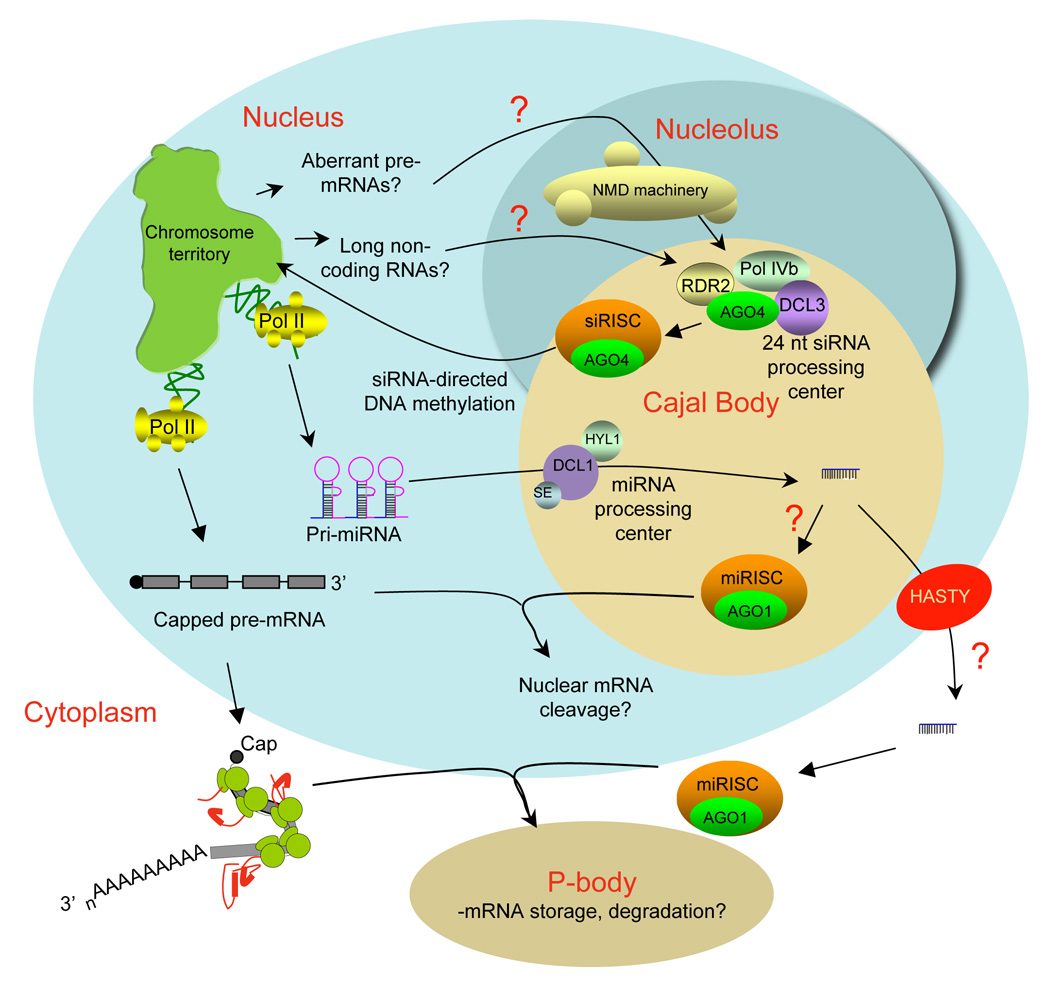
The role of Cajal bodies in miRNA and siRNA biogenesis and function in plants. In Arabidopsis, miRNA processing by DCL1 and 24 nt siRNA production by DCL3 occur within processing centers located in nucleolus-associated Cajal bodies rather than in the cytoplasm, as in animals. Multiple proteins of the nonsense-mediated decay pathway also localize within the nucleolus, suggesting that aberrant mRNAs or non-coding RNAs may be sensed and degraded in the nucleus, possibly feeding into the siRNA-directed DNA methylation pathway in order to silence the offending loci. It is possible that AGO1-mediated miRNA targeting of mRNAs or pre-mRNAs may take place primarily in the nucleus. However, the involvement of the Exportin 5 homolog, HASTY in some miRNA-mediated developmental phenomena suggests that at least some miRNAs function in the cytoplasm, presumably in the context of P-bodies, as in animals.
A miRNA processing center in plants is also associated with the nucleolus
Unlike animals that use Drosha and Dicer to produce miRNAs, Arabidopsis miRNA production is accomplished solely by DICER-LIKE 1 (DCL1) in a process assisted by HYL1, a dsRNA-binding protein that interacts with DCL1 and presumably plays a role analogous to PASHA in flies [23]. Also involved in miRNA biogenesis is a zinc finger protein SERRATE (SE), whose precise role in the process is unknown but whose mutation causes a general defect in miRNA production [24,25]. An Exportin 5 homolog, HASTY is thought to mediate the export of at least some miRNAs to the cytoplasm where miRNAs are expected to exert their negative regulation of mRNAs, presumably in the context of P-bodies, as in animals [26].
Recently, DCL1, HYL1 and SE were found to colocalize within nucleolus-associated bodies similar in appearance to DCL3-containing siRNA processing centers [27–30]. Moreover, fluorescently-tagged miRNA precursors colocalize with the DCL1, HYL1 and SE bodies (Figure 3), indicating that these bodies are sites of miRNA processing [27,28].
The plant DCL3 and DCL1 processing centers correspond to Cajal bodies
What are siRNA and miRNA processing centers doing in the nucleolus? The nucleolus is well-known as the site of ribosomal RNA (rRNA) synthesis, rRNA processing and ribosome assembly, a process that includes RNA polymerase I transcription, multiple pre-rRNA cleavages, and methylation and pseudouridylation (base modification) events that are guided by small nucleolar RNAs, or snoRNAs [31]. However, the nucleolus also participates in tRNA transcription and processing, mRNA maturation, RNA deamination by ADAR proteins and biogenesis or storage of signal recognition particle and telomerase ribonucleoprotein (RNP) complexes [31–33]. Therefore a nucleolar role in siRNA biogenesis is plausible. A related possibility tested by Li et al. [22] was that the DCL3/AGO4-containing siRNA processing center might be a Cajal body, named in honor of Ramon y Cajal, who referred to the structures as nucleolar accessory bodies [32,34–37]. Cajal bodies are common in the nucleolar periphery but can also be observed in the nucleoplasm (see Figure 1). They are highly dynamic structures, in a continual state of assembly/disassembly, that are implicated in trafficking RNA polymerase I, II and III transcription factors, assembly of snoRNA ribonucleoprotein (snoRNP) complexes, base modification of spliceosomal small nuclear RNAs (snRNA) guided by Cajal body-specific scaRNAs, and assembly of spliceosomal snRNP complexes involved in mRNA-splicing [32,34–36]. snoRNPs are thought to accumulate in Cajal bodies en route to the nucleolus whereas snRNPs are thought to traffic through Cajal bodies en route to nuclear speckles and spliceosomes [32,34]. Cajal bodies also play roles in the assembly and trafficking of telomerase, which is sequestered in the nucleolus until telomeres replicate in S phase [38] and of U7 snRNP, which functions in co-transcriptional histone mRNA 3' end processing [32,34].
The hypothesis that DCL3/AGO4 siRNA processing centers correspond to Cajal bodies has been tested using antibodies recognizing several Cajal body markers, specifically the 2,2,7-trimethyguanosine (TMG) cap on the 5' ends of spliceosomal snRNAs (except U6), the SmD3 protein (one of seven Sm proteins common to all spliceosomal snRNPs except U6 snRNP) and the U2B" protein that associates with U2 snRNP [22]. All three were found to colocalize with AGO4 at the nucleolar siRNA processing center. TMG, SmD3 and U2B" are also spliceosome components, but spliceosomes associate with sites of pol II transcription in the nucleoplasm and are not expected to be present in the nucleolus, from which pol II appears to be excluded [21]. Collectively, these findings indicate that the siRNA processing center is a Cajal body, an unexpected and new role for Cajal bodies [22].
The DCL1-containing bodies that also localize to the nucleolar periphery in Arabidopsis were initially thought to be unrelated to Cajal bodies because they do not colocalize with structures enriched for the Cajal body protein coilin [28,30]. The DCL1 bodies nonetheless colocalize with the SmB and SmD3 snRNP proteins [27], suggesting that Arabidopsis nuclei have Cajal bodies which lack coilin as well as Cajal bodies that contain coilin. An interesting parallel is that coilin is absent from the Drosophila genome, yet flies have Cajal bodies that contain nucleolar and snRNP components as well as distinct bodies that contain U7 snRNP and have been named Histone Locus Bodies [39].
P-bodies as sites of siRNA and miRNA action in animal cells
There is mounting evidence that siRNAs and miRNAs bring about mRNA degradation or translational arrest in cytoplasmic P-bodies where AGO proteins, miRNAs, target mRNAs and processing intermediates are all detected (see Figure 2) [40,41]. AGO proteins interact with P-body proteins that include GW182 and the DCP1/DCP2 decapping enzyme complex that removes the 7-methylguanosine cap protecting the 5' end of mRNAs [42]. Decapping facilitates 5'-to-3' degradation by the XRN1 exonuclease, which also localizes to P-bodies (Figure 2) [43]. Depletion of GW182 or DCP1/DCP2 in mammalian or Drosophila cells disrupts P-bodies and impairs the ability of miRNAs or siRNAs to inhibit translation [42,44–46]. Likewise, AGO2 mutations that impair siRNA binding and silencing inhibit AGO2 localization to P-bodies [42]. These, and other, studies strongly implicate P-bodies in miRNA and siRNA-mediated mRNA inactivation [40].
A nucleolus- P-body connection?
In addition to mediating miRNA and siRNA function, P-bodies are localization sites for proteins involved in nonsense-mediated mRNA decay (NMD; Figure 2). NMD is a quality control mechanism that recognizes premature nonsense or stop codons within an mRNA, and in mammals functions primarily to detect failed intron removal [47–49]. In collaboration with the exon joining complex (EJC) that marks properly fused exons, the NMD system recognizes incorrectly positioned stop codons and signals the elimination of problematic mRNAs through decapping, deadenylation and exonucleolytic degradation [47–49].
Interestingly, analysis of the Arabidopsis nucleolar proteome by mass spectrometry led to the unexpected realization that six or more EJC and NMD proteins are present in the nucleolus (see Figure 3), which was confirmed by the nucleolar localization of the proteins fused to GFP [50]. These results suggest that aspects of mRNA quality control may occur in the nucleolus as well as in cytoplasmic P bodies in plants.
Conclusions
A common thread connecting Cajal body functions is assembly and trafficking of ribonucleoprotein (RNP) complexes, with RISCs being yet another class of RNP linked to Cajal bodies. It seems likely that there are many kinds of Cajal bodies given the diverse functions attributed to them, suggesting a need for better nomenclature to differentiate among them. Presumably different types of Cajal-like bodies can be distinguished by unique immunological or protein markers, or combinations of markers. Protocols for Cajal body purification suggest that proteomic approaches could also be useful for identifying such markers.
In Arabidopsis it is intriguing that DCL3 siRNA processing centers colocalize with SmD3 [22], as do DCL1 miRNA processing centers [27], suggesting the possibility that DCL1 and DCL3 colocalize within an siRNA/miRNA processing supercenter. If so, the partial redundancy of Arabidopsis dicers in siRNA production may reflect crosstalk made possible by the close physical proximities of the RNA processing machineries.
Considering that EJC, NMD and siRNA pathway proteins associate with the Arabidopsis nucleolus, it is tempting to speculate that aberrant mRNAs or non-coding RNAs of heterochromatic regions might be recognized by the NMD pathway and fed into the 24 nt siRNA pathway (Figure 3). The transcripts would be degraded and the offending loci giving rise to aberrant RNAs could be silenced by siRNA-directed DNA methylation, shutting down further production of aberrant RNAs. Likewise, the production of miRNAs in the nucleolus by the DCL1 processing center may allow for certain miRNA-mediated RNA degradation events to occur in the nucleus. In this regard, it is intriguing that the multi-exonuclease complex known as the exosome, which is involved in rRNA processing as well as 3' to 5' degradation of deadenylated mRNAs, has both a nuclear form enriched in the nucleolus as well as a cytoplasmic form [51].
Other important questions in need of further research include knowing whether siRNA-or miRNA- RISCs carry out their chromatin or miRNA targeting functions in the context of Cajal bodies. In particular, do Cajal bodies participate in the transport of chromatin modifying RISCs to chromosomal sites of action? The localization of Cajal-like U7 snRNP-containing bodies at histone loci provides precedent for thinking that Cajal body-mediated RISC delivery to target loci is a distinct possibility.
Acknowledgments
We apologize to the many authors whose papers could not be cited due to limitations on the number of references; we hope that interested readers will avail themselves of the primary literature discussed in review articles that have been cited. Our work is supported by NIH grants R01GM60380 and R01GM077590 and the Monsanto □Company-Washington University Plant Biology Research Agreement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chapman EJ, Carrington JC. Specialization and evolution of endogenous small RNA pathways. Nat Rev Genet. 2007;8:884–896. doi: 10.1038/nrg2179. [DOI] [PubMed] [Google Scholar]
- 2.Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol. 2005;15:331–341. doi: 10.1016/j.sbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 4.Grewal SI, Elgin SC. Transcription and RNA interference in the formation of heterochromatin. Nature. 2007;447:399–406. doi: 10.1038/nature05914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaratiegui M, Irvine DV, Martienssen RA. Noncoding RNAs and gene silencing. Cell. 2007;128:763–776. doi: 10.1016/j.cell.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Huettel B, Kanno T, Daxinger L, Bucher E, van der Winden J, Matzke AJ, Matzke M. RNA-directed DNA methylation mediated by DRD1 and Pol IVb: a versatile athway for transcriptional gene silencing in plants. Biochim Biophys Acta. 2007;1769:358–374. doi: 10.1016/j.bbaexp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Wassenegger M. The role of the RNAi machinery in heterochromatin formation. Cell. 2005;122:13–16. doi: 10.1016/j.cell.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez F. Arabidopsis endogenous small RNAs: highways and byways. Trends Plant Sci. 2006;11:460–468. doi: 10.1016/j.tplants.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Brodersen P, Voinnet O. The diversity of RNA silencing pathways in plants. Trends Genet. 2006;22:268–280. doi: 10.1016/j.tig.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Joshua-Tor L. The Argonautes. Cold Spring Harb Symp Quant Biol. 2006;71:67–72. doi: 10.1101/sqb.2006.71.048. [DOI] [PubMed] [Google Scholar]
- 12.Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Mol Cell. 2007;26:611–623. doi: 10.1016/j.molcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Carmell MA, Hannon GJ. RNase III enzymes and the initiation of gene silencing. Nat Struct Mol Biol. 2004;11:214–218. doi: 10.1038/nsmb729. [DOI] [PubMed] [Google Scholar]
- 14.Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and Its D. melanogaster homolog are required for miRNA biogenesis. Curr Biol. 2004;14:2162–2167. doi: 10.1016/j.cub.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. Rna. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito K, Ishizuka A, Siomi H, Siomi MC. Processing of pre-microRNAs by the Dicer-1-Loquacious complex in Drosophila cells. PLoS Biol. 2005;3:e235. doi: 10.1371/journal.pbio.0030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Jiang F, Kalidas S, Smith D, Liu Q. Dicer-2 and R2D2 coordinately bind siRNA to promote assembly of the siRISC complexes. Rna. 2006;12:1514–1520. doi: 10.1261/rna.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pikaard CS. Cell Biology of the Arabidopsis Nuclear siRNA Pathway for RNA-directed Chromatin Modification. Cold Spring Harb Symp Quant Biol. 2006;71:473–480. doi: 10.1101/sqb.2006.71.046. [DOI] [PubMed] [Google Scholar]
- 19.Onodera Y, Haag JR, Ream T, Nunes PC, Pontes O, Pikaard CS. Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell. 2005;120:613–622. doi: 10.1016/j.cell.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Herr AJ, Jensen MB, Dalmay T, Baulcombe DC. RNA polymerase IV directs silencing of endogenous DNA. Science. 2005;308:118–120. doi: 10.1126/science.1106910. [DOI] [PubMed] [Google Scholar]
- 21. Pontes O, Li CF, Nunes PC, Haag J, Ream T, Vitins A, Jacobsen SE, Pikaard CS. The Arabidopsis chromatin-modifying nuclear siRNA pathway involves a nucleolar RNA processing center. Cell. 2006;126:79–92. doi: 10.1016/j.cell.2006.05.031. • In this paper, the authors define the localizations of proteins in the RNA-directed DNA methylation pathway, the order in which they work, and the existence of a nucleolus-associated siRNA processing center.
- 22. Li CF, Pontes O, El-Shami M, Henderson IR, Bernatavichute YV, Chan SW, Lagrange T, Pikaard CS, Jacobsen SE. An ARGONAUTE4-containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell. 2006;126:93–106. doi: 10.1016/j.cell.2006.05.032. • This paper shows that the nucleolus-associated 24 nt siRNA processing center colocalizes with several molecular markers of Cajal bodies, providing the first evidence that Cajal bodies are involved in siRNA metabolism.
- 23.Chen X. MicroRNA biogenesis and function in plants. FEBS Lett. 2005;579:5923–5931. doi: 10.1016/j.febslet.2005.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Liu Z, Lu F, Dong A, Huang H. SERRATE is a novel nuclear regulator in primary microRNA processing in Arabidopsis. Plant J. 2006;47:841–850. doi: 10.1111/j.1365-313X.2006.02835.x. [DOI] [PubMed] [Google Scholar]
- 25.Lobbes D, Rallapalli G, Schmidt DD, Martin C, Clarke J. SERRATE: a new player on the plant microRNA scene. EMBO Rep. 2006;7:1052–1058. doi: 10.1038/sj.embor.7400806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poethig RS, Peragine A, Yoshikawa M, Hunter C, Willmann M, Wu G. The function of RNAi in plant development. Cold Spring Harb Symp Quant Biol. 2006;71:165–170. doi: 10.1101/sqb.2006.71.030. [DOI] [PubMed] [Google Scholar]
- 27. Fujioka Y, Utsumi M, Ohba Y, Watanabe Y. Location of a Possible miRNA Processing Site in SmD3/SmB Nuclear Bodies in Arabidopsis. Plant Cell Physiol. 2007;48:1243–1253. doi: 10.1093/pcp/pcm099. • The authors show that DCL1, HYL1 and SE colocalize within a nucleolus-associated body that lacks Coilin but nonetheless contains the Cajal body markers SmD3 and SmB.
- 28. Fang Y, Spector DL. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr Biol. 2007;17:818–823. doi: 10.1016/j.cub.2007.04.005. • These authors show that DCL1, HYL1 and SE colocalize within a nucleolus-associated body that lacks Coilin.
- 29.Kurihara Y, Takashi Y, Watanabe Y. The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. Rna. 2006;12:206–212. doi: 10.1261/rna.2146906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song L, Han MH, Lesicka J, Fedoroff N. Arabidopsis primary microRNA processing proteins HYL1 and DCL1 define a nuclear body distinct from the Cajal body. Proc Natl Acad Sci U S A. 2007;104:5437–5442. doi: 10.1073/pnas.0701061104. • The authors show that HYL1 and DCL1 colocalize within a nucleolus-associated body that lacks Coilin.
- 31.Pederson T. The plurifunctional nucleolus. Nucleic Acids Res. 1998;26:3871–3876. doi: 10.1093/nar/26.17.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handwerger KE, Gall JG. Subnuclear organelles: new insights into form and function. Trends Cell Biol. 2006;16:19–26. doi: 10.1016/j.tcb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Olson MO, Dundr M, Szebeni A. The nucleolus: an old factory with unexpected capabilities. Trends Cell Biol. 2000;10:189–196. doi: 10.1016/s0962-8924(00)01738-4. [DOI] [PubMed] [Google Scholar]
- 34.Cioce M, Lamond AI. Cajal bodies: a long history of discovery. Annu Rev Cell Dev Biol. 2005;21:105–131. doi: 10.1146/annurev.cellbio.20.010403.103738. [DOI] [PubMed] [Google Scholar]
- 35.Shaw PJ, Brown JW. Plant nuclear bodies. Curr Opin Plant Biol. 2004;7:614–620. doi: 10.1016/j.pbi.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Stanek D, Neugebauer KM. The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze. Chromosoma. 2006;115:343–354. doi: 10.1007/s00412-006-0056-6. [DOI] [PubMed] [Google Scholar]
- 37.Kiss T, Fayet E, Jady BE, Richard P, Weber M. Biogenesis and intranuclear trafficking of human box C/D and H/ACA RNPs. Cold Spring Harb Symp Quant Biol. 2006;71:407–417. doi: 10.1101/sqb.2006.71.025. [DOI] [PubMed] [Google Scholar]
- 38.Lukowiak AA, Narayanan A, Li ZH, Terns RM, Terns MP. The snoRNA domain of vertebrate telomerase RNA functions to localize the RNA within the nucleus. Rna. 2001;7:1833–1844. [PMC free article] [PubMed] [Google Scholar]
- 39. Liu JL, Murphy C, Buszczak M, Clatterbuck S, Goodman R, Gall JG. The Drosophila melanogaster Cajal body. J Cell Biol. 2006;172:875–884. doi: 10.1083/jcb.200511038. • The authors describe the occurrence in flies of Cajal bodies that lack the canonical Cajal body protein, Coilin.
- 40.Jakymiw A, Pauley KM, Li S, Ikeda K, Lian S, Eystathioy T, Satoh M, Fritzler MJ, Chan EK. The role of GW/P-bodies in RNA processing and silencing. J Cell Sci. 2007;120:1317–1323. doi: 10.1242/jcs.03429. [DOI] [PubMed] [Google Scholar]
- 41. Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol. 2005;7:719–723. doi: 10.1038/ncb1274. • The authors show that mRNAs targeted for translational repression by miRNAs localize to P-bodies in a miRNA-dependent manner.
- 42. Liu J, Rivas FV, Wohlschlegel J, Yates JR, 3rd, Parker R, Hannon GJ. A role for the P-body component GW182 in microRNA function. Nat Cell Biol. 2005;7:1261–1266. doi: 10.1038/ncb1333. • Argonaute proteins are shown to physically interact and colocalize with the P-body protein GW182 providing a molecular basis for RISC-P-body associations.
- 43.Rehwinkel J, Behm-Ansmant I, Gatfield D, Izaurralde E. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. Rna. 2005;11:1640–1647. doi: 10.1261/rna.2191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakymiw A, Lian S, Eystathioy T, Li S, Satoh M, Hamel JC, Fritzler MJ, Chan EK. Disruption of GW bodies impairs mammalian RNA interference. Nat Cell Biol. 2005;7:1267–1274. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- 45.Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Z, Jakymiw A, Wood MR, Eystathioy T, Rubin RL, Fritzler MJ, Chan EK. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J Cell Sci. 2004;117:5567–5578. doi: 10.1242/jcs.01477. [DOI] [PubMed] [Google Scholar]
- 47.Conti E, Izaurralde E. Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr Opin Cell Biol. 2005;17:316–325. doi: 10.1016/j.ceb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Lejeune F, Maquat LE. Mechanistic links between nonsense-mediated mRNA decay and pre-mRNA splicing in mammalian cells. Curr Opin Cell Biol. 2005;17:309–315. doi: 10.1016/j.ceb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Amrani N, Sachs MS, Jacobson A. Early nonsense: mRNA decay solves a translational problem. Nat Rev Mol Cell Biol. 2006;7:415–425. doi: 10.1038/nrm1942. [DOI] [PubMed] [Google Scholar]
- 50. Pendle AF, Clark GP, Boon R, Lewandowska D, Lam YW, Andersen J, Mann M, Lamond AI, Brown JW, Shaw PJ. Proteomic analysis of the Arabidopsis nucleolus suggests novel nucleolar functions. Mol Biol Cell. 2005;16:260–269. doi: 10.1091/mbc.E04-09-0791. • The authors show that proteins of the exon-joining complex and nonsense-mediated decay pathway localize to the nucleolus in Arabidopsis.
- 51.Houseley J, LaCava J, Tollervey D. RNA-quality control by the exosome. Nat Rev Mol Cell Biol. 2006;7:529–539. doi: 10.1038/nrm1964. [DOI] [PubMed] [Google Scholar]
- 52.Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301. doi: 10.1038/35066075. [DOI] [PubMed] [Google Scholar]
- 53.Bartlett J, Blagojevic J, Carter D, Eskiw C, Fromaget M, Job C, Shamsher M, Trindade IF, Xu M, Cook PR. Specialized transcription factories. Biochem Soc Symp. 2006:67–75. doi: 10.1042/bss0730067. [DOI] [PubMed] [Google Scholar]


