Abstract
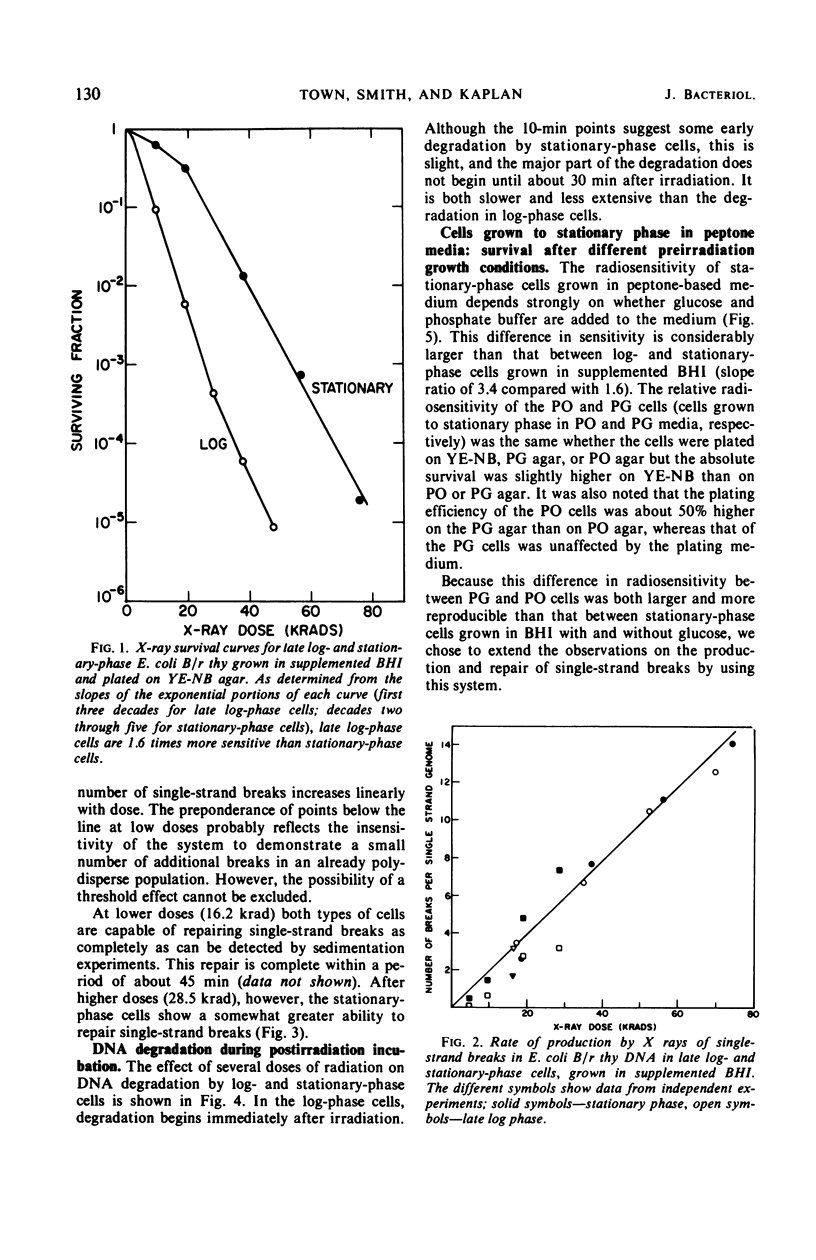
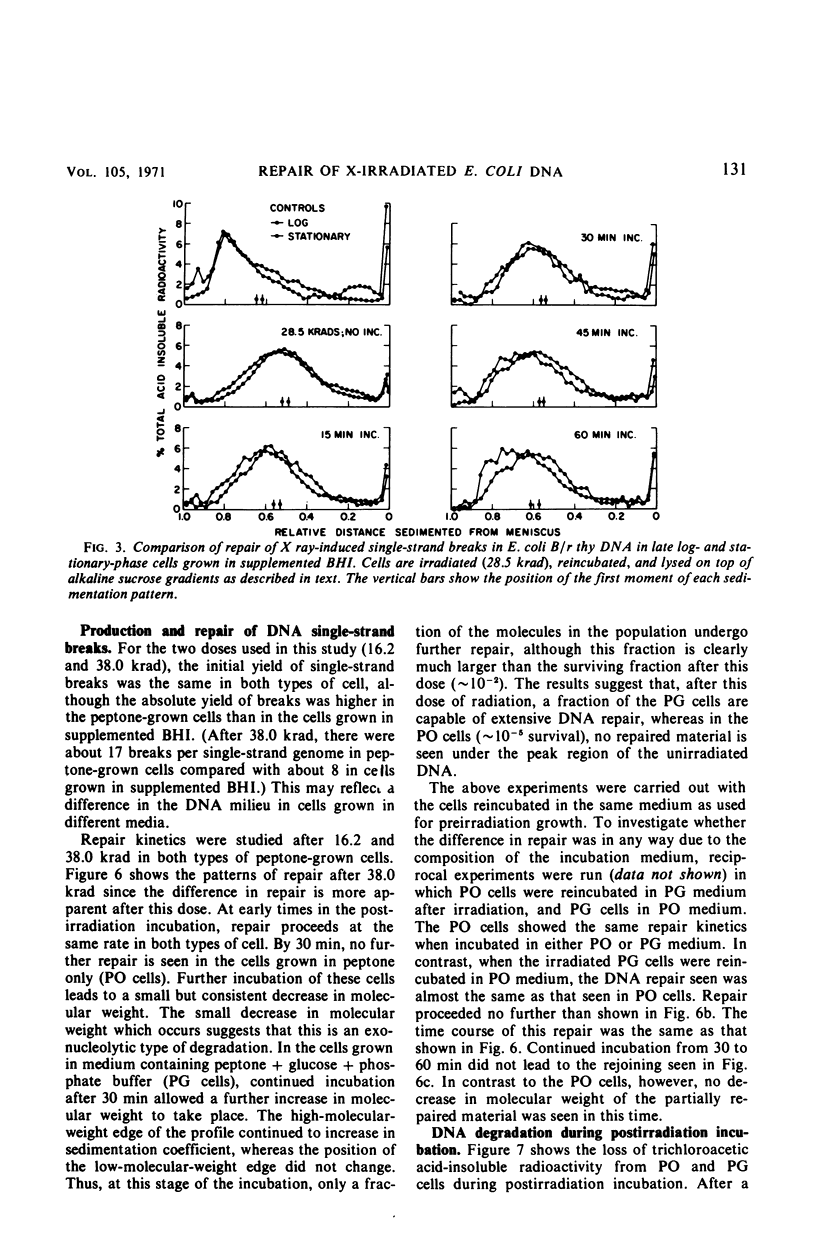
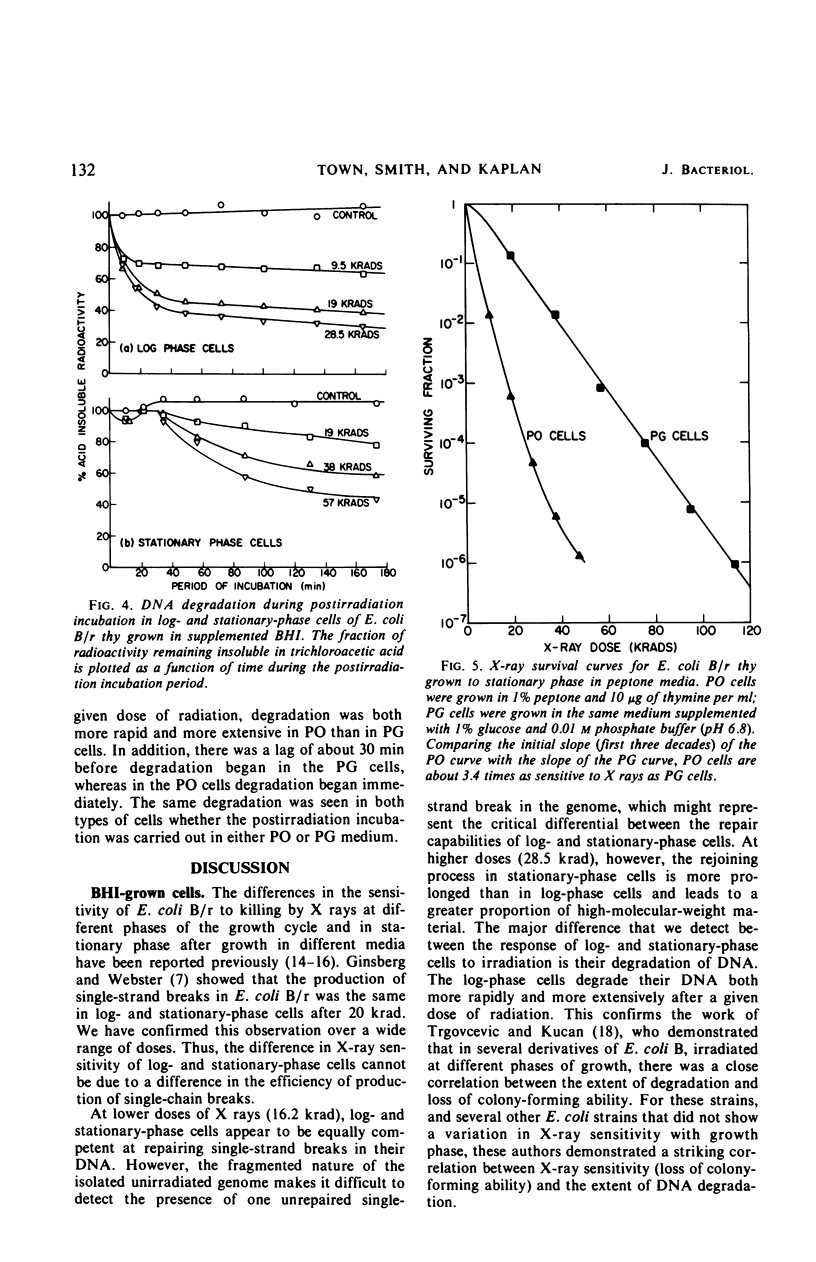
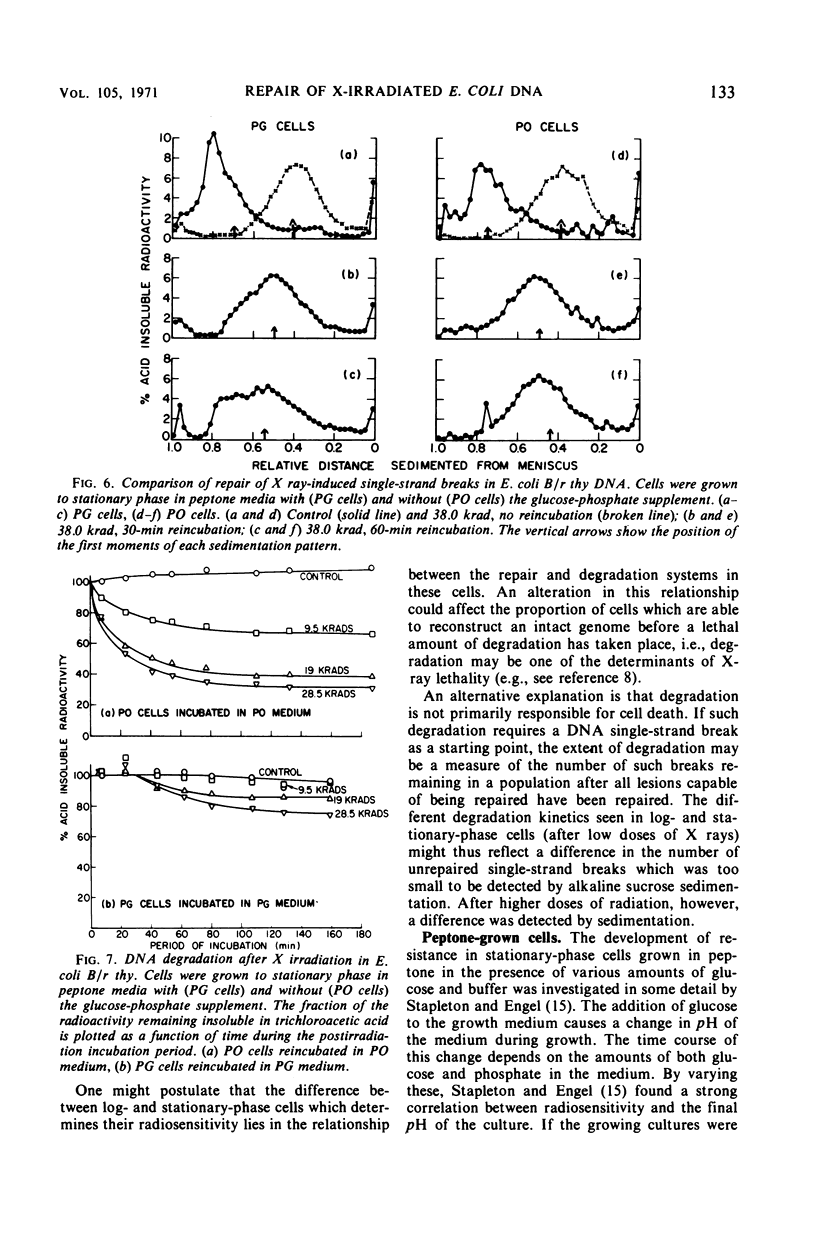
Late log-phase Escherichia coli B/r cells are 1.6 times more sensitive to killing by X rays than are stationary-phase cells when grown in Brain Heart Infusion (BHI) + glucose. The number of single-chain breaks formed per krad is the same for log- and stationary-phase cells. Stationary-phase cells show a somewhat greater ability to repair single-chain breaks (especially after high doses of X rays) than do log-phase cells. The rapidity and extent of postirradiation deoxyribonucleic acid (DNA) degradation are greater in log-phase cells than in stationary-phase cells. The enhanced viability exhibited by stationary-phase cells thus appears to correlate both with enhanced single-chain break repair and the reduced degradation of DNA. Cells grown to stationary phase in peptone medium (PO cells) are 3.4 times more sensitive to killing by X rays than cells grown to stationary phase in peptone medium supplemented with glucose and phosphate buffer (PG cells). The yield of single-strand breaks is the same for both types of cells (but the absolute yield is about two times higher than in the cells grown in BHI + glucose). The kinetics for the repair of single-chain breaks are the same for both types of cells for about 30 min. After this time period, further repair ceases in the PO cells but continues in the PG cells, provided that glucose is present in the medium. Postirradiation DNA degradation is both more rapid and more extensive in PO cells than in PG cells whether or not glucose is present in the postirradiation incubation medium. The survival of stationary-phase E. coli B/r grown in PO or PG medium is likewise unaffected by the presence of glucose in the plating medium, and thus correlates better with the lower DNA degradation seen in the PG cells than with the increased strand rejoining, since this latter process requires the presence of glucose.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Donch J., Chung Y. S., Greenberg J. Locus for radiation resistance in Escherichia coli strain B-r. Genetics. 1969 Feb;61(2):363–370. doi: 10.1093/genetics/61.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen B. S., Iyer P. S., Baptist J. E., Meyn R., Rodgers J. M. Glucose-induced resistance to gamma-rays in Escherichia coli. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18(2):159–172. doi: 10.1080/09553007014550961. [DOI] [PubMed] [Google Scholar]
- Ginsberg D. M., Webster H. K. Chemical protection against single-strand breaks in DNA of gamma-irradiated E. coli. Radiat Res. 1969 Aug;39(2):421–435. [PubMed] [Google Scholar]
- Hildebrand C. E., Pollard E. C. The study of ionizing radiation effects on Escherichia coli by density gradient sedimentation. Biophys J. 1969 Nov;9(11):1312–1322. doi: 10.1016/S0006-3495(69)86453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. doi: 10.1128/jb.103.1.49-54.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEVINGER R., HUISMAN P. A TWIN-TUBE 50-KVP BERYLLIUM-WINDOW X-RAY UNIT FOR MICROBIAL RADIOBIOLOGY. Radiat Res. 1965 Feb;24:357–367. [PubMed] [Google Scholar]
- Lett J. T., Caldwell I., Little J. G. Repair of x-ray damage to the DNA in Micrococcus radiodurans: the effect of 5-bromodeoxyuridine. J Mol Biol. 1970 Mar;48(3):395–408. doi: 10.1016/0022-2836(70)90053-7. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Stapleton G. E., Engel M. S. CULTURAL CONDITIONS AS DETERMINANTS OF SENSITIVITY OF ESCHERICHIA COLI TO DAMAGING AGENTS. J Bacteriol. 1960 Oct;80(4):544–551. doi: 10.1128/jb.80.4.544-551.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton G. E., Fisher W. D. Macromolecular synthesis and radiosensitivity in Escherichia coli. Radiat Res. 1967 Feb;30(2):173–185. [PubMed] [Google Scholar]
- Trgovcević Z., Kućan Z. Correlation between the breakdown of deoxyribonucleic acid and radiosensitivity of Escherichia coli. Radiat Res. 1969 Mar;37(3):478–492. [PubMed] [Google Scholar]
- Veatch W., Okada S. Radiation-induced breaks of DNA in cultured mammalian cells. Biophys J. 1969 Mar;9(3):330–346. doi: 10.1016/S0006-3495(69)86390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



