1. Introduction
The lateral hypothalamus (LH) plays a role in descending nociceptive modulation. Support for this claim comes from observations that antinociception occurs in the LH after microinjection of morphine (Fuchs and Melzack, 1995; Dafny et al., 1996; Franco and Prado, 1996) or glutamate (Behbehani et al., 1988), and following electrical stimulation in the cat (Carstens et al., 1983) and male rats (Dafny et al., 1996; Aimone and Gebhart, 1987). Additionally, stimulating the LH with the cholinergic agonist carbamyolcholine (carbachol) increases response latencies on the tail flick and foot withdrawal tests in female rats (Holden and Naleway 2001; Holden et al. 2002). However, the exact mechanism by which LH stimulation produces antinociception is not known.
Findings from previous studies are suggestive that serotonin plays a role in antinociception from LH stimulation. For example, electrical stimulation of the LH inhibits heat evoked responses in dorsal horn neurons, and this inhibition was blocked by systemic administration of the 5-HT antagonist methysergide blocks LH-induced antinociception (Carstens et al., 1983). Intrathecal administration of methysergide following electrical stimulation of the LH decreases response latencies on the tail flick and foot withdrawal tests in male rats (Aimone and Gebhart 1987). Stimulation of the LH with carbachol produces antinociception that is partially blocked by intrathecal 5-HT1A, 5-HT1B and 5-HT3 receptor antagonists in an acute thermal model in female rats (Holden et al. 2005). Although there is a small, but direct projection from the LH to the spinal cord (Hosoya, 1980), spinally descending serotonergic cells in the LH have not been identified (Bowker et al., 1981), indicating that LH stimulation activates intermediary nuclei that contain serotonin.
The most likely nuclei candidates for LH-mediated serotonin input to the spinal cord are found in the rostral ventromedial medulla (RVM; the nucleus raphe magnus and the nucleus reticularis gigantocellularis pars alpha), an area known to provide most of the serotonin to the spinal cord dorsal horn and to produce antinociception when activated (Dahlstrom A and Fuxe, 1965; Satoh et al., 1980; Bowker et al., 1981; Rivot et al., 1982; Jensen and Yaksh, 1984; Hammond et al., 1985; Ruda et al., 1986; Heinricher et al., 1999; Gilbert and Franklin, 2001; Hurley et al., 2003; Buhler et al., 2004). Inactivating the RVM by lidocaine microinjection or ibotenic acid lesion significantly increases the electrical stimulation levels needed in the LH to achieve antinociception in male rats (Aimone et al., 1988). However, electrical stimulation can activate fibers of passage as well as neurons in the area of stimulation, so this finding, while suggestive does not rule out involvement from areas other than the LH. Nor has this intermediary connection been established in female rats. Anatomically, there is evidence of an efferent connection from the LH to the RVM that is mainly ipsilateral (Berk and Finkelstein, 1982; Saper et al., 1979; Hosoya, 1980). Part of this connection may consist of substance P-immunoreactive (SP-ir) neurons, based on the observation that the LH contains SP-ir neuron profiles and the RVM contains SP-ir axonal fibers (Ljungdahl, et. al‥, 1978), but the connection has not been shown directly. Lastly, NK1 receptors that bind SP are found in the RVM (Makeham et al., 2005).
The objective of the present experiments was to determine whether the RVM is involved in LH-mediated antinociception in female rats. We used both anatomical and behavioral techniques to address the research question. We used retrograde tract tracing using FluoroGold combined with double label immunocytochemistry to determine whether neurons in the LH immunoreactive for SP project to the RVM. We also used the tail flick and foot withdrawal tests to measure responses to an acute thermal stimulus. We chose both tests because previous work has shown differences between tail and foot withdrawal latencies (Fang and Proudfit, 1996; Fang and Proudfit, 1998). Three sets of behavioral experiments addressed the research question. In the first set, carbachol 125 nmol in 0.5 µl normal saline, a dose previously determined to provide optimum antinociception in a lightly anesthetized acute preparation (Holden and Naleway, 2001; Holden et al., 2005), or normal saline for control was microinjected into the LH, and nociceptive responses were measured. Carbachol is a nonselective cholinergic receptor agonist that resists the actions of cholinesterase and produces activation of neurons at a number of intracerebral sites (Klamt and Prado, 1991). The LH contains both muscarinic and nicotinic receptors (Rainbow et al, 1984; Cortes et al., 1986; Oki et al., 2005), and we have shown that the actions of carbachol are mediated by cholinergic receptor activity in the LH (Holden et al., 2005). In the second set of experiments, we obtained carbachol-induced antinociception, then microinjected cobalt chloride a non-selective calcium channel blocker that blocks synaptic activity in the area of injection, L-703,606 a specific NK1 receptor antagonist, or normal saline for control into the RVM. In the third set of experiments, we microinjected cobalt chloride, L-703,606, or saline into the RVM without LH activation. Preliminary findings have been published as an abstract (Pizzi and Holden 2003).
2. Results
The location of SP-ir neuron profiles that project to the RVM
Retrograde transport of FluoroGold was used to identify the locations of neurons in the tuberal hypothalamus that project to the RVM. In six rats, the FluoroGold deposits included the nucleus raphe magnus and the nucleus reticularis gigantocellularis pars alpha in an area between the genu of the 7th nerve rostrally and the inferior olivary complex caudally (Fig. 1). None of the six injections was directly on the midline, but the core of the injection included both the raphe magnus and the reticularis gigantocellularis pars alpha. Analysis of these six cases showed that FluoroGold-containing neurons were located throughout the rostral-caudal extent of the tuberal hypothalamus, but were more concentrated in the LH, with labeling also in the posterior hypothalamus, the dorsomedial nucleus, and the dorsal hypothalamic area, located between the mammillothalamic tract and the third ventricle (Fig. 2). Relatively few neurons labeled in the anterior hypothalamic area. The pattern of labeling was bilateral, but the greatest concentration of FluoroGold labeled neuron profiles was found ipsilateral to the injection site.
Fig. 1.
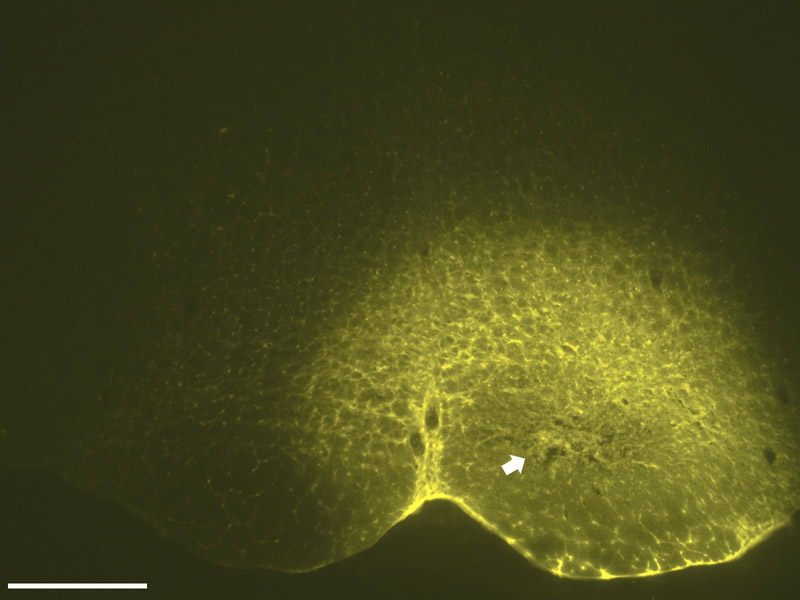
A photomicrograph (4X) of the FluoroGold injection site that resulted in retrograde labeling seen in Fig. 2 and Fig. 3. The center of the injection, characterized by a compacted core, lies dorsal to the pyramid and includes both the nucleus raphe magnus and the nucleus reticularis gigantocellularis pars alpha. A halo of neurons surrounds the core and represents local neurons that have been labeled by the tracer. Scale bar = 500 µm; distance from bregma approximately −11.60 mm.
Fig. 2.
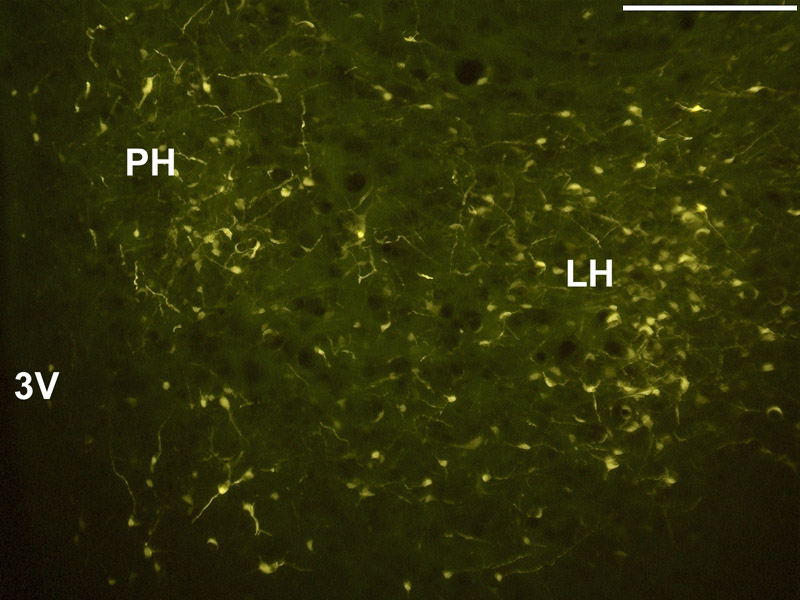
Neuron profiles in the hypothalamus retrogradely labeled by the FluoroGold deposit seen in Fig. 1. Abbreviations: 3V, third ventricle; LH, lateral hypothalamus; Ph, posterior hypothalamic area. Magnification = 10×; scale bar = 250 µm; distance from bregma approximately −3.80 mm.
An abundance of SP-ir terminals was seen throughout the area of the tuberal hypothalamus, while substance P-ir neuron profiles were found scattered through the rostrocaudal extent of the LH, the posterior hypothalamic area, the zona incerta and subincertal nucleus, and the dorsomedial and ventromedial hypothalamic nuclei. Most of the neuron profiles were between 20–30 µm in diameter. The intensity of SP-ir labeling varied from rat to rat, most likely due to technical differences and the effectiveness of the colchicine injection in reaching the neurons of interest. Preliminary studies indicated that, while labeling of terminals was seen without colchicine, no somatic labeling was observed in the absence of colchicine injection. No SP-ir labeling was seen in sections from the two control rats. Figure 3 shows that some of the neuron profiles labeled with FluoroGold were also labeled with SP-ir.
Fig. 3.
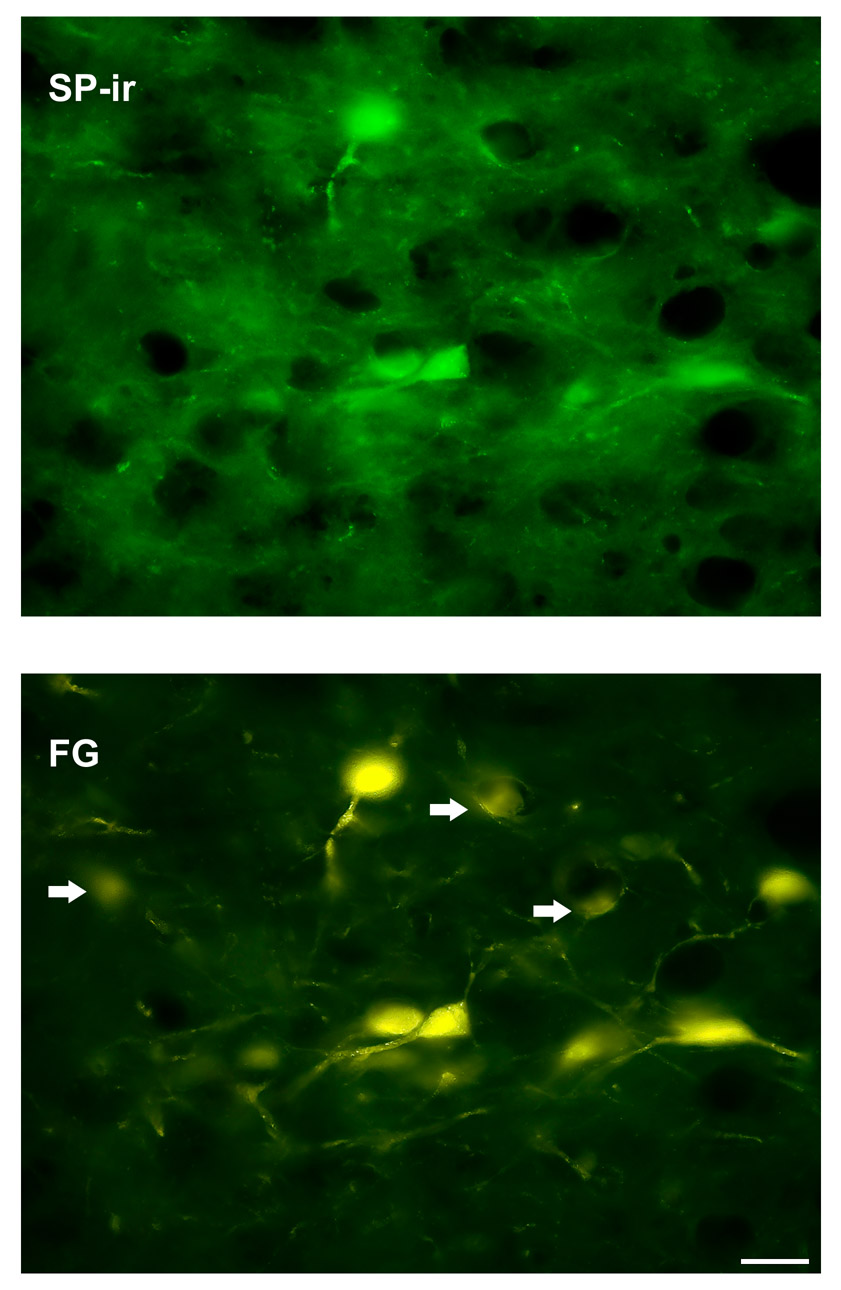
A high power (40×) magnification of neuron profiles in the LH immunoreactive for SP (top panel) co-localized with FluoroGold profiles (FG) as shown on the bottom panel. Arrows indicate FG labeled profiles that do not co-localize with SP-ir. Scale bar = 25 µm.
Carbachol microinjection injection in the LH
Of the 53 rats used in the three behavioral experiments, 11 were excluded because cannula placement occurred outside of the identifiable boundaries of the LH, RVM, or both. The first set of experiments demonstrated the time course for carbachol-induced antinociception. Fig. 4A is a schematic of microinjection sites and response latencies. Microinjection within the LH produced moderate to robust increases in response latencies. Injections in which the microinjector tip was ventral to the LH produced shorter response latencies. One microinjector placement in the amygdala also produced relatively short response latencies. To assure that nociceptive responses occurred only from LH stimulation, data were analyzed solely from experiments in which the injection site was located within the LH.
Fig. 4.
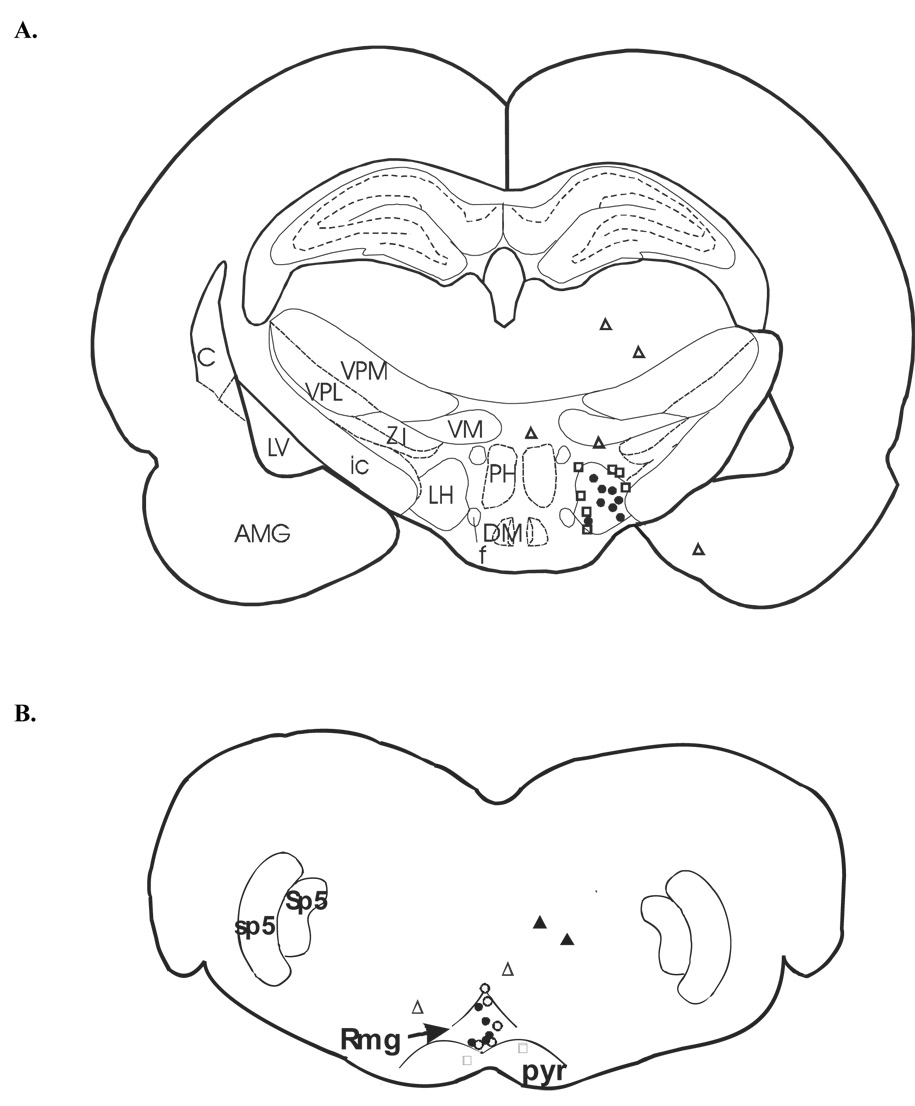
A schematic depiction of microinjection sites in rats receiving 125 nmol of carbachol (A). Mean response latencies were taken 5 min post injection and are represented as follows: filled circles, 4.5–6.0 sec; open squares, 3.0–4.5 sec; open triangles, 0.1–2.9 sec. Distance from bregma approximately −3.80 mm. (B) A schematic depiction of microinjection sites for rats receiving cobalt chloride (open symbols) or L-703,606 (closed symbols). Each symbol represents the mean response latency taken 5 min after microinjection of cobalt chloride or L-703, 606 and are represented as follows: open triangles, 4.5–6.0 sec; open squares, 3.0–4.4 sec; closed circles, 0.1–2.9 sec. Distance from bregma approximately −11.60 mm. Abbreviations: 7, facial nucleus; AMG, amygdala; C, caudate nucleus; DM, dorsomedial hypothalamic nucleus; f, fornix; ic, internal capsule; LV, lateral ventricle; LH, lateral hypothalamus; NGα, nucleus reticularis gigantocellularis, pars alpha; PH, posterior hypothalamus; pyr, pyramid; RMg, nucleus raphe magnus; sp5, spinal trigeminal tract; Sp5, spinal 5 nucleus, oral part. VM, ventromedial hypothalamic nucleus; VPL, ventral posteriolateral thalamic nucleus; ZI, zona incerta.
Microinjection of carbachol into the LH at sites similar to those shown in Fig. 4A produced moderate antinociception on both the tail flick test beginning at 1 min post injection and peaking at 5–10 min after microinjection, although the effect remained fairly stable for 30 min (F = 187.63(1,10), p <0.001; Fig. 5A). There was a significant interaction effect (p < 0.001), indicating that the effect of carbachol depended on the time the foot withdrawal measurement was taken. Compared to saline controls, rats receiving carbachol had significantly longer tail flick latencies (4.02 ± 0.09 vs. 2.2 ± 0.09 sec, resp.; p < 0.05).
Fig. 5.

(A) Following a baseline measurement at −5 min, carbachol microinjection into the LH produced antinociception as shown by an increase in tail flick latencies. Response latencies produced by microinjection of carbachol (closed circles; n = 6) or saline (open circles; n = 6) are expressed as mean tail flick latency values ± S.E.M., and plotted on the ordinate as a function of time min. Standard error bars may be obscured by the symbol. (B) Foot withdrawal latencies were also increased following carbachol microinjection (closed squares; n = 6) in the LH compared to vehicle controls (open squares; n = 6). Values for foot withdrawal latencies represent pooled data.
Carbachol microinjection also significantly increased foot withdrawal latencies (F = 134.14(1,10), p < 0.001; Fig. 5B). As with the tail, the effect of carbachol depended on time (p = 0.03). Rats with carbachol microinjection took significantly longer to withdraw their paws from the heat stimulus as compared to control rats (3.75 ± 0.09 vs. 2.21 ± 0.09 sec, resp.; p < 0.05). This set of experiments established the time course for LH-induced antinociception under conditions similar to those for subsequent experiments.
Blockade of LH-induced antinociception by cobalt chloride microinjection in the RVM
In the first of the second set of experiments, following a baseline measurement, carbachol was microinjected into the LH, and antinociception was established as shown by three withdrawal latency measurements (Fig. 6A, B). Cobalt chloride was then microinjected into the RVM at sites similar to those identified in Fig. 4B. Cobalt chloride injected into areas dorsal to the RVM or into the fiber tracts of the pyramids produced relatively longer response latencies. Microinjection of cobalt chloride in the RVM reduced tail flick response latencies to baseline levels, effectively abolishing LH-induced antinociception as compared to saline microinjection into the RVM (Two way repeated measures ANOVA, F = 23.1(2,12), p < 0.001). In contrast, physiological saline microinjected near the RVM had no effect on LH-induced antinociception (2.43 ± 0.19 sec vs. 4.00 ± 0.19 sec, cobalt chloride vs. saline, resp.; p < 0.05; Fig. 3A). There was a significant interaction effect (p = 0.002), indicating that the effect of carbachol was dependent on time.
Fig. 6.
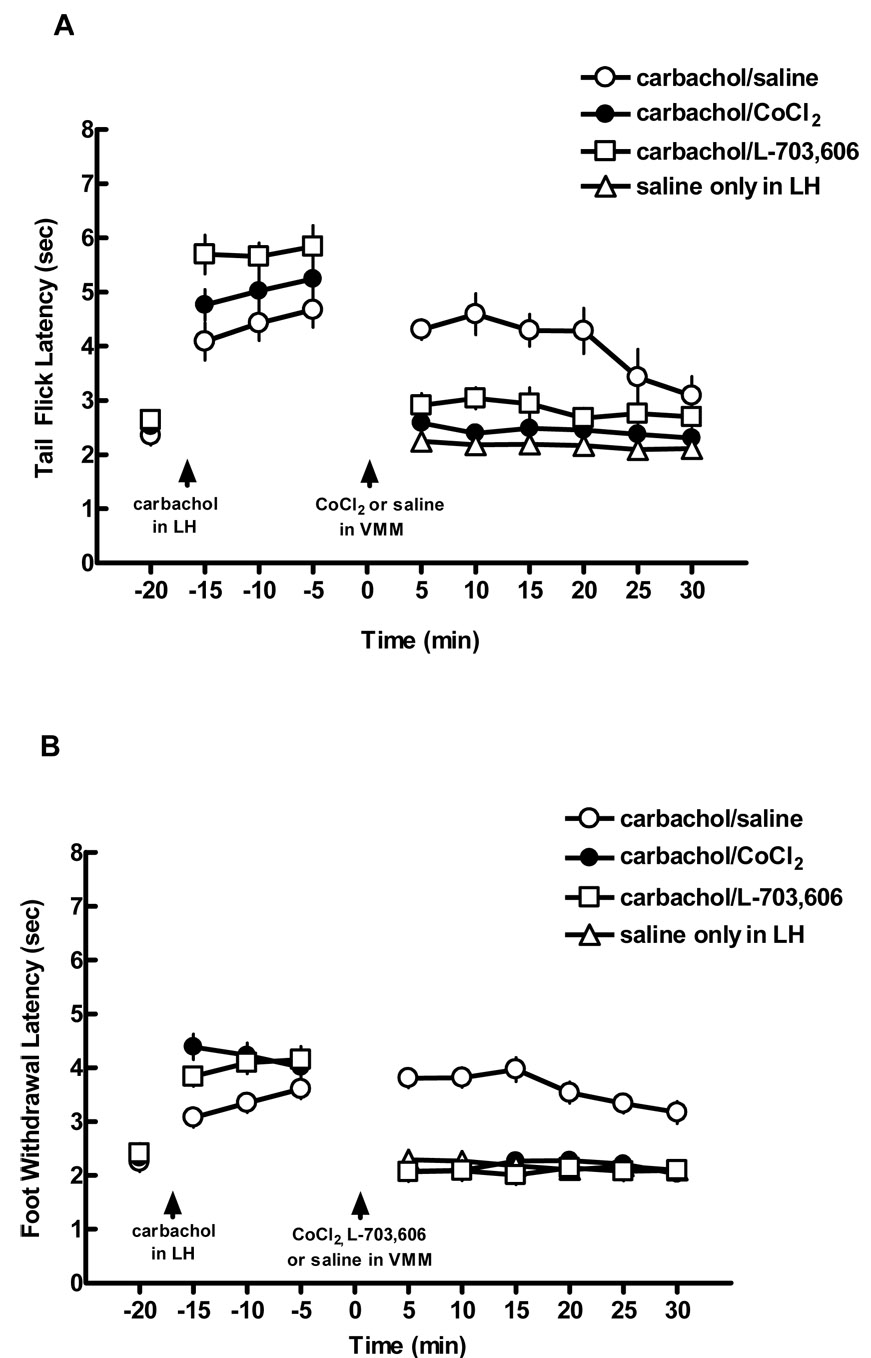
Antinociception produced by microinjection of carbachol in the LH was blocked by microinjection of cobalt chloride or L-703,606 in the RVM. (A) Following a baseline measurement at −20 min, carbachol was microinjected into the LH, and tail flick latencies were measured at −15, −10, and −5 min. Either cobalt chloride (n = 5), L-703,606 (n = 5) , or saline (n = 5) was injected into the RVM at time 0. For comparison purposes only, the open triangles illustrate response latencies when saline alone was microinjected into the LH at times 5–30 (data from Fig. 2). Mean latency values ± S.E.M. are plotted on the ordinate as a function of time min. (B) Cobalt chloride microinjected near into the RVM also blocked LH-mediated antinociception on the foot withdrawal test for the hind paws (closed squares) compared to saline microinjection into the RVM (open squares).
Fig. 6B shows the effect that microinjecting cobalt chloride had on foot withdrawal latencies. As with the tail flick latency, two way repeated measures ANOVA demonstrated a significant main effect (F = 35.09(2,12), p < 0.001, with a significant interaction effect (p < 0.001). Carbachol-induced antinociception was significantly reduced compared to saline controls following cobalt chloride administration (2.16 ± 0.12 vs. 3.43 ± 0.12 sec, resp.; p < 0.05).
Reversal of LH-induced antinociception by microinjection of the NK1 receptor antagonist L-703,606 in the RVM
A separate group of rats was used to determine whether blocking NK1 receptors in the RVM could reduce LH-induced antinociception, thereby providing evidence that substance P neurons play a role in this antinociceptive effect. As described in the previous section, carbachol was microinjected into the LH and three withdrawal latencies were measured. L-703,606 was then microinjected into the RVMin sites identified in Fig. 4B, and 5 min later, withdrawal latencies were again measured. As shown in Fig. 6A, the antinociceptive effect of carbachol administration in the LH was significantly decreased on the tail flick test within 5 min after microinjection (2.46 ± 0.19 vs. 4.00 ± 0.19 sec, L-703,606 vs. saline, resp.; p < 0.05). Two way repeated measures ANOVA, F = 23.1(2,12), p < 0.001) showed a main effect as well as an interaction effect (p = 0.002). In contrast, physiological saline had no effect on antinociception.
Fig. 6B shows the effect of microinjecting L-703,606 into the RVM on foot withdrawal latencies following carbachol-induced LH stimulation. A main effect was seen (F = 35.09(2,12), p < 0.001), with a significant interaction effect (p < 0.001). Withdrawal latencies were significantly reduced after L-703,606 microinjection compared to saline controls (2.15 ± 0.12 vs. 3.43 ± 0.12 sec, resp.; p < 0.05). Response latencies on both the tail flick and foot withdrawal tests following L-703,606 were similar to those seen following microinjection of cobalt chloride.
Microinjection of cobalt chloride or L-703,606 in the RVM without LH-induced antinociception
In the third set of experiments, cobalt chloride, L-703,606, or normal saline was microinjected into the RVM in the absence of LH-induced antinociception. Neither cobalt chloride nor L-703,606 produced significant changes in nociception compared to saline for either tail flick or foot withdrawal latencies (p > 0.05). This set of experiments demonstrated three things: that the connections between the LH and RVM were not tonically active; that the results of the experiments in which microinjection was made in the RVM were not due to the presence of a volume of liquid in the area; and that L-703,606 has no agonist properties.
3. Discussion
The data from the present study provide evidence to support the hypothesis that LH-induced antinociception is mediated in part by neurons in the LH that activate spinally projecting serotonergic neurons, either through direct synaptic contact or through activation of other neurons that activate spinally projecting serotonergic neurons in the RVM.
Our finding of SP-ir in the tuberal hypothalamic area is in line with the work of Ljungdahl, et. al. 1978, but was in no way meant to be a comprehensive study. Ljungdahl and colleagues (1978) indicated that colchicine injections at different locations in the ventricles were needed to fully identify the extent of SP-ir neurons through the hypothalamus. We gave a single injection of colchicine in the ventricle at approximately the same distance from bregma that we gave microinjections of carbachol. Our purpose was to determine whether neurons with SP-ir that project to the RVM were in located in the same area as our carbachol microinjections. Nor does our study provide conclusive evidence that carbachol is acting on these RVM projecting SP-ir neuron profiles. We could find no existing evidence in the literature that demonstrates the presence of cholinergic receptors co-localized with SP-ir neurons in the LH or elsewhere. However, our anatomical findings provide evidence of a possible anatomical substrate for our behavioral studies.
Because stimulating the LH produces antinociception that is partially blocked by systemic (Carstens et al., 1983) or intrathecal serotonin receptor subtype antagonists (Holden et al., 2005), and blockade of RVM function decreases LH-induced antinociception (Aimone and Gebhart, 1988), it is reasonable to suggest that LH-induced antinociception occurs in part from connections with the RVM when coupled with our anatomical findings. Findings of the present study alone, in which the most complete blockade of LH-induced antinociception from cobalt chloride occurred from microinjections where the cannula tip was in the RVM, are inconclusive. However, when added to existing evidence, these results support the proposal that the LH forms a connection with spinally projecting RVM neurons, and extends the findings of Carstens et al, (1983) and Aimone and Gebhart (1988) to include female rats and to include chemical activation of the LH, a method that is different from electrical stimulation.
In the present study, LH-induced antinociception was blocked by administration of cobalt chloride in a dose shown to block synaptic activity in the area of microinjection (Kretz, 1984; Nuseir et al., 1999; Holden et al., 2002). This finding is suggestive that unidentified LH neurons send axons to the RVM and activate serotonergic neurons that then alter nociceptive responses in the spinal cord dorsal horn. An important finding of the present study is that L-703,606 blocked LH-induced antinociception. The antagonist L-703,606 demonstrates an affinity specifically for NK1 receptors (Watling, 2001), and blockade of LH-induced antinociception by L-703,606 provides evidence to support the hypothesis that carbachol-stimulated substance P neurons in the LH project to the RVM and activate NK1 receptors. These receptors may then activate spinally projecting serotonergic neurons that inhibit incoming nociceptive responses in the dorsal horn.
However, recent reports are suggestive that NK1 receptors do not co-localize with serotonin-containing cell bodies or dendrites in the RVM in some rodent species. Specifically, co-localization of NK1 receptors on serotonin-containing neurons occurs in the central linear nucleus and the dorsal raphe nucleus, but not in the RVM, of male OFA rats (Leger et al., 2002). Such co-localization has also been reported in the dorsal raphe of male SV129 mice and male Sprague-Dawley rats (Lacoste et al., 2006), but the RVM was not included in this latter study. It is not clear whether NK1 receptors co-localized with serotonin-immunoreactive neurons in the RVM of Sprague-Dawley rats, the type used in the present study, nor have female rodents been used in the reported immunohistochemical studies.
An alternative hypothesis to a direct synaptic projection from substance P-containing neurons in the LH to serotonergic RVM neurons is that LH neurons innervate interneurons in the RVM that then activate serotonergic neurons. Support for this hypothesis comes from studies that identify neurons in the RVM that both activate and inhibit nociception in the dorsal horn (Neubert et al., 2004; Heinricher et al., 2001; Heinricher et al., 1999). For example, neurons have been identified in the RVM that are immunoreactive for glutamate decarboxylase and inhibit nociception (Winkler et al., 2006). Substance P axons from LH neurons could act at NK1 receptors on excitatory interneurons that in turn activate serotonin-containing neurons to produce antinociception in the dorsal horn. Blockade of NK1 receptors would then produce effects similar to those seen in Experiment 2 of the present study (Fig. 6). Furthermore, SP-ir neurons are located in the RVM (Menetrey and Basbaum 1987), and a connection between either SP-ir or unidentified neurons from the LH and these SP neurons is within the realm of possibility.
A second alternative hypothesis to a direct substance P connection from the LH to the RVM is that the LH projects to the periaqueductal gray PAG (Pelosi et al., 2006; Pajolla et al., 2005; Behbehani et al., 1988), which sends a major projection to, and activates, the RVM (Javanmardi et al., 2005, Heinricher et al., 2004; Behbehani and Fields, 1979). Part of that projection is from SP-ir neurons (Zeng et al., 1991; Beitz, 1982). It is possible that an LH-to-PAG-to-RVM connection could be responsible for the findings of the present experiments. Further anatomical studies would shed more light on the precise circuitry involved in the behavioral outcomes found in the present study.
In the present study, we did not observe qualitative differences between the tail flick and foot withdrawal latencies in any of the three experiments, nor did we observe any significant left/right foot differences. Although the projection from the LH to the RVM is mainly ipsilateral (Berk and Finkelstein, 1982; Saper et al., 1979; Hosoya, 1980), and we observed a heavier innervation of labeled neurons on the side ipsilateral to the FluoroGold injection site, descending serotonergic input to the dorsal horn is widely distributed. The lack of significant left/right foot differences in the present study corresponds with the bilateral effect seen in other studies from our laboratory following unilateral LH stimulation (Holden and Naleway, 2001; Holden et al., 2005). Small quantitative left and right paw differences following unilateral microinjection of galanin in the tuberal LH have been observed, but the overall effect in both paws was antinociceptive (Sun et al., 2004). We also reported small but statistically significant differences in left and right foot withdrawal latencies following LH stimulation (Holden et al., 2002), but the differences likely were due to technical issues rather than a real behavioral effect. The existing evidence supports the conclusion that unilateral LH stimulation produces a bilateral effect on foot withdrawal responses in the acute thermal pain model.
Recent work has shown that stimulating the LH produces hypotension in awake rats (Pajolla et al., 2005). We did not monitor blood pressure during our studies, and do not know whether carbachol microinjection lowered the blood pressure in our lightly anesthetized rats, although it is possible. A number of studies identify hypotension as a side effect of antinociceptive agents. For example, systemic administration of a brain-penetrating neurotensin analog in rhesus monkeys (Fantegrossi et al., 2005) and morphine administration in rats (Mahinda et al., 2004) produce hypotension as well as antinociception. The question at issue for the present study is whether hypotension, if it did occur, could produce the results we attribute to cobalt chloride or L-703,606. Rats that received carbachol microinjection in the LH and saline in the RVM did not have a decrease in withdrawal latencies on either the tail flick or foot withdrawal tests, compared to rats receiving cobalt chloride or L-703,606, so it is unlikely that our findings can be attributed to changes in blood pressure.
In summary, the results of the present study may be combined with the results from other studies to provide converging anatomical and functional evidence in support of the hypothesis that the LH mediates antinociception in part through either a direct SP connection with serotonin neurons or with excitatory interneurons that activate serotonin neurons in the RVM based on the following: 1) Electrical stimulation of the LH or microinjection of carbachol in the LH produce antinociception mediated by spinally projecting serotonergic neurons (Carstens et al., 1983; Aimone and Gebhart, 1987; Aimone et al., 1988; Holden et al., 2005). 2) The RVM provides most of the serotonin found in the spinal cord dorsal horn (Dahlstrom A and Fuxe, 1965; Satoh et al., 1980; Bowker et al., 1981; Jensen and Yaksh, 1984; Hammond et al., 1985; Ruda et al., 1986; Heinricher et al., 1999; Gilbert and Franklin, 2001; Hurley et al., 2003; Buhler et al., 2004). 3) An efferent connection from the LH to the RVM exists (Berk and Finkelstein, 1982; Saper et al., 1979; Hosoya, 1980) and we identified neuron profiles immunoreactive for SP that project to the RVM (Fig. 3). 4) NK1 receptors that bind SP are found in the RVM (Makeham et al., 2005), but whether these receptors co-localize with serotonin containing neurons is inconclusive. 5) Glutamate neurons that inhibit nociceptive responses exist in the RVM (Winkler et al., 2006), as do SP-ir neurons (Menetrey and Basbaum, 1987). 6) Antinociception produced by LH stimulation is blocked by microinjection of either cobalt chloride or the NK1 receptor antagonist L-703,606 (Fig. 6) in the RVM.
Taken together, these lines of converging evidence support the hypothesis that activation of the LH produces antinociception through either a direct or a multisynaptic SP connection with spinally projecting serotonin neurons in the RVM. The full role of the LH in descending nociceptive modulation remains to be determined, but this converging evidence is suggestive that activation of the LH produces a cascade of events that alters nociceptive responses in a variety of ways. It is possible that therapeutics aimed at activating the LH could ultimately produce improved clinical pain management via existing nociceptive modulatory pathways.
4. Experimental Procedures
The Institutional Animal Care Committee at the University of Illinois at Chicago approved the experimental protocols used in this study. The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals NIH Publications No. 90-23, revised 1978. All efforts were made to minimize animal suffering, reduce the numbers of animals used, and use alternatives to in vivo experiments.
Animals
Female Sprague-Dawley rats 250–350 g Charles River, Portage, MI were used in this study because our previous work was done in females. All rats were housed in cages that allowed free access to food and water, and rats were maintained on a 12-hour day/night schedule. Twenty rats were used for the anatomical study. For the behavioral study, rats were randomly assigned to groups, and no two rats were taken from the same cage on the same day to reduce the possibility of estrous cycle influence. A total of fifty-three rats were used for the behavioral study as reported, and each rat was used only once. Tail flick and foot withdrawal latencies were measured on each animal.
Retrograde tracing experiments
This experiment used the retrograde tracer FluoroGold and immunocytochemical methods to determine the locations of SP-ir neuron profiles in the LH that project to the RVM in the brainstem.
Tracer iontophoresis
Each of 20 rats was deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p) and surgically prepared using aseptic technique. A glass micropipette with a tip diameter of 25–30 µm was filled with 2% FluoroGold (Fluorochrome, Inc., Englewood, CO) solution and lowered into the RVM through a burr hole to a point defined by the following stereotaxic coordinates: 1.5 mm from bregma, lateral +1.7 mm, vertical +1.5 mm, incisor bar set at −2.5 mm, with the angle of the pipette adjusted 10° to bypass the venous sinus. FluoroGold was iontophorectically deposited using 15 µA positive current pulses that were 500 ms in duration and delivered at a rate of 0.5 Hz for 10 min. The pipette was kept in place for an additional 60 s before removal. Two weeks later, each rat was anesthetized with pentobarbital and 125 µg of colchicine (Sigma Aldrich Chemical Co., St. Louis, MO) in 25 µl of normal saline was injected into the left lateral ventricle. Approximately 24 h later, rats were anesthetized with pentobarbital and the brains fixed by perfusion through the aorta with the following solutions: 100 ml of normal saline followed by 200 ml of cold 4% paraformaldehyde in 0.1 M phosphate buffered saline (PB; pH 7.4), and 100 ml of 10% sucrose in 0.1 M PB.
Tissue processing
The brains were removed, postfixed for 1 h in 4% phosphate-buffered paraformaldehyde, and stored in a solution of 20% sucrose in 0.1 M PB for three to four days. Forty-micrometer transverse sections were cut on a cryostat microtome and alternating sections were placed in cold phosphate-buffered saline (PBS) for 10 min and processed as free-floating sections. Three ten-min washes in PBS were done between each step unless otherwise noted. With the exception of the primary antisera, all steps were done at room temperature with gentle agitation. To increase membrane permeability and decrease non-specific staining, sections were washed in two 5-min washes in 0.1 M PB that contained 0.3% Triton X-100 and 3% normal goat serum. The sections through the LH were incubated for eight days at 4°C in a solution that contained primary antisera directed against SP (rabbit anti SP; Chemicon International, Inc., Temecula, CA) diluted 1:50,000 in PBS containing 0.5% Triton X-100. Following washes with PBS, SP-ir neurons were labeled by incubating sections for 60 min with a solution that contained a goat anti-rabbit secondary antibody conjugated with Bodipy (1:200: Molecular Probes, Carlsbad, CA) and 0.5% Triton X-100 in PBS. Sections were then rinsed, mounted on gel-coated slides and coverslipped with DPX mounting medium (BDH Laboratory Supplies, Poole, England). Alternate sections from two rats were processed as indicated above, but the primary antibody was omitted for control.
Fluorescence microscopy
Labeled cells were visualized used epifluorescence microscopy. Photomicrographs were made using a Spot RT digital camera and the data acquisition software Metamorph. Adjustments were made to each micrograph for contrast, brightness and sharpness.
Behavioral Experiments
Three sets of behavioral experiments were done to address the research question. In the first set, carbachol or normal saline was microinjected into the LH using a dose previously determined to provide optimum antinociception in a lightly anesthetized acute preparation (Holden and Naleway, 2001; Holden et al., 2002; Holden et al., 2005), and nociceptive responses were measured. In the second set of experiments, carbachol-induced antinociception was obtained, followed by microinjection of cobalt chloride, L-703,606, or normal saline for control into the RVM. In the third set of experiments, cobalt chloride, L-703,606, or normal saline was microinjected into the RVM in the absence of LH stimulation.
Analgesiometric testing procedures
To determine the effect of LH stimulation on thermal nociception, the tail flick and foot withdrawal tests were used. In these procedures, a focused beam of high intensity light from a 250-W projection bulb was directed at the dorsal surface of the rat's tail and the hairy surface of the hind feet. The tail and paws were blackened with ink to facilitate even heating of the skin surface. The time interval between the onset of skin heating and the withdrawal response was measured electronically. In the absence of a response, the skin heating was terminated after 8 s to prevent burning of the skin. Three response latencies were measured in succession at three places on the tail. The average of the three tail latency values was defined as the nociceptive response latency. For the foot withdrawal, a response latency was measured at one place on the hairy surface of each hind foot. Baseline response latencies of the paws and tail were approximately 2–3 s.
Experiment 1: Carbachol or saline microinjection in the LH
Each rat was lightly anesthetized with sodium pentobarbital 35 mg/kg, IP, and the scalp was infused with bupivacaine 0.25%; 0.10 ml, which provides local anesthetic relief for approximately 24 hr. Supplemental doses of pentobarbital were given during the procedure if the rats vocalized or moved without stimulation, but supplementation was rarely required. Immediately after anesthesia, the rats were immobilized in a stereotactic apparatus, and the midline scalp was shaved. Using aseptic technique, a 2-cm incision was made, and the muscle and fascia retracted. A 23-gauge stainless steel guide cannula was lowered into the region of the left LH through a burr hole to a location defined by the following stereotactic coordinates: −1.5 mm from bregma, lateral +1.7 mm, vertical +1.5 mm, incisor bar set at −2.5 mm. A 30-gauge stainless steel injection cannula was connected to a 10 µl syringe by a length of PE-10 polyethylene tubing filled with either saline or a solution of carbachol 125 nmol in normal saline injected in a volume of 0.5 µl; Sigma, St. Louis, MO. The injection cannula was then inserted into the guide cannula and extended approximately 3 mm beyond the end of the guide cannula. After a baseline latency measurement was taken and recorded, either saline or carbachol was injected into the LH over a 1-min period using an electronic syringe pump. The microinjector was left in place for an additional 60 s following completion of the microinjection to reduce flow of drug up the guide cannula. Response latencies were then measured at 5 min intervals for 35 min.
Experiment 2: Carbachol microinjection in the LH followed by microinjection of antagonists in the RVM
Female Sprague-Dawley rats Charles River; 275–350 g were randomly assigned to groups and prepared for microinjection in the LH as described in the previous paragraph. In addition, a second guide cannula was lowered into the RVM at the following coordinates, with the tip angled 10° to bypass the venous sinus: AP +2.64 mm from the vertex angle, lateral 0 mm, vertical −1.9 mm incisor bar set at −2.5 mm. After a baseline response latency, carbachol 125 nmol in normal saline injected in a volume of 0.5 µl; Sigma was microinjected into the LH, and three response latencies were measured at five minutes apart. Twenty min after carbachol injection, cobalt chloride 100 nM/ 0.5 µl, L-703,606 5 µg in 0.5 µl or saline was microinjected using an electronic syringe pump in the manner described above. Response latencies were then measured every five min for 35 min. Additional controls were done in which saline, cobalt chloride or L-703,606, at doses described previously, was injected near the RVM of LH stimulation.
Experiment 3: RVM antagonists only
In the final set of experiments, female Sprague-Dawley rats Charles River; 275–350 g were lightly anesthetized as described, and prepared with cannula placement in the RVM, as in the preceding experiment. The rats in experiment 3 did not receive intracerebral microinjections. Following a baseline latency, cobalt chloride 100 nM/0.5 µl, L-703,606 5 µg in 0.5 µl or saline for control, was injected into the RVM at a rate of 5 µl/min using an electronic syringe pump. Response latencies were then taken every 5 min for 45 min.
Histology
Following testing, animals were overdosed with sodium pentobarbital and decapitated, and their brains were taken and drop fixed in a solution of 10% neutral-buffered formalin. To determine the position of the microinjection sites relative to the LH and the RVM, 40-µm transverse brain sections were cut from blocks of tissue that contained the visible injection cannula tract using a cryostat microtome. The sections were rinsed in cold phosphate-buffered saline PBS, 10 mM, mounted on gel-coated slides, stained with 0.05% neutral red, dehydrated through a series of alcohols and xylenes, and coverslipped. The placement of the microinjection cannula was determined by locating the most ventral position of the cannula tip in serial sections by brightfield microscopy. Tracings of the appropriate sections were then made using the Neurolucida imaging system Microbrightfield, Colchester, VT. The tracings were compared with drawings from the atlas of Paxinos and Watson Paxinos and Watson 1986 to verify that the cannula was within the LH or the RVM.
Statistical analysis
Each treatment group consisted of 5 or 6 rats. Tail flick and foot withdrawal latencies are presented as the mean ± S.E.M. Statistical comparisons among treatment groups across several time points were made using two-way repeated measures ANOVA, and comparisons among means at specific time points were made using the Bonferroni test for multiple post-hoc comparisons. Paired t-tests were used to compare withdrawal latencies for left and right feet. Foot withdrawal latencies were not significantly different between left and right paws for any experiment, so response latencies were averaged for clarity.
Acknowledgements
This work is supported by USPHS grant NR04778 from the National Institute of Nursing Research. The authors express appreciation to Younhee Jeong for assistance in data collection and to Herb Proudfit for assistance with photomicrography. The authors thank Kevin Grandfield for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone LD, Bauer CA, Gebhart GF. Brain-stem relays mediating stimulation-produced antinociception from the lateral hypothalamus in the rat. J. Neurosci. 1988;8:2652–2663. doi: 10.1523/JNEUROSCI.08-07-02652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone LD, Gebhart GF. Spinal monoamine mediation of stimulation-produced antinociception from the lateral hypothalamus. Brain Res. 1987;403:290–300. doi: 10.1016/0006-8993(87)90066-7. [DOI] [PubMed] [Google Scholar]
- Aimone LD, Gebhart GF. Serotonin or excitatory amino acids in the medial medulla mediates stimulation-produced analgesia from the lateral hypothalamus in the rat. Brain Res. 1988;450:170–180. doi: 10.1016/0006-8993(88)91556-9. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979;170:85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Park MR, Clement ME. Interactions between the lateral hypothalamus and the periaqueductal gray. J. Neurosci. 1988;8:2780–2787. doi: 10.1523/JNEUROSCI.08-08-02780.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz A. The nuclei of origin of brain stem enkephalin and substance P projections to the rodent nucleus raphe magnus. Neuroscience. 1982;7:2753–2768. doi: 10.1016/0306-4522(82)90098-7. [DOI] [PubMed] [Google Scholar]
- Berk ML, Finkelstein JA. Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res. Bull. 1982;8:511–526. doi: 10.1016/0361-9230(82)90009-0. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KK, Coulter JD. Origins of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Res. 1981;226:187–199. doi: 10.1016/0006-8993(81)91092-1. [DOI] [PubMed] [Google Scholar]
- Buhler A, Proudfit HK, Gebhart GF. Separate populations of neurons in the rostral ventromedial medulla project to the spinal cord and to the dorsolateral pons in the rat. Brain Res. 2004;1016:12–19. doi: 10.1016/j.brainres.2004.04.060. [DOI] [PubMed] [Google Scholar]
- Carstens E, Fraunhoffer M, Suberg S. Inhibition of spinal dorsal horn neuronal responses to noxious skin heating by lateral hypothalamic stimulation in the cat. J Neurophys. 1983;50:192–204. doi: 10.1152/jn.1983.50.1.192. [DOI] [PubMed] [Google Scholar]
- Cortes R, Probst A, Tobler HJ, Palacios JM. Muscarinic cholinergic receptor subtypes in the human brain. II. Quantitative autoradiographic studies. Brain Res. 1986;362:239–253. doi: 10.1016/0006-8993(86)90449-x. [DOI] [PubMed] [Google Scholar]
- Dafny N, Dong QW, Prieto-Gomez C, Reyes-Vazquez J, Stanford J, Qiao JT. Lateral hypothalamus: site involved in pain modulation. Neuroscience. 1996;70:449–460. doi: 10.1016/0306-4522(95)00358-4. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe A. Evidence for the existence of monoamine neurons in the central nervous system. Acta Physiol Scand. 1965;64 suppl:1–64. [PubMed] [Google Scholar]
- Fang F, Proudfit KK. Spinal cholinergic and monoamine receptors mediate the antinociceptive effect of morphine microinjected in the periaqueductal gray on the rat tail, but not the feet. Brain Res. 1996;722:95–108. doi: 10.1016/0006-8993(96)00198-9. [DOI] [PubMed] [Google Scholar]
- Fang F, Proudfit HK. Antinociception produced by microinjection of morphine in the periaqueductal gray is enhanced in the rat foot, but not the tail, by intrathecal injection of alpha 1-adrenoceptor antagonists. Brain Res. 1998;790:14–24. doi: 10.1016/s0006-8993(97)01441-8. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W, Ko MC, Woods JH, Richelson E. Antinociceptive, hypothermic, hypotensive, and reinforcing effects of a novel neurotensin receptor agonist, NT69L, in rhesus monkeys. Pharmacol Biochem Behav. 2005;80:341–349. doi: 10.1016/j.pbb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Franco ACJ, Prado WA. Antinociceptive effects of stimulation of discrete sites in the rat hypothalamus: evidence for the participation of the lateral hypothalamus area in descending pain suppression mechanisms. Brazilian J. Med. Bio. Res. 1996;29:1531–1541. [PubMed] [Google Scholar]
- Fuchs P, Melzack R. Analgesia induced by morphine microinjection into the lateral hypothalamus of the rat. Exp Neurol. 1995;134:277–280. doi: 10.1006/exnr.1995.1058. [DOI] [PubMed] [Google Scholar]
- Gilbert A-K, Franklin K. GABAergic modulation of descending inhibitory systems from the rostral ventromedial medulla RVM. Dose-response analysis of nociception and neurological deficits. Pain. 2001;90:25–36. doi: 10.1016/s0304-3959(00)00383-3. [DOI] [PubMed] [Google Scholar]
- Hammond D, Tyce G, Yaksh TL. Efflux of 5-hydroxytriptamine and noradrenaline into spinal cord superfusates during stimulation of the rat medulla. J Physiol. 1985;359:151–162. doi: 10.1113/jphysiol.1985.sp015579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Martenson ME, Neubert MJ. Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain. 2004;110:419–426. doi: 10.1016/j.pain.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Farr DA. The role of excitatory amino acid transmission within the rostral ventromedial medulla in the antinociceptive actions of systemically administered morphine. Pain. 1999;81:57–65. doi: 10.1016/s0304-3959(98)00271-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-D-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain. 2001;92:129–138. doi: 10.1016/s0304-3959(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Hess A, Sergejeva M, Budinsky L, Zeilhofer HU, Brune K. Imaging of hyperalgesia in rats by functional MRI. Eur J. Pain. 2007;11:109–119. doi: 10.1016/j.ejpain.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Holden JE, Naleway E, Jeong Y. Stimulation of the lateral hypothalamus produces antinociception mediated by 5-HT1A, 5-HT1B and 5-HT3 receptors in the rat spinal cord dorsal horn. Neuroscience. 2005;135:1255–1268. doi: 10.1016/j.neuroscience.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Holden JE, Naleway E. Microinjection of carbachol in the lateral hypothalamus produces opposing actions on nociception mediated by a1- and a2 - adrenoceptors. Brain Res. 2001;911:27–36. doi: 10.1016/s0006-8993(01)02567-7. [DOI] [PubMed] [Google Scholar]
- Holden JE, Van Poppel A, Thomas SB. Antinociception from lateral hypothalamic stimulation may be mediated by NK1 receptors in the A7 catecholamine cell group in rat. Brain Res. 2002;953:195–204. doi: 10.1016/s0006-8993(02)03285-7. [DOI] [PubMed] [Google Scholar]
- Hosoya Y. The distribution of spinal projection neurons in the hypothalamus of the rat, studied with the HRP method. Experimental Brain Research. 1980;40:79–87. doi: 10.1007/BF00236665. [DOI] [PubMed] [Google Scholar]
- Hurley R, Banfor P, Hammond DL. Spinal pharmacology of antinociception produced by microinjection of mu or delta opioid receptor agonists in the ventromedial medulla of the rat. Neuroscience. 2003;118:789–796. doi: 10.1016/s0306-4522(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Javanmardi K, Parviz M, Sadr SS, Keshavarz M, Minaii B, Dehpour AR. Involvement of N-methyl-D-aspartate receptors and nitric oxide in the rostral ventromedial medulla in modulating morphine pain-inhibitory signals from the periaqueductal grey matter in rats. Clin. Exp. Pharmacol. Physiol. 2005;32:585–589. doi: 10.1111/j.1440-1681.2005.04234.x. [DOI] [PubMed] [Google Scholar]
- Jensen T, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984;321:287–297. doi: 10.1016/0006-8993(84)90181-1. [DOI] [PubMed] [Google Scholar]
- Klamt JG, Prado WA. Antinociception and behavioral changes induced by carbachol microinjection into identified sites of the rat brain. Brain Res. 1991;549:9–18. doi: 10.1016/0006-8993(91)90593-k. [DOI] [PubMed] [Google Scholar]
- Kretz R. Local cobalt injection: a method to discriminate presynaptic from postsynaptic neuronal activity. J. Neurosci. Methods. 1984;11:129–135. doi: 10.1016/0165-0270(84)90030-x. [DOI] [PubMed] [Google Scholar]
- Lacoste B, Riad M, Descarries L. Immunocytochemical evidence for the existence of substance P receptor NK1 in serotonin neurons of rat and mouse dorsal raphe nucleus. Eur. J. Neurosci. 2006;23:2947–2958. doi: 10.1111/j.1460-9568.2006.04833.x. [DOI] [PubMed] [Google Scholar]
- Leger L, Gay N, Cespuglio R. Neurokinin NK1- and NK3- immunoreactive neurons in serotonergic cell groups in the rat brain. Neurosci. Lett. 2002;323:146–150. doi: 10.1016/s0304-3940(01)02543-5. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A, Hokfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat-I. cell bodies and nerve terminals. Neuroscience. 1978;3:861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Mahinda T, Lovell BM, Taylor BK. Morphine-induced analgesia, hypotension, and bradycardia are enhanced in hypertensive rats. Anesth. Analg. 2004;98:1698–1704. doi: 10.1213/01.ANE.0000115148.03515.56. [DOI] [PubMed] [Google Scholar]
- Makeham J, Goodchild AK, Pilowsky PM. NK1 receptor activation in rat rostral ventrolateral medulla selectively attenuates somato-sympathetic reflex while antagonism attenuates sympathetic chemoreflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1707–R1715. doi: 10.1152/ajpregu.00537.2004. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Basbaum A. The distribution of substance P-enkephalin-and dynorphin-immunoreactive neurons in the medulla of the rat and their contribution to bulbospinal pathways. Neuroscience. 1987;23:173–187. doi: 10.1016/0306-4522(87)90281-8. [DOI] [PubMed] [Google Scholar]
- Neubert M, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Nuseir K, Heidenreich BA, Proudfit HK. The antinociception produced by microinjection of a cholinergic agonist in the ventromedial medulla is mediated by noradrenergic neurons in the A7 catecholamine cell group. Brain Res. 1999;822:1–7. doi: 10.1016/s0006-8993(98)01195-0. [DOI] [PubMed] [Google Scholar]
- Oki T, Takagi Y, Inagaki S, Taketo MM, Manabe T, Matsui M, Yamada S. Quantitative analysis of binding parameters of [3H]N-methylscopalamine in central nervous system of muscarinic acetylcholine receptor knockout mice. Mol. Brain Res. 2005;133:6–11. doi: 10.1016/j.molbrainres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Pajolla G, Tavares RF, Pelosi GG, Correa FM. Involvement of the periaqueductal gray in the hypotensive response evoked by L-glutamate microinjection in the lateral hypothalamus of unanesthetized rats. Auton Neurosci. 2005;122:84–93. doi: 10.1016/j.autneu.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain In Stereotactic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Pelosi G, Tavares R, Correa F. Rostrocaudal somatotopy in the neural connections between the lateral hypothalamus and the dorsal periaqueductal gray of the rat brain. Cell. Mol. Neurobiol. 2006;26:633–641. doi: 10.1007/s10571-006-9015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzi JA, Holden JE. Lateral hypothalamic-induced antinociception is mediated by a substance P connection with spinally-projecting neurons in the ventromedial medulla. Soc. Neurosci. Abstr. 2003;29 doi: 10.1016/j.brainres.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow T, Schwartz RD, Parsons B, Kellar KJ. Quantitative autoradiography of nicotinic [3H]acetylcholine binding sites in rat brain. Neurosci. Lett. 1984;50:193–196. doi: 10.1016/0304-3940(84)90485-3. [DOI] [PubMed] [Google Scholar]
- Rivot J, Chiang C, Besson J. Increase of serotonin metabolism within the dorsal horn of the spinal cord during nucleus raphe magnus stimulation, as revealed by in vivo electrochemical detection. Brain Res. 1982;238:117–126. doi: 10.1016/0006-8993(82)90775-2. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Bennett GJ, Dubner R, editors. Neurochemistry and neural circuitry in the dorsal horn. Prog. Brain Res. 1986;66:219–268. doi: 10.1016/s0079-6123(08)64606-3. [DOI] [PubMed] [Google Scholar]
- Saper C, Swanson L, Cowen W. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J. Comp. Neurol. 1979;183:689–706. doi: 10.1002/cne.901830402. [DOI] [PubMed] [Google Scholar]
- Satoh M, Akaike A, Nakazawa T, Takagi H. Evidence for involvement of separate mechanisms in the production of analgesia by electrical stimulation of the nucleus reticularis paragigantocellularis and nucleus raphe magnus in the rat. Brain Res. 1980;194:525–529. doi: 10.1016/0006-8993(80)91236-6. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li J, Yang BN, Yu LC. Antinociceptive effects of galanin in the rat tuberomammillary nucleus and the plasticity of galanin receptor 1 during hyperalgesia. J. Neurosci. Res. 2004;77:718–722. doi: 10.1002/jnr.20206. [DOI] [PubMed] [Google Scholar]
- Watling KJ, editor. The RBI Handbook of Receptor Classification and Signal Transduction. 4th Ed. Natick, MA: Sigma-RBI; 2001. [Google Scholar]
- Winkler C, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor KOR and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventromedial medulla. J. Neurophysiol. 2006;96:3465–3473. doi: 10.1152/jn.00676.2006. [DOI] [PubMed] [Google Scholar]
- Zeng S, Li YQ, Rao ZR, Shi JW. Projections from serotonin- and substance P-like immunoreactive neurons in the midbrain periaqueductal gray onto the nucleus reticularis gigantocellularis pars alpha in the rat. Neurosci Lett. 1991;131:205–209. doi: 10.1016/0304-3940(91)90614-y. [DOI] [PubMed] [Google Scholar]


