Abstract
Background
Injection drug users (IDUs) are at high risk of hepatitis B (HBV) infection, and HBV vaccination coverage in IDUs is low. Recent studies demonstrate that syringe exchange programs are effective venues to reach and immunize IDUs. The purpose of this paper was to determine if targeting IDUs for HBV vaccination through syringe exchange programs is economically desirable for the healthcare system and to assess the relative effectiveness of several different vaccination strategies.
Methods
Active IDUs in Chicago IL and Hartford and Bridgeport CT (N=1964) were recruited and screened through local syringe exchange programs, randomized to a standard (0, 1, 6 months) or accelerated (0, 1, 2 months) vaccination schedule, and followed from May 2003 to March 2006. Analyses were conducted in 2007. The vaccination program’s costs were balanced against future HBV-associated medical costs. Benefits in terms of prevented acute HBV infections and quality-adjusted life years were estimated based on a Markov model.
Results
HBV vaccination campaigns targeting IDUs through syringe exchange programs are cost-saving. The most cost-saving strategies include giving the first dose to everyone at screening, administering the vaccination under the accelerated schedule (0, 1, 2 months), and obtaining highly discounted vaccine from local health departments.
Conclusions
It is economically inappropriate to offer HBV screening in the absence of vaccination. Existing syringe exchange programs in the U.S. should include HBV vaccination.
Introduction
Since the first safe and effective hepatitis B vaccine was approved in 1982, vaccination campaigns have been successful in controlling hepatitis B virus (HBV) infection in the general U.S. population. However, HBV infection remains a major health threat to individuals who engage in high-risk behaviors, especially injection drug users (IDUs).1 Among the estimated 1.3 million IDUs in the U.S.,2–4 the prevalence of HBV infection ranges from 40% to 80%,5–12 and the incidence rate ranges between 8% and 12%.13–15 The Advisory Committee on Immunization Practices recommended vaccination of IDUs as long ago as 1991,16 but the current vaccination coverage among IDUs remains low.17–19 Vaccination programs targeting IDUs are uncommon, mainly due to a lack of funds, the lack of reliable delivery venues, and expectations of poor adherence to a complicated hepatitis B vaccination schedule.
The mortality and morbidity associated with HBV infection (e.g., treatment of serious acute HBV infection and illnesses associated with chronic infection) impose a sizable economic burden on the healthcare system. To date, no study has undertaken a comprehensive evaluation of the costs and benefits of a hepatitis B vaccination program that targeted IDUs. Previous studies have shown that hepatitis B vaccination targeting other high-risk groups is cost effective and even cost saving. The hepatitis B vaccination of prison inmates in the U.S. would realize savings for the healthcare system, although it would cost the prison system $415 per infection averted.20 A recent study in England and Wales found that the vaccination of high-risk adults in genitourinary clinics was more cost effective than universal infant vaccination.21 Also, U.S. and Italian studies yielded similar conclusions among hemodialysis patients.22,23 Therefore, hepatitis B vaccination targeting IDUs was anticipated to be cost effective or cost saving if IDUs could be successfully reached and immunized.
Analysis of recent studies revealed that it is possible to successfully reach and immunize IDUs by implementing vaccine programs through syringe exchange programs and providing modest payments to clients as incentives.24–26 It was hypothesized that adherence could also be increased by adopting an accelerated (2-month) hepatitis B vaccination schedule without a substantial reduction of immune protection.27 The purpose of the present study was to determine the economic and clinical consequences of vaccinating IDUs against hepatitis B after implementing these approaches. Two vaccination schedules (standard 0, 1, 6 months versus accelerated 0, 1, 2 months) and two screening strategies (giving the first dose only to susceptible people who return following screening versus giving the first dose to every participant at the screening visit) were compared. This study set out to ascertain whether hepatitis B vaccination campaigns targeting IDUs are economically desirable for the healthcare system and which screening strategy would be the most effective.
Methods
Study Population
The assessment of hepatitis B vaccination of IDUs was based on data from the Hepatitis Vaccine Study (HVS) of active IDUs in Chicago IL and Hartford and Bridgeport CT. Data were collected between May 2003 and March 2006. The primary inclusion criteria were that participants be aged≥18 years and had injected drugs in the past 30 days as indicated by self-report and the evidence of injection stigmata. The HVS research protocol was approved by the Yale Human Investigation Committee as well as the IRBs at DePaul University (Chicago) and the Hispanic Health Council (Hartford). Blood samples, collected at screening, were tested to determine HBV serostatus. First, Abbott Corzyme B® and Abbott Ausab® enzyme immunoassays (EIAs) were used to detect HBV core antibody (HBcAb) and HBV surface antibody (HBsAb), respectively. When necessary, sera were further tested with the Auszyme® EIA to detect HBV surface antigen (HBsAg), a marker for active infection. Among a total of 1964 recruits, 860 (44%) individuals were susceptible to HBV infection (i.e., their sero-samples tested negative for HBsAb, HBcAb, and HBsAg) and therefore were eligible to receive a vaccination. All others were excluded from the vaccination program.
Participants who returned for their serologic results and received the first dose of vaccine were randomized to either the standard (0, 1, and 6 months) or accelerated (0, 1, and 2 months) vaccination schedules. In Hartford and Bridgeport, all vaccinees received TWINRIX (GlaxoSmithKline, [GSK]). In Chicago, some of vaccinees received Recombvax (Merck) + HAVRIX (GSK) instead. Blood samples were collected again at the Dose-3 visit (i.e., Month 2 or Month 6) and at the exit visit (Month 7) to assess successful immunization rates for two doses and three doses, respectively. Those who reached or surpassed the immunization threshold of 10 mIU/ml of HBsAb were considered to be successfully immunized. Across the three sites, an average of $15 was paid to each participant at each visit as an incentive.
A total of 595 eligible participants (69.2% of the 860 who tested susceptible) returned and received the first dose of vaccine. Among those who received the first dose, 76.4% completed two doses, and 52.0% completed three doses in the standard arm; 78.1% completed two doses, and 63.6% completed three doses in the accelerated arm. The successful immunization rates of three doses in the standard and accelerated vaccination schedules were 85.7% and 78.3%, respectively. The overall successful immunization rate of two doses was 60%. More details of the setting of the HVS and the demographic details of the study population are available in Heimer et al.28
Model
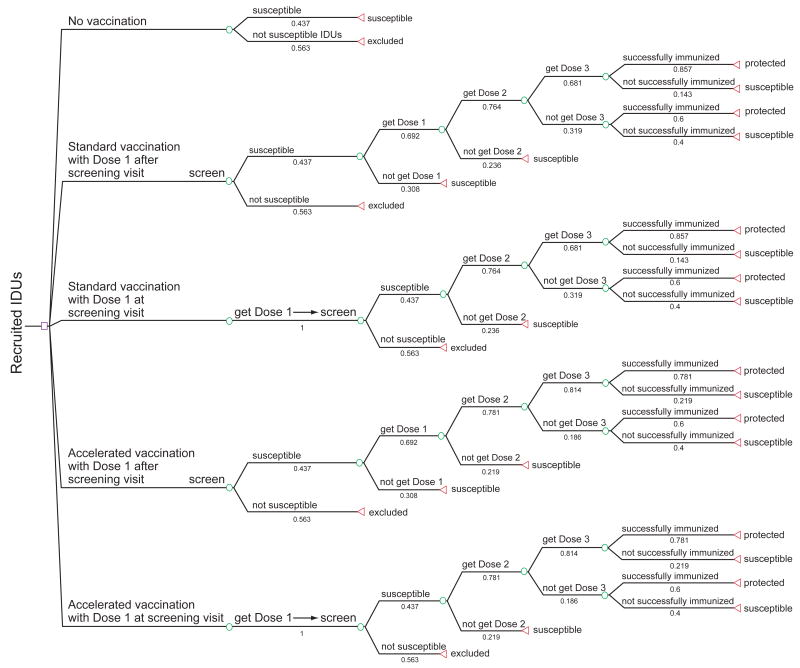
This study compared the economic performances of four vaccination strategies to that of a no-vaccination strategy in a cohort of 1964 IDUs (Figure 1). In the no-vaccination strategy, all susceptible IDUs were assumed to be at risk of HBV infection for the rest of their lives. The first vaccination strategy was standard vaccination with first dose after screening visit; this is the current standard recommended practice for high-risk populations,29 which assumed the standard 6-month vaccination schedule, with the first dose given only to susceptible IDUs after having received their serologic tests results. The second vaccination strategy was standard vaccination with first dose at screening visit, where every recruit was given the first dose at the screening visit (i.e., prior to obtaining serologic test results). For those who returned, second and third doses were given 1 and 6 months later only to those susceptible at screening.
Figure 1. Decision model for hepatitis B vaccination at syringe-exchange programs.
This figure depicts four vaccination strategies and the no-vaccination strategy considered in the model. Depending on the strategy chosen, participants would receive the first dose either prior to knowing their serologic results or after learning they are susceptible according to their serologic results; the third dose would be administered on either a standard or accelerated schedule. Once participants initiate vaccination, they either return for their follow-up doses or fail to complete the vaccine series. The completion rates, successful immunization rates, and percentage of the susceptible were based on data obtained from the HVS study. Those who remain susceptible as a result of failing to begin the vaccine series, to complete the vaccine series, or to become successfully immunized were entered into a Markov model simulating the natural history of HBV infection.
IDU, injection drug user
The third vaccination strategy was accelerated vaccination with first dose after screening visit, which is similar to the first strategy except that the third and final dose was given after 2 instead of 6 months. The fourth vaccination strategy was accelerated vaccination with first dose at screening visit, which gave the first dose to every recruit at the screening visit; the second and third doses were given 1 and 2 months later only to those susceptible at screening. IDUs successfully immunized in the four vaccination strategies included those successfully immunized after three doses and those successfully immunized after only two doses. Successfully immunized individuals were assumed to have a life-long protection against HBV. Individuals who completed the three-dose vaccine series but were not successfully immunized were assumed to have the same risk of infection as those who were unvaccinated.
By extending a Markov model simulating the natural history of HBV infection (Figure 2),21 the long-term clinical consequences and medical costs of those who remain susceptible as a result of failing to begin or complete the vaccine series or who fail to become successfully immunized were analyzed. The model was analyzed using TreeAge Pro 2007. A total of ten health states were followed in the model until all people in the cohort died; the analyses were conducted in 2007. A specified percentage of each state moves into other states according to a set of transition probabilities (Table 11,13–15,20,30–51) during a time interval called the Markov cycle. The Markov cycle in this model was set to 1 year. Because the annual rate of IDUs who permanently stop drug injection is unknown and recidivism is very common, it was assumed that the IDU cohort in this model was a closed population in the baseline analysis, and a range of permanent cessation rates was tested using the sensitivity analysis. Those IDUs who permanently stopped injection were assumed to have the same low risk of infection as the general population for the rest of their lives.
Figure 2. Markov model of disease states following HBV infection.
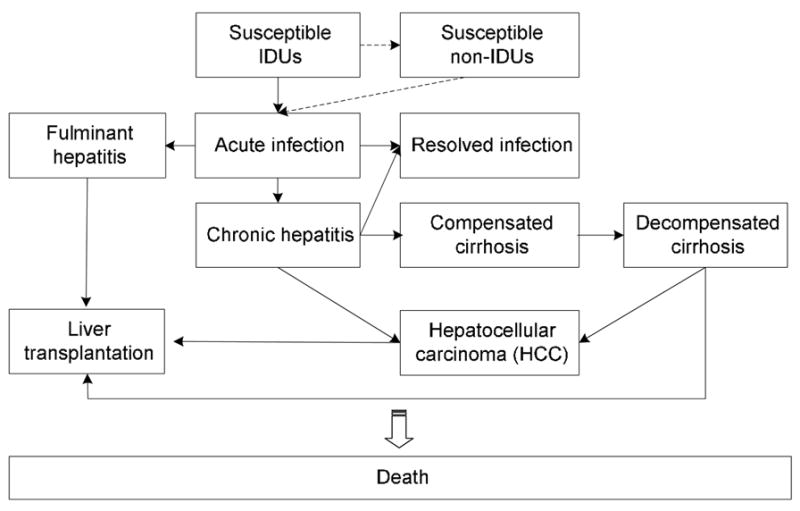
This figure shows the dynamic flow of health states included in the model. People who develop acute infections could have three possible manifestations: asymptomatic, non-hospitalized symptomatic, and hospitalized symptomatic. These three manifestations were not explicitly stated in the Markov model, but different medical costs would be considered in the analysis. Some acute infections can progress to fulminant hepatitis. Although most acute infections are resolved in adults, a percentage—about 5%—develop chronic infection. The chronic infections may progress to compensated cirrhosis, to hepatocellular carcinoma (HCC), or to both. Compensated cirrhosis may progress to decompensated cirrhosis and subsequent HCC. Patients with fulminant or chronic hepatitis may subsequently undergo liver transplantation. The dotted line in this model describes the dynamic of permanent cessation of drug injection in the sensitivity analysis.
Table 1.
Estimation for parameters in the Markov model
| Parameter | Baseline | Range | Sources |
|---|---|---|---|
| Susceptible IDUs | |||
| Probability of developing acute infection | 0.1 | 0.08–0.12 | 13–15 |
| Probability of stopping injecting drugs | 0 | 0–0.8 | Estimated |
| Probability of disease-free mortality | 0.035 | 0.033–0.081 | 30,31 |
| Susceptible non-IDUs | |||
| Probability of developing acute infection | 0.0004 | 0.0004–0.0006 | 1 |
| Probability of disease-free mortality | 0.017 | 0.013–0.017 | Estimated |
| Acute infection state | |||
| Proportion of asymptomatic infection | 0.6 | 20 | |
| Proportion of symptomatic infection | 0.4 | 20 | |
| Proportion of hospitalization | 0.12 | 20 | |
| Proportion of non-hospitalization | 0.88 | 20 | |
| Probability of developing fulminant hepatitis | 0.005 | 0.001–0.01 | 32,33 |
| Probability of developing chronic hepatitis | 0.05 | 0.02–0.1 | 34 |
| Probability of recovery | 0.94 | 35 | |
| Probability of disease-specific excess mortality | 0.005 | 0.005–0.01 | 35 |
| Fulminant hepatitis state | |||
| Probability of undergoing liver transplantation | 0.2 | 0.11–0.3 | 36 |
| Probability of disease-specific excess mortality | 0.7 | 0.6–0.8 | 37–39 |
| Chronic hepatitis state | |||
| Probability of developing compensated cirrhosis | 0.121 | 0.004–0.142 | 36 |
| Probability of developing HCC | 0.005 | 0.002–0.007 | 36 |
| Probability of recovery | 0.1 | 0.095–0.105 | 36 |
| Probability of disease-specific excess mortality | 0.025 | 0.018–0.036 | 33,40 |
| Compensated cirrhosis state | |||
| Probability of developing decompensatedcirrhosis | 0.044 | 0.028–0.083 | 41–43 |
| Probability of developing HCC | 0.024 | 0.013–0.055 | 44–47 |
| Probability of disease-specific excess mortality | 0.036 | 0.03–0.044 | 36 |
| Decompensated cirrhosis state | |||
| Probability of developing HCC | 0.024 | 0.013–0.055 | 44–47 |
| Probability of disease-specific excess mortality | 0.275 | 0.225–0.325 | 42,44,48 |
| Probability of undergoing liver transplantation | 0.018 | 0.015–0.024 | 36 |
| HCC state | |||
| Probability of disease-specific excess mortality | 0.429 | 0.259–0.503 | 36 |
| Probability of undergoing liver transplantation | 0.045 | 0.036–0.071 | 36 |
| Liver transplantation for fulminant | |||
| Probability of disease-specific excess mortality | 0.07 | 0.066–0.104 | 49–51 |
IDU, injection drug user; HCC, hepatocellular carcinoma
The model estimated the number of new acute HBV infections, quality-adjusted life years (QALYs), and the future medical costs in each strategy. QALYs and future medical costs were discounted at a 3% annual rate. The quality-of-life scale ranging from 0 (death) to 1 (full quality) for HBV-related illnesses was obtained from Kim et al.36 Economic results were summarized as the difference between the total costs (the sum of costs of the vaccination program and future medical costs) of each vaccination strategy and those costs incurred in the no-vaccination strategy (i.e., net cost). Benefits were expressed as acute HBV infections prevented and as QALYs gained.
Epidemiologic Data
The incidence of HBV and the transition probabilities used in the Markov model were estimated from the published literature (Table 1). It is known that the probabilities of progressing from acute to chronic infection and from chronic infection to cirrhosis differ between adults and children. Because participants in this study were all aged ≥18 years and the average age was 40, the probabilities used in this model were those characteristics of the adult group.
Costs
This economic evaluation was designed to assess vaccination strategies with respect to the allocation of healthcare funds, and therefore the analysis was conducted from the perspective of the healthcare sector. As such, only the direct medical costs of HBV-associated illnesses and the direct costs of the vaccination program targeting IDUs were considered in the model (Table 236,52,53). The medical costs associated with HBV morbidity and mortality by other diseases in co-infected individuals54 were not considered in this model. The annual medical costs of acute infections were obtained from Margolis et al.,52 and the annual medical costs of chronic infection and its associated illnesses were obtained from Kim et al.36 All these costs were updated to 2003 by using the medical care component of the Consumer Price Index. Vaccination program costs included (1) staff salaries and benefits associated with time spent on recruiting, serologic testing, and vaccine administration; (2) supply costs (e.g., serologic testing supplies, paper, and miscellaneous office supplies); (3) the cost of participant incentives; and (4) the cost of the vaccine. The baseline analysis assumed the availability of a discounted vaccine price of $10 (i.e., provided by health departments), but additional models using the standard retail vaccine price of $55 were analyzed. Future costs were discounted to 2003 at 3% per year.
Table 2.
Medical costs of hepatitis B-associated illnesses and vaccination costs
| Costs | Estimate
($ per person) |
Source |
|---|---|---|
| Annual medical costsa | ||
| Acute infectionb (one-time costs) | ||
| Nonhospitalized | 309 | 52 |
| Hospitalized | 9,173 | 52 |
| Fulminant | 17,407 | 52 |
| Chronic carrier | 1,003 | 36 |
| Compensated cirrhosis | 5,666 | 36 |
| Decompensated cirrhosis | 29,800 | 36 |
| Hepatocellular carcinoma | 26,425 | 36 |
| Liver transplantation | ||
| First year | 328,407 | 36 |
| Follow-up years | 31,681 | 36 |
| HBV-unrelated medical expenditure | 2,416 | 53 |
| Costs of vaccination program | ||
| Recruiting | 42 | Estimated |
| Blood drawing | 15 | Estimated |
| Vaccine administering | 13 | Estimated |
| Serologic test cost | 34 | Estimated |
| Supply cost | 8 | Estimated |
| Payment per visit | 15 | Estimated |
| Vaccine | 10–55 per dose | Estimated |
All medical costs are expressed in 2003 U.S. dollars.
Only medical costs for acute infection are one-time costs.
Sensitivity Analysis
Sensitivity analyses examined the effect of variations in the probabilities of disease progression, the incidence rate of acute infection, the percentage of the susceptible IDUs, vaccine completion rates, successful immunization rates, the rates at which IDUs stop injecting, and access to medical care.
Results
Baseline Results
Table 3 presents the baseline results for each strategy. It shows that the accelerated vaccination schedule prevented 17% more acute infections and gained 14%–20% more QALYs per person than the standard vaccination schedule. Also, the vaccination strategy with the first dose administered to everyone at the screening visit prevented 45% more acute infections and saved 43%–50% more QALYs per person than the strategy that administered the first dose only to susceptible persons who returned for their test results. Compared with the no-vaccination strategy, the net costs of the four vaccination strategies were all cost saving. (i.e., negative net costs). The accelerated vaccination schedule realized 32%–51% more savings than the standard vaccination schedule, and the strategy with the first dose administered to everyone at the screening visit realized 99%–127% more savings than the strategy that administered the first dose only to susceptible persons. Therefore, the accelerated vaccination schedule with the first dose given at the screening visit was the most cost-saving and cost-effective strategy. It should be remembered that the vaccination programs analyzed here were furnished with vaccine from local health departments that obtain their vaccine from the CDC for approximately $10 per dose. If vaccines were obtained at retail, the cost would be $55 per dose. This higher vaccine price decreased the magnitude of savings, but it did not change the cost-saving result and the relative order of magnitude of the savings of the four vaccination strategies.
Table 3.
The baseline cost–benefit result of the syringe exchange program-delivered hepatitis B vaccination involving 1964 IDUs in Chicago IL and Hartford and Bridgeport CT
| Strategy | Acute infections prevented | Discounted QALYs Gained | Discounted HBV-related medical costs ($) | Program cost ($) | Net cost ($) | ||
|---|---|---|---|---|---|---|---|
| $10 vaccine | $55 vaccine | $10 vaccine | $55 vaccine | ||||
| No vaccination | 0 | 0 | 1,414,526 | 0 | 0 | / | / |
| Standard vaccination with first dose after screening | 225 | 0.07 | 914,508 | 342,052 | 403,207 | −157,967 | −96,812 |
| Accelerated vaccination with first dose after screening | 264 | 0.08 | 827,333 | 348,926 | 413,636 | −238,267 | −173,557 |
| Standard vaccination with first dose at screening | 326 | 0.10 | 690,815 | 364,783 | 503,129 | −358,928 | −220,582 |
| Accelerated vaccination with first dose at screening | 382 | 0.12 | 565,811 | 374,717 | 518,192 | −473,999 | −330,524 |
HBV, hepatitis B virus; IDU, injection drug user; QALY, quality-adjusted life year
Sensitivity Analysis
When each disease-progression factor was varied within a plausible range (Table 1), none changed the relative order of the number of acute infections prevented or the dominant position of the accelerated vaccination with the first dose at screening. Among the variables tested, the total cost of each strategy was most sensitive to the probabilities of developing chronic hepatitis from acute infection, developing compensated cirrhosis from chronic hepatitis, and mortality rates.
Successful immunization rates in IDUs were 10% lower than those in healthy young adults.27 But the results were less sensitive to the successful immunization rates than to the vaccine-completion rates. When both two-dose and three-dose successful immunization rates increased by 10%, the savings of the four vaccination strategies increased by 4% to 10%, and the benefits increased by 2% to 3%, respectively. When both two-dose and three-dose completion rates increased by 10%, the savings of the four vaccination strategies increased by 24% to 52%, and the benefits increased by 15% to 18%, respectively.
A lower susceptibility rate, a lower incidence rate, or both, decreased the number of acute infections prevented and the savings realized from each vaccination strategy. The CDC recommendation is that the prevaccination screening may be considered when the prevalence of HBV infection is >20%.55 The analyses in this paper determined that the standard vaccination program, with the first dose given after the screening visit, was cost saving when susceptibility was not less than 25% (i.e., prevalence is not greater than 67%) and the vaccination and laboratory costs did not exceed $500,000. When the susceptibility rate was less than 17% (i.e., prevalence greater than 75%) or the annual incidence of acute infection was lower than 2.5%, the future HBV-related medical costs saved by the four vaccination strategies did not offset the vaccination program costs, and the four vaccination strategies were no longer cost saving compared with the no-vaccination strategy.
When the cessation rate of drug injection was considered in the model (i.e., when the annual percentage of IDUs permanently stopping drug injection increased), an increase in QALYs gained was found. This is because the life-saving effect of hepatitis B vaccination diminished due to the high mortality rate of IDUs, and the effect is more obvious in the general population due to the relatively lower mortality rate. When injection-cessation rates increased, there was a decrease in the number of acute infections prevented and in the magnitude of savings in each vaccination strategy. This is attributed to the significantly lower HBV incidence rate in the general population. When more than 29% of IDUs permanently stopped drug injection per year, all four vaccination strategies were no longer cost saving compared with the no-vaccination strategy.
In the baseline analysis, it was assumed that 100% of IDUs had access to medical care after they were infected. The sensitivity analysis demonstrated that all four vaccination strategies were cost saving compared to the no-vaccination strategy once more than 70% of IDUs had access to medical care. When less than 46% of IDUs had access to medical care, all four vaccination strategies were no longer cost-saving, because 54% of medical costs were no longer spent.
Discussion
This study demonstrated that integrating hepatitis B vaccination into existing syringe exchange programs would realize an economic benefit for the healthcare system. The most cost-saving and cost-effective vaccination strategy included giving the first dose to all screened participants prior to knowing their serological results and administering the vaccination under the accelerated schedule (0, 1, 2 months); this strategy saved almost a half-million dollars, realized a gain of 0.12 QALYs per person, and prevented 382 acute HBV infections for a cohort of 860 susceptible IDUs.
The cost saving of a hepatitis B vaccination program as demonstrated in this study was based on several factors. First, the target population had a comparatively high incidence rate of infection.13–15 Second, a high proportion (44%) of the target population remained susceptible to infection. Third, the medical costs of chronic HBV-associated illnesses are expensive, ranging from $1003 to $328,407 per person per year.36 Fourth, the sensitivity analysis indicated that at least 46% of the target population should have access to medical care when they get infected. Although health data were not collected in this study, 79.7% active IDUs in Connecticut are insured (unpublished data, 2002). However, it should be noted that having health insurance does not guarantee access to medical care. Fifth, the program is cost saving if less than 29% of IDUs permanently stop drug injecting annually. To our knowledge, there is no information in the literature concerning permanent injection-cessation rates. In fact, this rate would be difficult if not impossible to ascertain; it is imagined that a 29% cessation rate surpasses the actual rate. Economic benefit would be expected when implementing similar vaccination campaigns in other U.S. cities that satisfy the above conditions.
In addition, this study demonstrated that an investment in hepatitis B vaccination targeting IDUs through syringe exchange programs compared favorably to other HBV interventions targeting other high-risk groups in the U.S. The vaccination of hemodialysis patients was found to cost $261 per patient from the perspective of the healthcare sector.23 A recent study showed that routine hepatitis B vaccination costs $6.80 per adult client through HIV counseling and testing sites, and costs $7.40 per adult client through sexually transmitted disease clinics from the societal perspective.36 Only one previous hepatitis B vaccination study with the perspective of the healthcare sector also identified cost savings, and it demonstrated that providing hepatitis B vaccine for prison inmates would realize a saving of $45,000,000 for 381,646 inmates, approximately $118 per inmate involved.20 In contrast, the most cost-saving strategy in the current study could save $473,999 for 1964 IDUs, approximately $241 per IDU involved.
These estimates are conservative and likely underestimate the benefits of a hepatitis B vaccination program for IDUs. Only participants who were successfully protected by completing two or three doses were considered in this model. But those who completed only one dose were found to have successful immunization rates ranging from 5.4% to 20.4% in healthy adults in previous studies.56 Although the one-dose successful immunization rate would probably be lower among IDUs, one might reasonably expect some increase in protection by factoring that rate into the model. Furthermore, occult HBV infection was not taken into account in the current study’s model. A previous study showed that those with occult HBV infection might have higher probabilities of developing cirrhosis and hepatocellular carcinoma,57 which may incur greater medical costs for infected people compared to vaccinated people and increase the savings from vaccination. Moreover, because most HBV transmission is from individuals engaged in high-risk behaviors, the benefits of vaccination will be greater if secondary transmission to non-IDUs is considered in the model. In addition, the average age of IDUs in this study was 40. More savings would be realized by enrolling younger IDUs, because they may have a higher likelihood of developing protective antibody levels.58 The cohort effect of universal vaccination (implemented by the U.S. Public Health Service in the 1990s) should be considered in future models.
In conclusion, existing syringe exchange programs in the U.S. should include hepatitis B vaccination programs because they are effective in protecting IDUs against HBV infection and are economically beneficial to the healthcare system. This logic can be extended to any program or service that comes into repeated contact with high HBV-incidence populations such as IDUs.
Acknowledgments
The authors would like to thank National Institute on Drug Abuse (R01-DA14502) for funding support. The authors also thank the staff of the project: Clifton W. Sanchez and Nayda de la Rosa in Chicago, Gregory Rivera and Migdalia Texidor-Huertas in Hartford, and Anthony Givens and Erin Curtin in Bridgeport. The authors are also grateful for the valuable input from David A. Paltiel concerning modeling issues, particularly the costing part. The project is indebted to the syringe exchange programs in Chicago, Hartford, and Bridgeport that agreed to participate and refer their clients.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the U.S. : recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR16):1–31. [PubMed] [Google Scholar]
- 2.Aceijas C, Stimson GV, Hickman M, Rhodes T. Global overview of injecting drug use and HIV infection among injecting drug users. Aids. 2004;18(17):2295–303. doi: 10.1097/00002030-200411190-00010. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SR, Tempalski B, Cooper H, et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. J Urban Health. 2004;81(3):377–400. doi: 10.1093/jurban/jth125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmberg SD. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86(5):642–54. doi: 10.2105/ajph.86.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter H, Robinson G, Hanlon C, Hailwood C, Massarotto A. Prevalence of hepatitis B and C infection in a methadone clinic population: implications for hepatitis B vaccination. N Z Med J. 2001;114(1136):324–6. [PubMed] [Google Scholar]
- 6.Cook PA, McVeigh J, Syed Q, Mutton K, Bellis MA. Predictors of hepatitis B and C infection in injecting drug users both in and out of drug treatment. Addiction. 2001;96(12):1787–97. doi: 10.1046/j.1360-0443.2001.961217878.x. [DOI] [PubMed] [Google Scholar]
- 7.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86(5):655–61. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine OS, Vlahov D, Koehler J, Cohn S, Spronk AM, Nelson KE. Seroepidemiology of hepatitis B virus in a population of injecting drug users. Association with drug injection patterns. Am J Epidemiol. 1995;142(3):331–41. doi: 10.1093/oxfordjournals.aje.a117639. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Zetina J, Kerndt P, Ford W, Woerhle T, Weber M. Prevalence of HIV and hepatitis B and self-reported injection risk behavior during detention among street-recruited injection drug users in Los Angeles County, 1994–1996. Addiction. 2001;96(4):589–95. doi: 10.1080/09652140020031638. [DOI] [PubMed] [Google Scholar]
- 10.Murrill CS, Weeks H, Castrucci BC, et al. Age-specific seroprevalence of HIV, hepatitis B virus, and hepatitis C virus infection among injection drug users admitted to drug treatment in 6 U.S. cities. Am J Public Health. 2002;92(3):385–7. doi: 10.2105/ajph.92.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuel MC, Doherty PM, Bulterys M, Jenison SA. Association between heroin use, needle sharing and tattoos received in prison with hepatitis B and C positivity among street-recruited injecting drug users in New Mexico, USA. Epidemiol Infect. 2001;127(3):475–84. doi: 10.1017/s0950268801006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steffen T, Blattler R, Gutzwiller F, Zwahlen M. HIV and hepatitis virus infections among injecting drug users in a medically controlled heroin prescription programme. Eur J Public Health. 2001;11(4):425–30. doi: 10.1093/eurpub/11.4.425. [DOI] [PubMed] [Google Scholar]
- 13.Hagan H, Snyder N, Hough E, et al. Case-reporting of acute hepatitis B and C among injection drug users. J Urban Health. 2002;79(4):579–85. doi: 10.1093/jurban/79.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Des Jarlais DC, Diaz T, Perlis T, et al. Variability in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among young injecting drug users in New York City. Am J Epidemiol. 2003;157(5):467–71. doi: 10.1093/aje/kwf222. [DOI] [PubMed] [Google Scholar]
- 15.Hagan H, McGough JP, Thiede H, Weiss NS, Hopkins S, Alexander ER. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol. 1999;149(3):203–13. doi: 10.1093/oxfordjournals.aje.a009792. [DOI] [PubMed] [Google Scholar]
- 16.The Advisory Committee on Immunization Practices (ACIP) Update on adult immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1991;40(RR12):1–94. [PubMed] [Google Scholar]
- 17.Carey J, Perlman DC, Friedmann P, et al. Knowledge of hepatitis among active drug injectors at a syringe exchange program. J Subst Abuse Treat. 2005;29(1):47–53. doi: 10.1016/j.jsat.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Kuo I, Mudrick DW, Strathdee SA, Thomas DL, Sherman SG. Poor validity of self-reported hepatitis B virus infection and vaccination status among young drug users. Clin Infect Dis. 2004;38(4):587–90. doi: 10.1086/381440. [DOI] [PubMed] [Google Scholar]
- 19.Kuo I, Sherman SG, Thomas DL, Strathdee SA. Hepatitis B virus infection and vaccination among young injection and non-injection drug users: missed opportunities to prevent infection. Drug Alcohol Depend. 2004;73(1):69–78. doi: 10.1016/j.drugalcdep.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Pisu M, Meltzer MI, Lyerla R. Cost-effectiveness of hepatitis B vaccination of prison inmates. Vaccine. 2002;21(3–4):312–21. doi: 10.1016/s0264-410x(02)00457-7. [DOI] [PubMed] [Google Scholar]
- 21.Williams JR, Nokes DJ, Anderson RM. Targeted hepatitis B vaccination—a cost effective immunisation strategy for the UK? J Epidemiol Community Health. 1996;50(6):667–73. doi: 10.1136/jech.50.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabrizi F, Di Filippo S, Marcelli D, et al. Recombinant hepatitis B vaccine use in chronic hemodialysis patients. Long-term evaluation and cost-effectiveness analysis. Nephron. 1996;72(4):536–43. doi: 10.1159/000188935. [DOI] [PubMed] [Google Scholar]
- 23.Saab S, Weston SR, Ly D, et al. Comparison of the cost and effectiveness of two strategies for maintaining hepatitis B immunity in hemodialysis patients. Vaccine. 2002;20(25–26):3230–5. doi: 10.1016/s0264-410x(02)00249-9. [DOI] [PubMed] [Google Scholar]
- 24.Altice FL, Bruce RD, Walton MR, Buitrago MI. Adherence to hepatitis B virus vaccination at syringe exchange sites. J Urban Health. 2005;82(1):151–61. doi: 10.1093/jurban/jti016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Des Jarlais DC, Fisher DG, Newman JC, et al. Providing hepatitis B vaccination to injection drug users: referral to health clinics vs on-site vaccination at a syringe exchange program. Am J Public Health. 2001;91(11):1791–2. doi: 10.2105/ajph.91.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seal KH, Kral AH, Lorvick J, McNees A, Gee L, Edlin BR. A randomized controlled trial of monetary incentives vs. outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug Alcohol Depend. 2003;71(2):127–31. doi: 10.1016/s0376-8716(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 27.Marsano LS, Greenberg RN, Kirkpatrick RB, et al. Comparison of a rapid hepatitis B immunization schedule to the standard schedule for adults. Am J Gastroenterol. 1996;91(1):111–5. [PubMed] [Google Scholar]
- 28.Heimer R, Grau LE, Singer M, et al. Hepatitis B virus prevalence and vaccination rates among Hispanic injection drug users participating in a vaccination campaign operated at syringe exchange programs. J Drug Issues. In press. [Google Scholar]
- 29.Clover RD, Zimmerman RK. The 2003 recommended adult immunization schedule. Advisory Committee on Immunization Practices. American Academy of Family Physicians. American College of Obstetricians and Gynecologists. American College of Physicians. American Society of Internal Medicine. CDC. Am Fam Physician. 2002;66(12):2329–30. 33–5. [PubMed] [Google Scholar]
- 30.Galai N, Safaeian M, Vlahov D, Bolotin A, Celentano DD. Longitudinal patterns of drug injection behavior in the ALIVE Study cohort, 1988–2000: description and determinants. Am J Epidemiol. 2003;158(7):695–704. doi: 10.1093/aje/kwg209. [DOI] [PubMed] [Google Scholar]
- 31.Vlahov D, Wang CL, Galai N, et al. Mortality risk among new onset injection drug users. Addiction. 2004;99(8):946–54. doi: 10.1111/j.1360-0443.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 32.Karvountzis GG, Redeker AG, Peters RL. Long term follow-up studies of patients surviving fluminant viral hepatitis. Gastroenterology. 1974;67(5):870–7. [PubMed] [Google Scholar]
- 33.Nielsen JO. Clinical course and prognosis of acute hepatitis. Ann Clin Res. 1976;8(3):151–7. [PubMed] [Google Scholar]
- 34.McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151(4):599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 35.CDC. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50(RR5):1–43. [PubMed] [Google Scholar]
- 36.Kim SY, Billah K, Lieu TA, Weinstein MC. Cost effectiveness of hepatitis B vaccination at HIV counseling and testing sites. Am J Prev Med. 2006;30(6):498–506. doi: 10.1016/j.amepre.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Chan PC, Chen HL, Kong MS, et al. Factors affecting the mortality of pediatric fulminant hepatic failure in relation to hepatitis B virus infection. J Gastroenterol Hepatol. 2005;20(8):1223–7. doi: 10.1111/j.1440-1746.2005.03923.x. [DOI] [PubMed] [Google Scholar]
- 38.Garfein RS, Bower WA, Loney CM, et al. Factors associated with fulminant liver failure during an outbreak among injection drug users with acute hepatitis B. Hepatology. 2004;40(4):865–73. doi: 10.1002/hep.20383. [DOI] [PubMed] [Google Scholar]
- 39.Kato Y, Nakata K, Omagari K, et al. Clinical features of fulminant hepatitis in Nagasaki Prefecture, Japan. Intern Med. 2001;40(1):5–8. doi: 10.2169/internalmedicine.40.5. [DOI] [PubMed] [Google Scholar]
- 40.Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology. 1999;29(3):971–5. doi: 10.1002/hep.510290312. [DOI] [PubMed] [Google Scholar]
- 41.Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53(5):744–9. doi: 10.1136/gut.2003.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gheorghe L, Iacob S, Simionov I, et al. Natural history of compensated viral B and D cirrhosis. Rom J Gastroenterol. 2005;14(4):329–35. [PubMed] [Google Scholar]
- 43.Marcellin P, Castelnau C, Martinot-Peignoux M, Boyer N. Natural history of hepatitis B. Minerva Gastroenterol Dietol. 2005;51(1):63–75. [PubMed] [Google Scholar]
- 44.Alward WL, McMahon BJ, Hall DB, Heyward WL, Francis DP, Bender TR. The long-term serological course of asymptomatic hepatitis B virus carriers and the development of primary hepatocellular carcinoma. J Infect Dis. 1985;151(4):604–9. doi: 10.1093/infdis/151.4.604. [DOI] [PubMed] [Google Scholar]
- 45.Beasley R, Hwang L-Y, editors. Overview on the epidemiology of hepatocellular carcinoma. Baltimore: Williams & Wilkins; 1991. [Google Scholar]
- 46.Colombo M, de Franchis R, Del Ninno E, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325(10):675–80. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 47.Obata H, Hayashi N, Motoike Y, et al. A prospective study on the development of hepatocellular carcinoma from liver cirrhosis with persistent hepatitis B virus infection. Int J Cancer. 1980;25(6):741–7. doi: 10.1002/ijc.2910250609. [DOI] [PubMed] [Google Scholar]
- 48.D’Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31(5):468–75. doi: 10.1007/BF01320309. [DOI] [PubMed] [Google Scholar]
- 49.Benner A, Lyden E, Rogge J, Weaver L, Mukherjee S. Outcomes of liver transplantation for hepatitis B: a single-center study of 35 patients. Transplant Proc. 2004;36(9):2741–3. doi: 10.1016/j.transproceed.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 50.Wai CT, Da Costa M, Sutedja D, et al. Long-term results of liver transplant in patients with chronic viral hepatitis-related liver disease in Singapore. Singapore Med J. 2006;47(7):588–91. [PubMed] [Google Scholar]
- 51.Wai CT, Lee YM, Selamat DS, et al. Liver transplantation in Singapore 1990–2004. Singapore Med J. 2006;47(7):580–3. [PubMed] [Google Scholar]
- 52.Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA. 1995;274(15):1201–8. [PubMed] [Google Scholar]
- 53.Bureau of Labor Statistics. Comsumer expenditure in 2003. Report 986. Washington DC: U.S. Dept. of Labor; 2005. [Google Scholar]
- 54.Singer M, Clair S. Syndemics and public health: reconceptualizing disease in bio-social context. Med Anthropol Q. 2003;17(4):423–41. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- 55.Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the U.S.: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55(RR16):1–33. quiz CE1–4. [PubMed] [Google Scholar]
- 56.Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. 2003;63(10):1021–51. doi: 10.2165/00003495-200363100-00006. [DOI] [PubMed] [Google Scholar]
- 57.Brechot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Brechot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34(1):194–203. doi: 10.1053/jhep.2001.25172. [DOI] [PubMed] [Google Scholar]
- 58.Hadler SC, Margolis HS. Hepatitis B immunization: vaccine types, efficacy, and indications for immunization. Curr Clin Top Infect Dis. 1992;12:282–308. [PubMed] [Google Scholar]