Abstract
Background
Fluoroquinolones and tetracyclines can penetrate epithelial cells, but the mechanism by which they cross the plasma membrane is unclear. In this study, a cell line derived from oral epithelium was used as a model to demonstrate a role for active transport.
Methods
Transport of ciprofloxacin and minocycline by confluent cell monolayers was assayed by measuring the increase in cell-associated fluorescence.
Results
Uptake of both agents was saturable and was inhibited at low temperatures. At 37° C, the cells transported ciprofloxacin and minocycline with Km values of 351 and 133 μg/ml, respectively, and maximum velocities of 5.11 and 13.4 ng/min/μg cell protein, respectively. When ciprofloxacin and minocycline were removed from the extracellular medium, the intracellular levels of both agents decreased. Ciprofloxacin efflux from loaded cells occurred more rapidly than with minocycline. Cells accumulated intracellular drug levels that were at least 8-fold higher than extracellular levels for ciprofloxacin and at least 40-fold higher for minocycline. Transport of ciprofloxacin and minocycline was significantly influenced by pH and was most favorable at pH 7.7 and 7.2, respectively. While ciprofloxacin transport was Na+-independent, minocycline transport was strongly inhibited when sodium in the medium was replaced with choline. Transport of both agents was inhibited by a variety of organic cations, but the pattern of inhibition was different. Papaverine, phenylephrine and doxycycline competitively inhibited minocycline transport, but inhibited ciprofloxacin transport by a noncompetitive mechanism.
Conclusions
Epithelial cells take up ciprofloxacin and minocycline via different active transport systems. These transporters may play an important role in enhancing the effectiveness of these agents against invasive pathogens.
Keywords: fluoroquinolone, tetracycline, antimicrobial chemotherapy, invasive infection
Introduction
While only a small proportion of bacterial pathogens are capable of invading host cells, invasive infections are among the most difficult to eradicate. When pathogens enter mammalian cells, they can elude the host response and gain access to a rich nutrient supply in the host's cytoplasm. Invasive bacteria can survive and multiply within membrane-bound vacuoles or in the cytoplasm of the host cell. In the oral cavity, the virulence of the periodontal pathogens Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis is enhanced by their ability to invade epithelial cells (1–4). Their tendency to invade soft tissue makes them difficult to eliminate by scaling and root planing alone.
One logical approach for eradicating these pathogens is to introduce an antimicrobial agent into invaded epithelial cells. In cases that undergo continued periodontal breakdown after scaling and root planing, antimicrobial chemotherapy is frequently used as an adjunct to enhance the elimination of pathogens (5). Penicillins and cephalosporins are not ideal choices for treating invasive infections because they don’t easily penetrate the plasma membrane. However, fluoroquinolones and tetracyclines can enter epithelial cells (6), where they can potentially kill intracellular pathogens. Fluoroquinolones terminate chromosomal replication and interfere with bacterial cell division and gene expression (7). They are highly active against most aerobic and facultative Gram-negative bacteria and exhibit good activity against Gram-positive bacteria (8). Tetracyclines have an unusually wide antibacterial spectrum, which is related to their inhibition of ribosomal protein synthesis (9). Most strains of A. actinomycetemcomitans are highly susceptible to ciprofloxacin in vitro (10, 11). Tetracyclines inhibit A. actinomycetemcomitans, P. gingivalis and most other periodontal microorganisms at concentrations attainable in vivo (12,13).
Based on previous studies, it is unclear whether fluoroquinolones and tetracyclines are actively transported into epithelial cells. However, many cationic and zwitterionic prescription drugs are actively translocated into the cell interior by a ubiquitous family of organic cation transporters. We hypothesized that active transporters mediate the uptake of these antimicrobial agents. It was impractical to test this hypothesis with primary human gingival epithelial cell cultures, which divide only a few times in culture. Oral squamous cell carcinomas (SCC), the malignant variant of oral squamous epithelial cells, share many of their functions and features. The SCC-25 cell line was used as a model of oral squamous epithelium to characterize the transport of ciprofloxacin and minocycline in the present study. It does not require a fibroblast feeder layer (14), which can potentially interfere with epithelial transport assays.
Methods
Cell culture
SCC-25 cells (CRL-1628)* were seeded into 24 well tissue culture plates in 50% Dulbecco’s modified Eagle’s medium, 50% Ham’s F12 medium# containing 10% heat-inactivated fetal bovine serum& and 0.4 μg/ml hydrocortisone&. They were seeded at 9000 cells/well, were fed on the fourth day, and grew to form a confluent monolayer within seven days. Cell protein was measured by the method of Bradford (15).
Assay of fluoroquinolone and tetracycline transport by SCC cells
Transport was assayed by measuring cell-associated fluoroquinolone or tetracycline fluorescence. For both agents, the cluster-tray method of Gazzola et al (16) was used to transport. Multiwell culture plates containing confluent cell monolayers were washed five times with Hanks balanced salts solution# (HBSS), overlaid with 0.2 ml/well HBSS, and warmed to 37° C prior to assay. In the ciprofloxacin transport assays, 0.2 ml of warm HBSS containing twice the desired final fluoroquinolone concentration was simultaneously added to each well with a multi-channel pipette. After incubation at 37° C for the indicated times, the fluoroquinolone solutions were quickly removed and each well was rapidly washed four times with HBSS. Cell monolayers were lysed in 1 ml of 100 mM glycine (pH 3.0) with a scraper. The lysate was centrifuged (13,000 × g, 6 min) and its fluorescence was measured as previously described (17).
Transport of tetracyclines was assayed using a similar approach, except that 0.2 ml of warm HBSS containing twice the desired final antibiotic concentration was simultaneously added to 0.2 ml of warm HBSS in each well. The assays were terminated by aspirating the antibiotic solution and washing the plate four times with HBSS. For minocycline transport assays, cells were lysed in 1 ml of water and added to 1 ml of ethylene glycol containing 200 mM citric acid and 200 mM magnesium acetate (18). The fluorescence of the lysate was measured at excitation and emission wavelengths of 380 and 480nm, respectively. Calibration plots were constructed to relate fluorescence values to cell antibiotic content, which was normalized to cell protein.
To determine the affinity and maximal velocity of transport, the kinetics of transport were measured during the linear initial phase of transport (0 to 3 minutes) and analyzed by the Lineweaver-Burk (double reciprocal) method. A program× was used to derive Km and Vmax values from least-squares regression lines obtained with the plotted data. Lineweaver-Burk analysis was also used to determine the inhibition constant (Ki) and characterize the mechanisms of transport inhibition produced by organic cations.
Intracellular volume measurements
The intracellular water space was measured by equilibrating cell suspensions with [3H]-water% (5 μCi/ml) at 37° C (19). After equilibration for 10 minutes, the cells were rapidly pelleted through oil as previously described (20). [14C]-inulin% (2 μCi/ml) was used as a marker of extracellular water trapped between pelleted cells.
Results
As indicated by the linear Lineweaver-Burk plots in Figure 1, the kinetics of minocycline and ciprofloxacin transport by SCC monolayers obeyed the Michaelis-Menten equation. At 37° C, the cells transported minocycline with a Km of 133 μg/ml and a Vmax of 13.4 ng/min/μg cell protein. Minocycline was taken up with a higher affinity and at a higher velocity than doxycycline or its parent compound, tetracycline (Table 1). Its transport was dramatically inhibited when the temperature was reduced to 2° C (inset, Figure 1). Among the fluoroquinolones, ciprofloxacin was transported with Km of 351 μg/ml and a Vmax of 5.11 ng/min/μg. Ofloxacin was taken up with higher affinity and a slightly lower velocity. Moxifloxacin, which is more active against anaerobes (21), was taken up with a higher affinity and velocity. Ciprofloxacin transport was highly temperature dependent between 4° and 37° C (r = 0.97, data not shown). Although the affinity of transport was relatively low, the cells accumulated substantial intracellular levels of minocycline and ciprofloxacin. When incubated to equilibrium with extracellular antibiotic concentrations of 1 μg/ml, the observed intracellular concentrations were 8.39 μg/ml for ciprofloxacin and 40.5 μg/ml for minocycline (Table 2).
Figure 1.
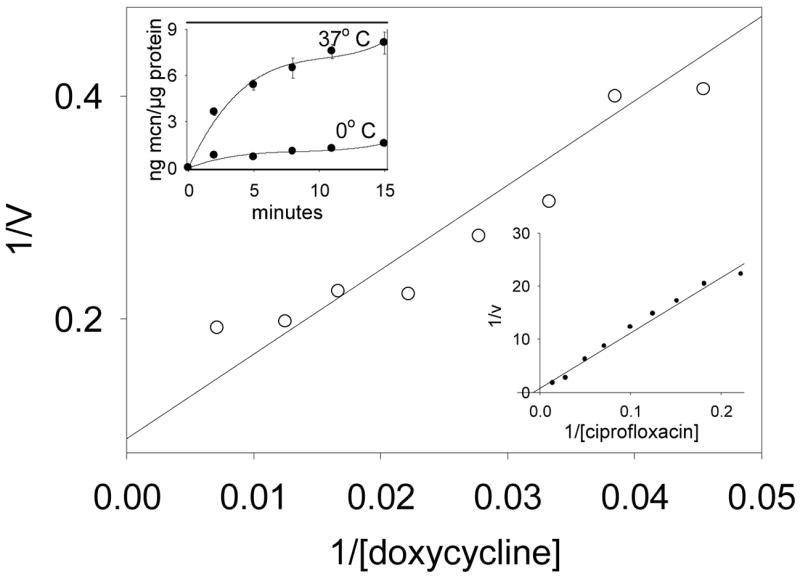
A representative Lineweaver-Burk plot of minocycline transport by SCC-25 cells at 37° C. Left inset: Temperature-dependence of minocycline accumulation by SCC cells. Cell monolayers were incubated in HBSS at 37° and 2° C. Minocycline (10 μg/ml) was added and uptake was monitored over the indicated time intervals. Data are representative of three experiments. Right inset: A representative plot of ciprofloxacin transport at 37° C.
Table 1.
Kinetic constants for transport of four antimicrobial agents by SCC-25 cells
| Agent | Km (μg/ml) | Vmax (ng/min/μg protein) |
|---|---|---|
| minocycline | 133 ± 14.3 | 13.4 ± 1.35 |
| doxycycline | 507 ± 38.6 | 5.33 ± 0.62 |
| tetracycline | 592 ± 59.8 | 5.86 ± 0.56 |
| ciprofloxacin | 351 ± 12.7 | 5.11 ± 0.32 |
| ofloxacin | 221 ± 20.6 | 4.24 ± 0.23 |
| moxifloxacin | 244 ± 8.43 | 8.68 ± 0.69 |
Transport activity was assayed during the rapid initial phase of uptake. The kinetic data was analyzed with the Lineweaver-Burk method to determine the kinetic constants.
Table 2.
Cellular/extracellular concentration ratios observed in SCC-25 cells
| Extracellular concentration | Cellular/extracellular for ciprofloxacin | Cellular/extracellular for Minocycline |
|---|---|---|
| 1 μg/ml | 8.39 ± 0.28 | 40.5 ± 3.10 |
| 3 μg/ml | 8.27 ± 0.47 | 43.3 ± 4.96 |
| 10 μg/ml | 8.46 ± 0.37 | 50.8 ± 5.41 |
Intracellular volume was determined by incubating cell suspensions with [3H]-water. Cells were then separated from the medium by pelleting through an oil cushion. A correction for trapped extracellular water was applied by incubating with [14C]-inulin. In these studies, extracellular concentration had no significant effect on the C/E ratio (P > 0.4, repeated measures ANOVA).
When ciprofloxacin or minocycline was removed from the extracellular medium after the SCC cells were loaded to equilibrium, both agents were translocated in the reverse direction. Their efflux was monitored by the decrease in cell-associated ciprofloxacin or minocycline fluorescence. Consistent with its lower affinity and velocity of transport into cells, the efflux of ciprofloxacin was more rapid than for minocycline (Figure 2). Efflux of half the intracellular content occurred within 3 minutes for ciprofloxacin and 7 minutes for minocycline. After 25 minutes of efflux, only 6% of the original intracellular ciprofloxacin content remained inside the cells, while 32% of the intracellular minocycline content remained. Only 13% of the original minocycline content was still inside the cells after 60 minutes (data not shown).
Figure 2.
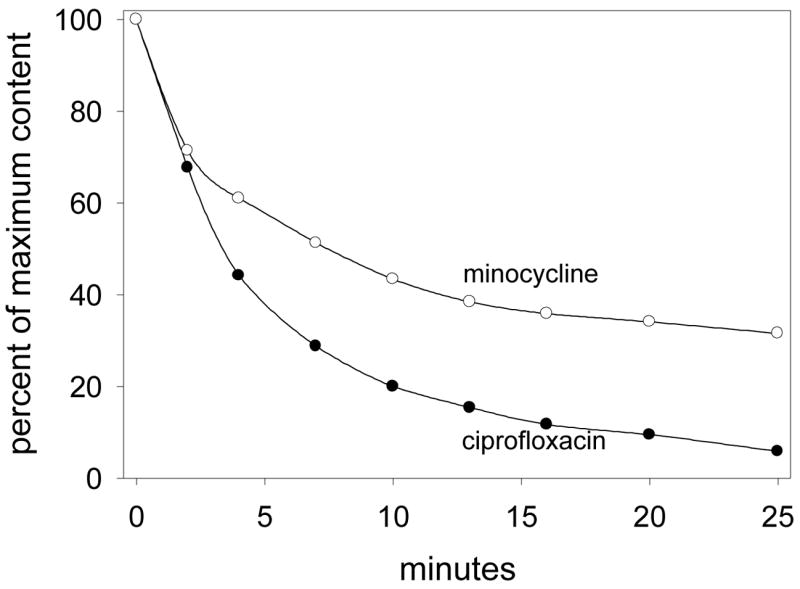
Efflux of ciprofloxacin and minocycline from loaded SCC cells. Cells were loaded to a steady state concentration by incubation with HBSS containing 35 μg/ml ciprofloxacin or minocycline for 15 minutes at 37° C. These solutions were rapidly aspirated and replaced with 37° C HBSS. The decrease in intracellular antimicrobial content was monitored over 25 minutes.
Ciprofloxacin transport by SCC cells was similar in the presence and absence of sodium. In contrast, minocycline transport was strongly inhibited when choline chloride was substituted for sodium chloride in the extracellular medium (Figure 3). To determine the effect of pH on transport, the kinetics of uptake were analyzed over the pH range of 6.2 to 8.2. For both ciprofloxacin and minocycline, pH had a significant effect on the efficiency of transport (as assessed by the Vmax/Km ratio) (P < 0.05, ANOVA, Figure 4). The efficiency of ciprofloxacin transport was nearly 40% lower at pH 6.2 and 8.2 than at neutral pH (P > 0.05, Dunnett’s test). For minocycline, the efficiency was only about 20% lower at pH 8.2 than at pH 7.2, but it was 55% lower at pH 6.2 (P < 0.05, Dunnett’s test). To further characterize the mechanisms of ciprofloxacin and minocycline transport in SCC cells, we examined the pattern of inhibition produced by several different organic cations (Table 3). Adenine competitively inhibited the transport of ciprofloxacin and minocycline with a Ki value of 2.7 mM. Diazepam failed to inhibit ciprofloxacin transport at a concentration of 2 mM, but produced potent competitive inhibition of minocycline transport (Ki = 0.50 mM). Several other cationic agents, including papaverine, histamine, phenylephrine and doxycycline, inhibited ciprofloxacin transport through a noncompetitive mechanism, but produced competitive inhibition of minocycline transport.
Figure 3.
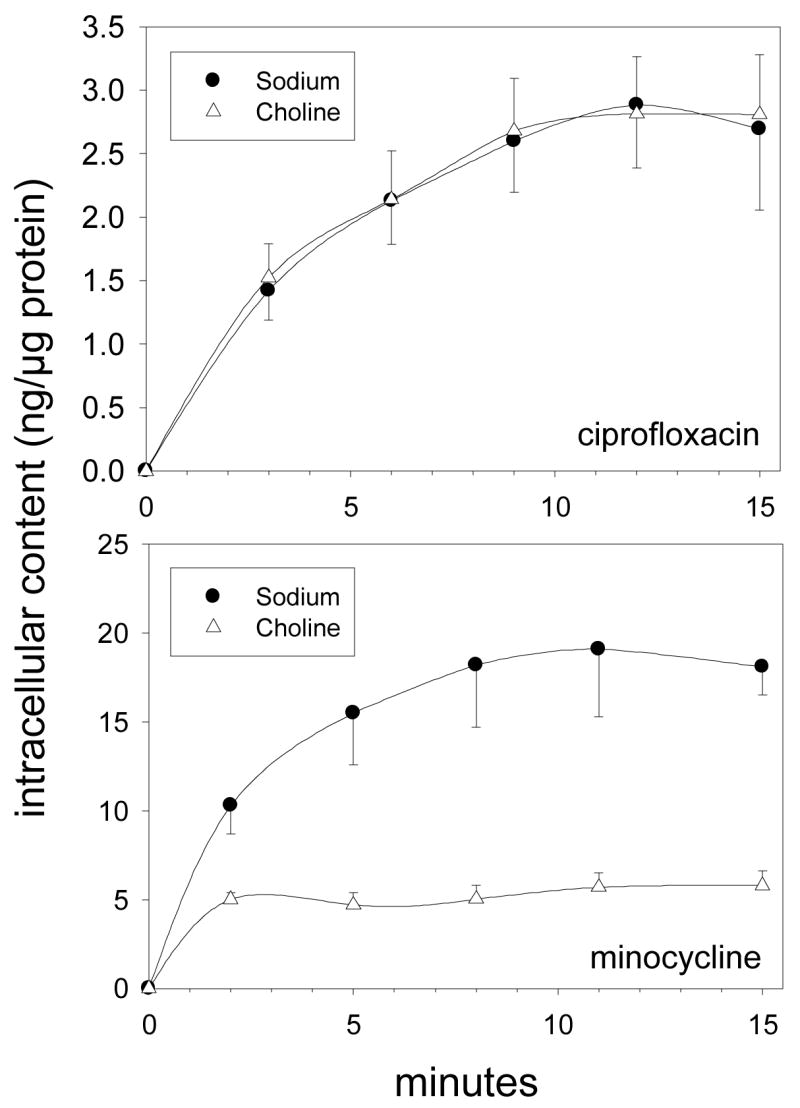
Time course of ciprofloxacin and minocycline accumulation by SCC-25 cells in the presence and absence of Na+. Confluent monolayer cell cultures were washed and overlaid with balanced salts solutions containing NaCl or choline chloride. Intracellular accumulation of ciprofloxacin or minocycline (30 μg/ml) was assayed at 37° C. Data are presented as the mean ± standard errors of three experiments.
Figure 4.
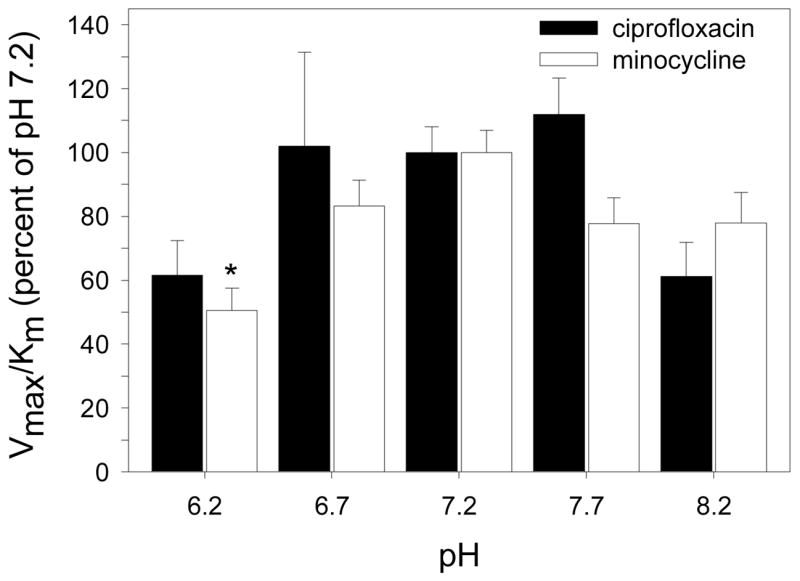
The pH dependence of ciprofloxacin and minocycline transport by SCC-25 cells. Confluent monolayer cell cultures were washed with PBS and overlaid with HBSS adjusted to the appropriate pH. The kinetics of ciprofloxacin and minocycline transport were assayed at 37° C. The data were converted to percent of Vmax/Km observed at pH 7.2 and presented as the means ± standard errors of three experiments. Changes in pH produced a significant treatment effect on ciprofloxacin and minocycline transport (P < 0.05, ANOVA ). For ciprofloxacin, no significant differences in transport were noted between pH 7.2 and other groups. For minocycline, the pH 6.2 group was significantly different from the pH 7.2 group (* indicates P < 0.05 in Dunnett’s test).
Table 3.
Inhibition of ciprofloxacin and minocycline transport by organic cations
| Agent | Inhibition of ciprofloxacin transport (Ki) | Inhibition of minocycline transport (Ki) |
|---|---|---|
| adenine | competitive (2.7 ± 0.4 mM) | competitive (2.7 ± 0.3 mM) |
| papaverine | noncompetitive (2.8 ± 0.4 mM) | competitive (1.7 ± 0.2 mM) |
| histamine | noncompetitive (7.0 ± 0.6 mM) | competitive (3.7 ± 0.5 mM) |
| phenylephrine | noncompetitive (0.90 ± 0.1 mM) | competitive (1.1 ± 0.2 mM) |
| diazepam | no inhibition at 2 mM | competitive (0.50 ± 0.1 mM) |
| doxycycline | noncompetitive (1.0 ± 0.2 mM) | competitive (0.53 ± 0.1 mM) |
Transport was assayed in the presence and absence of the indicated agents as described in the methods. The kinetic data was analyzed with the Lineweaver-Burk method to determine the mechanism of inhibition and the inhibitory constant (Ki) with respect to ciprofloxacin or minocycline
Discussion
Our findings provide evidence that oral epithelial cells are capable of actively transporting fluoroquinolones and tetracyclines. The transport of ciprofloxacin and minocycline was temperature-dependent and exhibited Michaelis-Menten kinetics. Transport of both agents was concentrative, and the cellular to extracellular (C/E) concentration ratios were relatively high (>8 for ciprofloxacin, >40 for minocycline). Previous studies have shown that other types of cells are capable of concentrating fluoroquinolones and tetracyclines. With respect to ciprofloxacin, the C/E ratio observed in this study was similar to that reported for fluoroquinolone uptake by neutrophils, macrophages and McCoy epithelial cells (22, 23). However, the C/E ratio observed for minocycline was much higher than the value previously reported for the uptake of tetracyclines by neutrophils (24).
Previous studies have shown that inflamed periodontal pockets are more alkaline (pH 7.7 to 8.7) than healthy gingival crevices (25–27). Based on our findings, this alkaline shift could potentially inhibit the transport of ciprofloxacin and minocycline by crevicular epithelial cells, but the effect may not be significant. Despite their sensitivity to pH, SCC cells were capable of transporting both agents over a relatively broad pH range. In other respects, there were important differences in the transport of these two agents. Ciprofloxacin transport was sodium-independent, while minocycline transport was inhibited in the absence of sodium. Further, differences were noted in their susceptibility to inhibition by organic cations. Adenine was a competitive inhibitor of ciprofloxacin transport by SCC cells, but several other cationic agents (including doxycycline) inhibited ciprofloxacin transport through a noncompetitive mechanism. All of these agents produced competitive inhibition of minocycline transport. This suggests that epithelial cells use different transport systems to take up ciprofloxacin and minocycline. Ciprofloxacin, which possesses structural features that resemble purines, could potentially share an uptake path with adenine. Adenine interacts with certain members of the nucleoside and nucleobase transporter families, which allow cells to import the biosynthetic precursors for nucleic acids and ATP, respectively (28). Minocycline and other tetracyclines possess cationic primary and tertiary amine groups. Minocycline transport was competitively inhibited by papaverine, phenylephrine, diazepam and histamine, which are substrates of organic cation transporters. This family of transporters has a ubiquitous distribution and interacts with a broad spectrum of prescription drugs and cationic compounds (29, 30).
Locally delivered tetracyclines and fluoroquinolones have been used as adjuncts in periodontal therapy (31–33). Although local delivery devices yield high antibiotic levels in the periodontal pocket, it is uncertain whether these agents are taken up by the pocket epithelium. The results of this study suggest that they are. Cultured SCC and epithelial cells attach to plastic tissue culture substrates via their basal surface. This impairs transport across the basal surface, which is the usual route by which nutrients and systemically administered agents are taken up. Thus, the main route of antibiotic uptake in our model of oral epithelial transport is across the apical surface, which is the same direction locally delivered antibiotics could potentially enter pocket epithelial cells. Based on the efficiency of transport observed in this model (as assessed by the Vmax/Km ratio), minocycline is most capable of penetrating epithelium, followed by moxifloxacin, ciprofloxacin and tetracycline. It is likely that these agents are taken up at least as efficiently across the basal epithelial surface as across the apical surface. Based on our findings, it is reasonable to predict that systemically administered fluoroquinolones and tetracyclines accumulate inside pocket epithelial cells at concentrations that are several-fold higher than those in the subjacent gingival connective tissue. Although these agents translocate out of epithelial cells when their extracellular concentrations decrease, the efflux of minocycline occurs more slowly than for ciprofloxacin. Thus, epithelial cells can more easily sustain effective intracellular levels of minocycline as its concentration decreases in the interstitial fluid.
Although bacterial susceptibility to inhibition is a primary requirement for successful antimicrobial chemotherapy, antimicrobial agents must be able to penetrate infection sites to produce their inhibitory effects. To inhibit invasive pathogens like A. actinomycetemcomitans and P. gingivalis, antimicrobial agents must attain effective levels inside an infected host cell. The present study provides evidence that oral epithelial cells possess active transporters that are capable of accumulating fluoroquinolones and tetracyclines. It is likely that they play an important role in enhancing the effectiveness of these agents against intracellular bacteria. Characterization of these transporters provides a better understanding of the distribution of antimicrobial agents in the gingiva. These studies also complement the significant volume of work that has already been done to define the antibiotic susceptibilities of periodontal pathogens and delineate their mechanisms for invading soft tissue.
Acknowledgments
This investigation was supported by USPHS research grants DE00338 and DE12601 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
Footnotes
American Type Culture Collection, Manassas, VA
GIBCO Division, Invitrogen Corporation, Rockville, MD
Sigma Chemical Company, St. Louis, MO
EnzPack for Windows (BioSoft, Ferguson, MO)
NEN Life Science Products, Boston, MA
References
- 1.Meyer DH, Sreenivasan PK, Fives-Taylor PM. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:2719–2726. doi: 10.1128/iai.59.8.2719-2726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer DH, Lippmann JE, Fives-Taylor PM. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun. 1996;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamont RJ, Oda D, Persson RE, Persson GR. Interaction of Porphyromonas gingivalis with gingival epithelial cells maintained in culture. Oral Microbiol Immunol. 1992;7:364–367. doi: 10.1111/j.1399-302x.1992.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 4.Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;64:2988–2997. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotic therapy in periodontics. Periodontol 2000. 1996;10:45–78. doi: 10.1111/j.1600-0757.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 6.Chadwick PR, Mellersh AR. The use of a tissue culture model to assess the penetration of antibiotics into epithelial cells. J Antimicrob Chemother. 1987;19:211–220. doi: 10.1093/jac/19.2.211. [DOI] [PubMed] [Google Scholar]
- 7.Bryan LE, Bedard J, Wong S, Chamberland S. Quinolone antimicrobial agents: Mechanism of action and resistance development. Clin Invest Med. 1989;12:14–19. [PubMed] [Google Scholar]
- 8.Sanders CC. Microbiology of fluoroquinolones. In: Sanders WE, Sanders CC, editors. Fluoroquinolones in the Treatment of Infectious Diseases. Glenview, IL: Physicians and Scientists Publishing; 1990. pp. 1–27. [Google Scholar]
- 9.Goldman RA, Hasan T, Hall CC, Strycharz WA, Cooperman BS. Photoincorporation of tetracycline into E. coli ribosomes. Identification of the major proteins photolabeled by native tetracycline and tetracycline photoproducts and implications for the inhibitory action of tetracycline on protein synthesis. Biochemistry. 1983;22:358–367. doi: 10.1021/bi00271a020. [DOI] [PubMed] [Google Scholar]
- 10.Pavicic MJ, van Winkelhoff AJ, de Graaff J. In vitro susceptibilities of Actinobacillus actinomycetemcomitans to a number of antimicrobial combinations. Antimicrob Agents Chemother. 1992;36:2634–2638. doi: 10.1128/aac.36.12.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajukanta R, Asikainen S, Saarela M, Alaluusua S, Jousimies-Somer H. In vitro antimicrobial susceptibility of different serotypes of Actinobacillus actinomycetemcomitans. Scand J Dent Res. 1993;101:299–303. doi: 10.1111/j.1600-0722.1993.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 12.Walker CB, Gordon JM, McQuilkin SJ, Niebloom TA, Socransky SS. Tetracycline: levels achievable in gingival crevice fluid and in vitro effects on subgingival organisms. II. Susceptibilities of periodontal bacteria. J Periodontol. 1981;52:613–616. doi: 10.1902/jop.1981.52.10.613. [DOI] [PubMed] [Google Scholar]
- 13.Walker CB, Pappas JD, Tyler KZ, Cohen S, Gordon JM. Antibiotic susceptibilities of periodontal bacteria. In vitro susceptibilities to eight antimicrobial agents. J Periodontol. 1985;56(Suppl):67–74. doi: 10.1902/jop.1985.56.11s.67. [DOI] [PubMed] [Google Scholar]
- 14.Rheinwald JF, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Gazzola GC, Dall’Asta V, Franchi-Gazzola R, White MF. The cluster-tray method for rapid measurement of solute fluxes in adherent cultured cells. Anal Biochem. 1981;115:368–374. doi: 10.1016/0003-2697(81)90019-1. [DOI] [PubMed] [Google Scholar]
- 17.Walters JD, Zhang F, Nakkula RJ. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrob Agents Chemother. 1999;43:2710–2715. doi: 10.1128/aac.43.11.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lever M. Improved fluorometric determination of tetracyclines. Biochem Med. 1972;6:216–222. doi: 10.1016/0006-2944(72)90041-5. [DOI] [PubMed] [Google Scholar]
- 19.Garraffo R, Jambou D, Chichmanian RM, Ravoire S, Lapalus P. In vitro and in vivo ciprofloxacin pharmacokinetics in human neutrophils. Antimicrob Agents Chemother. 1991;35:2215–2218. doi: 10.1128/aac.35.11.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bounds SJ, Nakkula R, Walters JD. Fluoroquinolone transport by human monocytes: Characterization and comparison to other cells of myeloid lineage. Antimicrob Agents Chemother. 2000;44:2609–2614. doi: 10.1128/aac.44.10.2609-2614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein E, Citron DM, Hudspeth M, Gerardo SH, Merriam CV. In vitro activity of BAY 12–8039, a new 8-methoxyquinolone, compared to activities of 11 other oral antimicrobial agents against 390 aerobic and anaerobic bacteria isolated from human and animal bite wound skin and soft tissue infection in humans. Antimicrob Agents Chemother. 1997;41:1552–1557. doi: 10.1128/aac.41.7.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascual A, Garcia I, Ballesta S, Perea EJ. Uptake and intracellular activity of trovafloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1997;41:274–277. doi: 10.1128/aac.41.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascual A, Garcia I, Ballesta S, Perea EJ. Uptake and intracellular activity of moxifloxacin in human neutrophils and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1999;43:12–15. doi: 10.1128/aac.43.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabler WL. Fluxes and accumulation of tetracyclines by human blood cells. Res Commun Chemical Pathol Pharmacol. 1991;72:39–51. [PubMed] [Google Scholar]
- 25.Kleinberg I, Hall G. pH and depth of gingival crevices in different areas of the mouths of fasting humans. J Periodont Res. 1968;3:109–117. doi: 10.1111/j.1600-0765.1969.tb01955.x. [DOI] [PubMed] [Google Scholar]
- 26.Borden SM, Golub LM, Kleinberg I. The effect of age and sex on the relationship between crevicular fluid flow and gingival inflammation in humans. J Periodont Res. 1977;12:160–165. doi: 10.1111/j.1600-0765.1977.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 27.Bickel M, Cimasoni G. The pH of human crevicular fluid measured by a new microanalytical technique. J Periodont Res. 1985;20:35–40. doi: 10.1111/j.1600-0765.1985.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 28.Griffith DA, Jarvis SM. Nucleoside and nucleobase transport systems of mammalian cells. Biochim Biophys Acta. 1996;1286:153–181. doi: 10.1016/s0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 29.Koepsell H. Organic cation transporters in intestine, kidney, liver and brain. Annu Rev Physiol. 1998;60:243–266. doi: 10.1146/annurev.physiol.60.1.243. [DOI] [PubMed] [Google Scholar]
- 30.Dresser MJ, Zhang L, Giacomini KM. Molecular and functional characteristics of cloned human organic cation transporters. Pharmaceutical Biotechnology. 1999;12:441–469. doi: 10.1007/0-306-46812-3_15. [DOI] [PubMed] [Google Scholar]
- 31.Goodson J, Offenbacher S, Farr D, et al. Periodontal disease treatment by local drug delivery. J Periodontol. 1985;56:265–272. doi: 10.1902/jop.1985.56.5.265. [DOI] [PubMed] [Google Scholar]
- 32.Williams RC, Paquette DW, Offenbacher S, et al. Treatment of Periodontitis by local administration of minocycline microspheres: A controlled trial. J Periodontol. 2001;72:1535–1544. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- 33.Kimura S, Toda H, Shimabukuro Y, et al. Topical chemotherapy in human periodontitis using a new controlled-release insert containing ofloxacin. 1. Microbiological observation. J Periodont Res. 1991;26:33–41. doi: 10.1111/j.1600-0765.1991.tb01623.x. [DOI] [PubMed] [Google Scholar]


