Abstract
Background The objectives were to estimate the prevalence of hepatitis A among children and adolescents from the Northeast and Midwest regions and the Federal District of Brazil and to identify individual-, household- and area-levels factors associated with hepatitis A infection.
Methods This population-based survey was conducted in 2004–2005 and covered individuals aged between 5 and 19 years. A stratified multistage cluster sampling technique with probability proportional to size was used to select 1937 individuals aged between 5 and 19 years living in the Federal capital and in the State capitals of 12 states in the study regions. The sample was stratified according to age (5–9 and 10- to 19-years-old) and capital within each region. Individual- and household-level data were collected by interview at the home of the individual. Variables related to the area were retrieved from census tract data. The outcome was total antibodies to hepatitis A virus detected using commercial EIA. The age distribution of the susceptible population was estimated using a simple catalytic model. The associations between HAV infection and independent variables were assessed using the odds ratio and corrected for the random design effect and sampling weight. Multilevel analysis was performed by GLLAMM using Stata 9.2.
Results The prevalence of hepatitis A infection in the 5–9 and 10–19 age-group was 41.5 and 57.4%, respectively for the Northeast, 32.3 and 56.0%, respectively for the Midwest and 33.8 and 65.1% for the Federal District. A trend for the prevalence of HAV infection to increase according to age was detected in all sites. By the age of 5, 31.5% of the children had already been infected with HAV in the Northeast region compared with 20.0% in the other sites. By the age of 19 years, seropositivity was ∼70% in all areas. The curves of susceptible populations differed from one area to another. Multilevel modeling showed that variables relating to different levels of education were associated with HAV infection in all sites.
Conclusion The study sites were classified as areas with intermediate endemicity area for hepatitis A infection. Differences in age trends of infection were detected among settings. This multilevel model allowed for quantification of contextual predictors of hepatitis A infection in urban areas.
Keywords: Hepatitis A infection, multilevel analysis, Brazil, risk factors, contextual effects
Introduction
Hepatitis A infection is found all over the world, and high prevalence is associated with poor socioeconomic conditions and diverse epidemiological patterns.1,2 In the past decade, hepatitis A seroprevalence has declined due to improved sanitation. In highly endemic areas, young children are the most frequently affected population and, in developed countries, the majority of symptomatic cases occur among adolescents and adults, travelers, men who have sex with men and injecting drug users.3,4 After exposure to HAV, individuals develop antibodies for all strains that remain with them throughout their lives. Highly effective anti-hepatitis A vaccines have been licensed since the mid-90s in the United States and it has been recommended for all children aged between 12 and 23 months.4,5 In low endemic countries and in specific outbreaks, the World Health Organization (WHO) recommends vaccination according to the epidemiology of hepatitis A in the respective region. In highly endemic areas, HAV vaccination is not recommended1 and in areas with intermediate endemicity, use of the vaccine depends on epidemiological situation, target groups and cost-benefit analysis.4,5
Brazil has been considered a country with high endemicity for HAV according to a review of seroprevalence studies (1980–2002), conducted mainly among restricted populations of Southeast region. Higher prevalences were detected in the North and Northeast regions compared with the South and Southeast.6 Comparison of HAV seroprevalence in six Latin-American countries (México, Chile, Venezuela, Brazil, Argentina and Dominican Republic) has suggested a changing epidemiological pattern, shifting from high to intermediate endemicity for HAV.7 In general, surveillance data on HAV incidence are limited because of underreporting and lack of laboratory confirmation and case investigation.8 In this respect, the Brazilian surveillance system is no exception. The HAV vaccine is not universally recommended for the National Immunization Program and only a small proportion of the population has access to it. Poor sanitation, lack of water supply, low family income and daycare attendance are known risk factors for hepatitis A infection and viral transmission. A multilevel approach, taking into account both individual- and household-level, in addition to socioeconomic census tract variables, is particularly effective in distinguishing vulnerable groups and environmental risk factors of several infections in urban areas.9,10 A national population-based survey was launched by the Brazilian Ministry of Health and Research Institutes of the University of Pernambuco to estimate the HAV infection in urban areas, with the view to evaluating the epidemiological settings where vaccination should be considered as a preventive measure. The main goal of this research was to estimate HAV prevalence using a population-based study of the capitals of states in the Northeast and Midwest regions and in the Federal District of Brazil. The study also identifies predictive factors for hepatitis A infection among children and adolescents, using a multilevel approach.
Subjects and methods
Study design and sites
A population-based household survey was conducted in all the capital cities of states in the Northeast and Midwest regions, as well as the Federal District, from September 2004 to June 2005. The Northeast region comprises the following nine states (with their respective capitals in parenthesis): Bahia (Salvador), Sergipe (Aracaju), Alagoas (Maceio), Pernambuco (Recife), Paraiba (João Pessoa), Rio Grande do Norte (Natal), Ceara (Fortaleza), Piaui (Teresina), Maranhão (São Luis). The total population of these capital cities for the 5–9 and 10–19 year age groups were 943 033 and 2 054 539, respectively.11 The Midwest region comprises three states: Goias (Goiania), Mato Grosso do Sul (Campo Grande) and Mato Grosso (Cuiaba). The population for the same age groups in the Midwest region was 397 740 and 866 536 inhabitants, respectively.11 The Federal District (Brasilia city) is located in the Midwest region and the populations for the above-mentioned age groups were 182 537 and 387 831, respectively.
Subjects and assessment of variables
The criteria for inclusion were that subject be aged between 5 and 9 and 10 and 19 years and resident in one of the capitals cities.11 All participants or guardians (in the case of children under 13 years) were interviewed by trained health personnel using a structured questionnaire. Individual- and household-level data were collected during household visits. The following area-level variables were retrieved from a subset of IBGE census data: level of education of head of household (average years of schooling), literacy of head of household (%), household headed by a woman (%), head of the household being a woman and illiterate (%), individuals in the households who are both literate and aged 15–19 years (%), water supply coverage (%) and solid waste collection coverage (%).3
Outcome variable and laboratory test
During the household visit, blood samples were collected, transported and stored according to standard procedures. Total antibodies to hepatitis A virus were detected using commercial enzyme immunoassay kit (HAVAB EIA; Abbott laboratories, Abbot Park IL) in central public health laboratories. Laboratory tests were validated and quality control was monitored. Seropositive results were taken to indicate current or past HAV infection (ever infected). Borderline results (0.15%) and the inadequate samples were excluded from the analysis.
Sampling strategy
The study sample was selected using stratified multistage cluster sampling based on IBGE census data for the year 2000. The census tract is the smallest geographic unit available for which socioeconomic data on residents with similar economic characteristics are available and comprises approximately 300 households (approx. equal to 1000 inhabitants).11,12
The study population was stratified into two age-groups (5 to 9- and 10 to 19-years-old) as well as by State capital. The sample was obtained in three stages. To make the census tracts more homogeneous with respect to the population, primary sampling units (PSU) were created. An ordered list of PSUs was then drawn up, taking into account the number of years of schooling of the head of the household and a systematic sample was taken with probability proportional to size. In the second stage, block units were selected also with probability proportional to size. The third stage involved systematic sampling of households within each block using a list of residential addresses.11 All current residents of the selected households within the 5–19 years age bracket were included in the hepatitis A study. Census tract maps were provided to guide the field workers.
Sample size
The sample size was calculated on the basis of an expected prevalence of HAV-infection of 30% and α < 0.05. The variance was estimated at 20% of the prevalence value for the Northeast and Midwest regions and 30% for the Federal District. Within each region and age stratum, the sample was proportionate with respect to the different capital cities.
Data analysis
The overall prevalence of seropositivity and 95% CI were calculated for each study site (the Northeast, the Midwest and the Federal District) by age-group corrected for the design effect.13 The age-specific distribution of the population susceptible to HAV infection was estimated using a simple catalytic model to estimate the force of infection.
The association between HAV infection and independent variables was assessed using the Odds ratio (OR), a 95% CI and a P-value. The OR was corrected by random design effect due to the study design. Weighting was also applied because different sampling fractions were adopted for the two age groups. First, univariate analysis was performed separately for each age group. As the risk factors identified were similar in the two populations, they were combined to increase the statistical power of the study. Weighting was used in order to correct the difference in sampling fraction between age groups.
Individual-, household- and area-level variables that were associated to the outcome in univariate analysis (P < 0.10) were successively included in a multilevel model. Generalized Linear and Latent Mixed Models (GLLAMM) were used to perform the multilevel analysis using Stata 9.2.14
Ethical issues
Written consent was obtained from all participants and, in the case of minors, from their legal guardian. The project was submitted to the National Research Ethics Committee (CONEP) of the Brazilian National Health Council and to local research ethics committees in each site.
Results
Overall, 1937 individuals aged 5–19 years were tested for total HAV antibodies and 923 tested positive (47.7%). The prevalence of anti-HAV in the 5–9 years age group was: 41.4% (95% CI 34.4–48.6) in the Northeast region; 32.3% (95% CI 25.6–39.1) in the Midwest region and 33.8% (95% CI 22.4–45.3) in the Federal District. In the 10–19 years age group, the prevalence of HAV soropositivity was 57.4% (95% CI 50.3–64.6); 56.0% (95% CI 49.2–62.7) and 65.1% (95% CI 55.3–74.8) for Northeast, Midwest and Federal District, respectively.
Table 1 compares the main characteristics of individual-, household- and area-level variables for the study sites. Similar proportions for females (∼50%) and school attendance (∼90%) were observed in all settings, but the percentage of illiterate children ≥7 years and adolescents were twice as high in the Northeast region (5.1%) compared with other sites. So far as household-level data were concerned, ∼95% of those in the Federal District area received regular water supply. There was a gradient, from high to low, from the Federal District to Midwest (89.5%) and Northeast (66.9%) regions. Half of children and adolescents from the State capitals in the Northeast and Midwest lived in households with no sewerage. In contrast, around 90% of the households in the Federal District were connected to sewage disposal services. All sites had >90% coverage of solid waste disposal, although the Northeast region had the lowest coverage. The area-based variables showed similar results for all sites with the exception of a higher proportion of illiterate heads of household (13.0%) and of the illiterate female heads of households (17.4%) in the Northeast. It is also interesting to note that approximately one-third of the families identified a woman as the head of the household in the three sites studied.
Table 1.
Individual-, household- and area-level characteristics among children and adolescents, Northeast region, Midwest region and Federal district, Brazil, 2005
| Study sites |
|||
|---|---|---|---|
| Characteristic | Northeast n = 634a | Centralwest n = 703a | Federal district n = 600a |
| Individual level n (%)b | |||
| Female | 314 (49.5) | 356 (50.6) | 302 (50.6) |
| 5- to 9-years-old | 294 (46.4) | 310 (44.1) | 328 (54.7) |
| 10- to 19-years-old | 340 (53.6) | 396 (55.9) | 272 (45.3) |
| HAV vaccine reported | 58 (11.1) | 29 (4.9) | 7 (1.2) |
| School attendance | 574 (90.5) | 623 (88.6) | 558 (93.0) |
| Illiterate individuals ≥7 years | 24 (5.1) | 13 (2.4) | 9 (2.2) |
| Household level n (%)b | |||
| Home ownership | 529 (83.8) | 491 (70.5) | 399 (68.3) |
| Water supply | 584 (92.4) | 656 (94.3) | 590 (98.3) |
| Pipe water inside the house | 532 (84.4) | 585 (84.1) | 582 (97.0) |
| Regular water supply | 423 (66.9) | 623 (89.5) | 577 (95.1) |
| Sewage disposal | 310 (49.1) | 373 (53.6) | 520 (87.3) |
| Solid waste disposal | 574 (90.8) | 677 (97.3) | 598 (99.5) |
| Area level—median percentage (0.25; 0.75) | |||
| Illiterate head of household | 13.0 (7.1; 20.6) | 6.5 (3.3; 11.1) | 8.4 (6.2; 12.6) |
| Illiterate female head of household | 17.4 (8.0; 26.1) | 10.5 (5.8; 18.1) | 11.6 (7.1; 18.9) |
| Mean level of schooling of head of the household (years) | 6.0 (4.8; 7.8) | 6.7 (5.7; 8.3) | 6.3 (5.4; 7.6) |
| 15–19 years literate individuals | 96.9 (93.8; 98.4) | 99.2 (98.4; 100) | 98.6 (97.1; 100.0) |
| Female head of household | 33.5 (28.1; 39.2) | 28.8 (23.8; 34.3) | 33.3 (24.6; 39.2) |
| Households with water supply | 97.9 (92.1; 99.7) | 95.3 (84.5; 99.4) | 99.7 (98.5; 100.0) |
| Households with solid waste collection | 98.5 (83.5; 100.0) | 99.6 (96.5; 100) | 100.0 |
| Individuals per household | 4.0 (3.7; 4.2) | 3.5 (3.4; 3.8) | 3.9 (3.7; 4.0) |
aNumbers refer to the sample size for individual and household variables.
bPercentages were calculated excluding missing values.
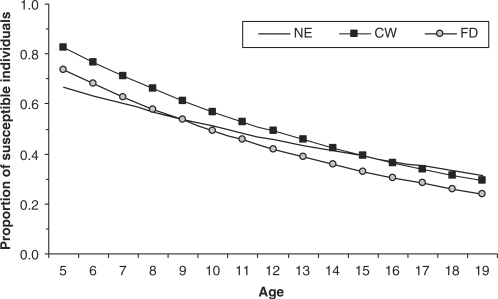
There was an upward trend of HAV infection according to age in all sites. By the age of 5, 31.5% of the children had already been infected with HAV in the Northeast region, while, in the other two sites, around 20.0% of children of this age were infected. However, by the age of 19 years, the prevalence of seropositivity was ∼70% in all sites. Figure 1 shows the distribution of the susceptible population according to age using a simple catalytic model. The Midwest and Federal District show parallel curves showing similar slopes with an incidence of 7.4% per 100 person/year in the Midwest and 8.0% per 100/year in the Federal District. In the Northeast region, the prevalence of current or past infection was higher in younger age groups with ∼50% of individuals infected by the age of 10 and an estimated incidence of 5.3 per person/year.
Figure 1.
Susceptible population for Hepatitis A infection data using a simple catalytic model, Northeast, Midwest and Federal District, Brazil, 2005
Similar frequencies for HAV antibodies were detected in males and females in all study sites. On univariate analysis, an individual's level of education was shown to be a predictor of HAV infection in all settings (Table 2). Table 3 shows that—at household-level—the head of household, schooling and income were negatively associated with the infection of children and adolescents with HAV. These household-based variables were consistently related to infection in all sites. Also, an inverse association was found between HAV infection and the variables related to environmental conditions (water supply, garbage collection) and education (years of schooling or illiteracy) of the head of the family (Table 4).
Table 2.
Hepatitis A seropositivity, odds ratio and 95% CI for the association between individual variables and hepatitis A, Northeast region, Midwest region and Federal district, Brazil, 2005
| Individual level variables | Northeast |
Midwest |
Federal district |
|||
|---|---|---|---|---|---|---|
| n (%)a,b | ORc (95% CI) | n (%)a | ORc (95% CI) | n (%)a,b | ORc (95% CI) | |
| Sex | ||||||
| Female | 314 (50.9) | 1.00 | 356 (45.8) | 1.00 | 302 (50.7) | 1.00 |
| Male | 320 (48.5) | 0.94 (0.68–1.30) | 347 (45.2) | 1.07 (0.76–1.51) | 298 (49.3) | 0.88 (0.61–1 26) |
| Literacyd | ||||||
| Yes | 492 (50.8) | 1.00 | 581 (48.5) | 1.00 | 454 (54.2) | 1.00 |
| No | 34 (61.8) | 1.52 (0.72–3.23) | 23 (56.5) | 1.71 (0.44–6.60) | 28 (46.4) | 0.72 (0.34–1.54) |
| Schoolingd | ||||||
| Illiterate | 62 (53.2) | 1.00 | 60 (55.0) | 1.00 | 32 (43.7) | 1.00 |
| ≤8 years | 409 (51.8) | 0.78 (0.37–1.67) | 381 (42.3) | 0.52 (0.24–1.10) | 348 (51.1) | 1.47 (0.61–3.54) |
| 9–11 years | 42 (50.0) | 0.76 (0.31–1.86) | 106 (63.2) | 0.98 (0.40–2.43) | 69 (66.7) | 2.28 (0.94–5.58) |
| University | – | – | 54 (61.1) | 0.85 (0.34–2.11) | 30 (60.0) | 1.80 (0.52–6.18) |
aPrevalence of anti-HAV antibodies.
bPercentages were calculated excluding missing values.
cOdds ratio corrected by random effect and weight sampling.
dFor children ≥7 years of age.
Table 3.
Association between household level variables and hepatitis A infection, Northeast region, Midwest region and Federal district, Brazil, 2005
| Northeast | Midwest | Federal district | |
|---|---|---|---|
| Household level variables | Odds ratioa (95% CI) | Odds ratioa (95% CI) | Odds ratioa (95% CI) |
| Literate head of household | |||
| Yes | 1.00 | 1.00 | 1.00 |
| No | 2.03 (1.02–4.05) | 3.78 (0.83–17.3) | 2.98 (0.87–10.2) |
| Level of education of head of the household | |||
| Illiterate | 1.00 | 1.00 | 1.00 |
| Incomplete Basic level | 0.48 (0.26–0.89) | 1.13 (0.44–2.88) | 0.78 (0.32–1.88) |
| Completed Basic level | 0.30 (0.14–0.65) | 0.54 (0.21–1.36) | 0.67 (0.29–1.55) |
| Secondary level | 0.17 (0.08–0.38) | 0.27 (0.11–0.70) | 0.30 (0.11–0.79) |
| Head of household in paid employment | |||
| Yes | 1.00 | 1.00 | 1.00 |
| No | 1.10 (0.70–1.74) | 0.80 (0.58–1.10) | 0.98 (0.62–1.57) |
| Income of head of householdb | |||
| <1 | 1.00 | 1.00 | 1.00 |
| 1 to <2 | 0.60 (0.36–0.98) | 1.09 (0.57–2.10) | 1.15 (0.63–2.11) |
| 2 to 3 | 0.27 (0.13–0.58) | 0.52 (0.21–1.28) | 1.48 (0.78–2.80) |
| 3 to <5 | 0.19 (0.08–0.46) | 0.36 (0.17–0.73) | 0.82 (0.31–2.20) |
| 5 to 10 | 0.22 (0.07–0.69) | 0.46 (0.20–1.08) | 0.41 (0.21–0.80) |
| >10 | 0.06 (0.02–0.24) | 0.34 (0.13–0.91) | 0.36 (0.14–0.92) |
| Home ownership | |||
| Yes | 1.00 | 1.00 | 1.00 |
| No | 0.92 (0.55–1.53) | 1.25 (0.82–1.90) | 0.68 (0.41–1.11) |
| Water supply | |||
| Yes | 1.00 | 1.00 | 1.00 |
| No | 1.92 (0.90–4.10) | 1.16 (0.50–2.71) | 0.64 (0.19–2.21) |
| Piped water in home | |||
| Yes | 1.00 | 1.00 | 1.00 |
| No | 1.07 (0.57–2.00) | 1.91 (1.08–3.35) | 3.09 (0.63–15.1) |
| Lack of water supply | |||
| Never | 1.00 | 1.00 | 1.00 |
| At least once per month | 1.59 (0.82–3.06) | 1.11 (0.29–4.30) | 0.82 (0.24–276.0) |
| Rarely | 1.43 (0.82–2.48) | 0.78 (0.35–1.77) | 0.92 (0.46–1.80) |
| Sewage disposal | |||
| Public system | 1.00 | 1.00 | 1.00 |
| Septic tank | 1.52 (0.97–2.40) | 1.19 (0.72–1.95) | 2.52 (0.97–7.50) |
| Other destination | 2.63 (0.90–7.64) | – | – |
| Solid waste disposal | |||
| Collected by public service | 1.00 | 1.00 | – |
| Other destination | 1.23 (0.55–2.78) | 1.33 (0.53–3.31) | – |
aOdds ratio corrected by random effect and weight sampling.
bIncome minimum wage.
Table 4.
Association between area-level variables and hepatitis A infection, Northeast region, Midwest region and Federal district, Brazil, 2005
| Northeast | Central western | Federal district | |
|---|---|---|---|
| Area-level variables | Odds ratioa (95% CI) | Odds ratioa (95% CI) | Odds ratioa (95% CI) |
| Illiterate head of household (%) | 1.05 (1.03–1.08) | 1.10 (1.05–1.16) | 1.09 (1.03–1.16) |
| Illiterate female head of household (%) | 1.13 (1.06–1.21) | 1.27 (1.13–1.44) | 1.04 (0.99–1.09) |
| Schooling of head of household (mean years) | 0.79 (0.71–0.89) | 0.81 (0.73–0.89) | 0.73 (0.62–0.85) |
| 15–19 years literate individuals living in the household (%) | 0.93 (0.87–1.00) | 0.76 (0.64–0.89) | 0.65 (0.64–0.89) |
| Female head of household (%) | 0.97 (0.95–1.00) | 0.98 (0.94–1.02) | 1.02 (0.99–1.05) |
| Water supply coverage (%) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | 0.98 (0.97–0.99) |
| Solid waste collection coverage (%) | 0.98 (0.96–0.99) | 0.97 (0.94–0.99) | 0.97 (0.96–0.97) |
aCorrected by random effect and weight sampling.
The multilevel model showed that individual, household- and area-level variables are independently associated with HAV infection. Age is an important predictor of outcome. Additionally, education variables that were collected through household-level interviews or retrieved from IBGE census tract data were associated with HAV infection in all sites (Table 5).
Table 5.
Multilevel model for individual-, household- and area-level variables associated with Hepatitis A infection, Northeast region, Midwest region and Federal district, Brazil, 2005
| Northeast |
Centralwest |
Federal district |
||||
|---|---|---|---|---|---|---|
| Odds ratio | Odds ratio | Odds ratio | ||||
| Characteristics | (95% CI) | P-value | (95% CI) | P-value | (95% CI) | P-value |
| Individual level | ||||||
| Age | 1.13 (1.06–1.20) | 0.001 | 1.23 (1.16–1.30) | 0.001 | 1.30 (1.20–1.40) | 0.001 |
| Schooling | ||||||
| Illiterate and basic level | 1.00 | |||||
| Secondary level or more | 0.44 (0.23–0.85) | 0.015 | ||||
| Household level | ||||||
| Income of head of householda | ||||||
| <2 | 1.00 | 1.00 | ||||
| ≥2 | 0.32 (0.16–0.61) | 0.001 | 0.51 (0.30–0.87) | 0.013 | ||
| Schooling of head of household | ||||||
| Illiterate | 1.00 | |||||
| Basic level | 0.44 (0.23–0.87) | 0.018 | ||||
| Secondary level | 0.33 (0.14–0.80) | 0.014 | ||||
| Area level | ||||||
| Illiterate head of household (%) | 1.10 (1.04–1.17) | 0.001 | ||||
| Schooling of head of household (mean number years) | 0.86 (0.77–0.97) | 0.014 | 0.72 (0.61–0.84) | 0.001 | ||
| Water supply coverage (%) | 0.99 (0.98–0.99) | 0.001 | ||||
| Solid waste collection coverage (%) | 0.98 (0.97–0.99) | 0.011 | 0.99 (0.98–0.99) | 0.001 | ||
aIncome minimum wage.
Discussion
The evidence that 40% of children and adolescents tested positive for HAV total antibodies allows the Northeast and Midwest regions and Federal District to be classified as areas with intermediate endemicity. Most previous studies of HAV infection in Brazil were conducted using selected groups or restricted areas, and have shown wide variations in prevalence according to the age and socioeconomic characteristics.2,6,7,15–17
Data from the Brazilian national surveillance system reported 25 275 cases of hepatitis A in 200518 yielding an incidence rate of 13.7 cases per 100 000 inhabitants. Considering the underreporting of the acute hepatitis A cases, the Pan American Health Organization has estimated an incidence of 130 cases per 100 000 inhabitants.18 Our data from an unbiased sample of the base population, therefore, provides better estimates of current or past HAV exposure.
The endemicity of hepatitis A is considered to be low in developed countries such as the United States, Canada and Western Europe, where relatively few children are infected.5,8 The prevalence of hepatitis A antibody in the general US population was 31.3%, ranging from 9.4% among individuals aged 6–11 years to 74.6% among those ≥70 years, as reported by the Third National Health and Nutrition Examination Survey (1988–1994).19
In our study, the age-distribution showed that HAV infection increased with age in all sites, as is to be expected for infectious diseases that are dependent on environmental exposures.2,7,13 However, there was a distinct age-specific trend for the two macro-regions. In the Northeast region, a higher prevalence of seropositivity was detected at 5 years of age compared to the other settings, which is consistent with this region's lower indicators of socio-economic conditions (as identified in this study and by census tract data). The likely explanation is that higher incidence of HAV infection occurs in the first years of life (0–4 years), which leads to a higher prevalence by the age of 5. However, by the age of 19 years, similar frequencies for susceptible populations (∼30%) were detected in all sites. The slope increased less steeply in the Northeast as indicated by the lower force of infection (5.1 per 100 person/year) in the group aged 5–19 years. The curves for age-specific HAV susceptible populations in the Midwest region and Federal District were similar. These results seem consistent with those from the Federal District which is a city located within the Midwest region and these two settings have similar socioeconomic conditions.
It is well known in the hepatitis A epidemiology that there is an inverse relationship between socio-economic conditions and HAV seropositivity, particularly in less developed countries.2,3,7,17,20 Univariate analysis at household-level carried out by our study indicated that schooling and income of the head of the household were predictors in all settings. At area level, the education of the head of the household, the head of the household being a woman, water and solid waste collection were also found to be common risk factors for hepatitis A infection. However, multilevel analysis produced different epidemiological profiles. Age remained an associated individual-based risk factor in all three sites investigated, but individual schooling was associated with HAV infection only in the most developed site (the Federal District). At the household-level, income and schooling of the head of the family were independently associated with the area with the lowest level of socioeconomic indicators, the Northeast. At area level, schooling of the head of household was a predictor of infection in all three areas. Lack of solid waste disposal was associated with the outcome for Midwest region and Federal District, while water supply coverage was a predictor of hepatitis A infection only in the Federal District. Paradoxically, only education (household- and area-level) and income (household-level) remained as predictors in the Northeast region. In the other settings with higher socioeconomic conditions, area-level variables related to sanitation seem to predict infection.
We are aware that this study has some limitations. The exclusion of children below 5 years of age meant that it was not possible to quantify the force of infection in the first years of life. This exclusion criterion was justifiable for operational reasons and by the need to minimize refusal to participate in the study. Rural and less-populated areas were not included, since the study was designed to determine the overall HAV prevalence rates in the State capitals. Therefore, the reported figures are representative of HAV infection among children and adolescents in highly urbanized areas only. However, the average prevalence for each setting may mask clusters of populations with high and low susceptibility in some parts of the cities. Additionally, the biological marker (total anti-HAV) applied does not make it possible to differentiate between current/previous exposure (ever exposed) and vaccinated individuals. However, few individuals included in our study had been vaccinated.
The current evidence that an important proportion of the population of young people is susceptible to HAV infection has a number of public health implications. These susceptible individuals will be at risk of acquiring hepatitis A later in life, leading to a more severe disease. Several strategies have been employed to delivery hepatitis A vaccination in intermediate endemicity areas such as those covered by our study. These have ranged from vaccination during or after outbreaks, targeting specific settings of the population, restricted, extensive or universal vaccination.21 In Brazil, the current HAV vaccine recommendation is to restrict it to high-risk groups such as those with chronic liver disease, including HBV and HCV; HIV-positive children under 13 years, liver transplant patients and so forth. Our study, therefore, was conducted in the pre-vaccination era and few individuals included had a history of previous vaccination.
A reduction in adult morbidity and the burden of disease has been reported as one of the advantages of universal vaccination in low and intermediate endemic areas.3 Analysis of cost-effectiveness is essential for evaluating the feasibility of this public health policy.22
The results of our multilevel analysis showed that social deprivation, measured using a proxy marker shapes the risk of acquiring HAV infection. There is, therefore, still room for improvement with regard to sustainable measures such as sanitation and socioeconomic conditions related to poverty. To date, this is the first HAV population-based survey using a representative sample of children and adolescents in two large Brazilian areas. The differences observed between regions provide a comprehensive picture of individual-, household- and area-based risk factors associated to HAV transmission. Such predictors furnish important information for drawing up public health recommendations. This approach provides a useful tool for measuring inequalities and highlighting risk-related contextual factors over and beyond the effect of individual risk factors.
Acknowledgements
We are grateful for the financial support of Brazilian Ministry for Health, Pan American Health Organization and for administrative support of University of Pernambuco Foundation. The authors were partially supported by CNPq (scholarship 307963/2004-7 to C.M.T.M. and 300917/2006-6 to R.A.A.X. and 303049/2007-3 to M.R.A.C.)
Conflict of interest: None declared.
KEY MESSAGES.
Overall prevalence of 40% among children and adolescent classifies Northeast and Midwest regions areas with intermediate endemicity for HAV.
Our findings showed an upward trend of HAV infection with increasing age with differences in the force of infection according to settings.
The study revealed the importance of individual and contextual factors associated with HAV infection in inner cities.
Appendix
Hepatitis Study Group: Zulma Medeiros (Centro de Pesquisa Aggeu Magalhães—FIOCRUZ), Demócrito Miranda Filho (Universidade de Pernambuco), Maria Mabel Melo (LACEN-PE), Conceição Sá (Hemocentro—HEMOPI-PI), Carlos Henrique Nery Costa (Universidade Federal do Piauí), Arnaldo de Jesus Dominici (Universidade Federal do Maranhão), Maria das Graças Aragão (Universidade Federal do Maranhão), Elizabeth de Souza Lima (LACEN-MA), José Milton de Castro Lima (Universidade Federal do Ceará), José Wellington Oliveira Lima (FUNASA-CE), Maria do Carmo Vidal Gadelha Lima (LACEN-CE), Gilmar Amorim de Sousa (Universidade Federal do Rio Grande do Norte), Márcia Araújo Barreto (Universidade Federal do Rio Grande do Norte), Manoel Jaime Xavier Filho (Universidade Federal da Paraíba), Jória Viana Guerreiro (Universidade Federal da Paraíba), Dayse Mércia Cavalcanti de Oliveira (FUNASA-AL), Maria Rosileide Bezerra Alves (Secretaria Estadual de Saúde de Alagoas), Ivoneide Moreira de Oliveira Barros (LACEN-PB), Tereza Virgínia Silva B.Nascimento (Universidade Federal de Sergipe), Lúcia Maria Sayde de Azevedo Tavares (FUNASA-SE), Raymundo Paraná Ferreira Filho (Universidade Federal da Bahia), José Tavares-Neto (Universidade Federal da Bahia), Maria Alice Sant'Anna Zarife (LACEN-BA), Rodrigo Sebba Aires (Secretaria Estadual de Saúde de Goiás), Flúvia Pereira A. da Silva (Secretaria Municipal de Saúde de Goiania), Beatriz Maranhão Bariani (LACEN-GO), José Ivan Aguiar (Universidade Federal do Mato Grosso do Sul), Eugênio Barros (Secretaria Municipal de Saúde—Campo Grande), Gilza Bastos dos Santos (LACEN-MS), Francisco Souto (Universidade Federal do Mato Grosso), Cor Jesus Fernando Fontes (Universidade Federal do Mato Grosso), Virgínia Correia de Azevedo (LACEN-MT), Roberto de Melo Dusi (Secretaria Estadual de Saúde - DF), Lídia Maria Pinto Luna (LACEN-DF), Gabriela Coral (Fundação Faculdade de Ciências Médicas de Porto Alegre), Airton Tetelbom Stein (Fundação Faculdade de Ciências Médicas de Porto Alegre), José Carlos da Fonseca (Universidade Federal do Amazonas), Leila Melo Brasil (Fundação Medicina Tropical do Amazonas), Kátia Biscuola de Campos (Programa Nacional de Prevenção e Controle das Hepatites Virais/SVS/Ministério da Saúde).
References
- 1.WHO. 2008. Jan 30, pp. 38–42. Hepatitis A vaccine: WHO position paper. Weekly Epidemiological Record 2000.
- 2.Struchiner CJ, de Almeida LM, de Azevedo RS, Massad E. Hepatitis A incidence rate estimates from a pilot seroprevalence survey in Rio de Janeiro, Brazil. Int J Epidemiol. 1999;28:776–81. doi: 10.1093/ije/28.4.776. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004;132:1005–22. doi: 10.1017/s0950268804002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal P. Cost-effectiveness of hepatitis A vaccination in children, adolescents, and adults. Hepatology. 2003;37:44–51. doi: 10.1053/jhep.2003.50016. [DOI] [PubMed] [Google Scholar]
- 5.Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev. 2006;28:101–11. doi: 10.1093/epirev/mxj012. [DOI] [PubMed] [Google Scholar]
- 6.Vitral CL, Gaspar AM, Souto FJ. Epidemiological pattern and mortality rates for hepatitis A in Brazil, 1980–2002–a review. Mem Inst Oswaldo Cruz. 2006;101:119–27. doi: 10.1590/s0074-02762006000200001. [DOI] [PubMed] [Google Scholar]
- 7.Tapia-Conyer R, Santos JI, Cavalcanti AM, et al. Hepatitis A in Latin America: a changing epidemiologic pattern. Am J Trop Med Hyg. 1999;61:825–29. doi: 10.4269/ajtmh.1999.61.825. [DOI] [PubMed] [Google Scholar]
- 8.Bell BP, Shapiro CN, Alter MJ, et al. The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies. J Infect Dis. 1998;178:1579–84. doi: 10.1086/314518. [DOI] [PubMed] [Google Scholar]
- 9.Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health. 2000;21:171–92. doi: 10.1146/annurev.publhealth.21.1.171. [DOI] [PubMed] [Google Scholar]
- 10.Merlo J. Multilevel analytical approaches in social epidemiology: measures of health variation compared with traditional measures of association. J Epidemiol Community Health. 2003;57:550–52. doi: 10.1136/jech.57.8.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Brazilian Institute of Geography and Statistics (IBGE) Demographic Census 2000: characteristics of the population and households, results from the population universe. Available from: www.ibge.gov.br/home/estatistica/populacao/censo2000/default.shtm.
- 12.Ximenes RA, Martelli CM, Souza WV, et al. [Surveillance of endemic diseases in urban areas: the interface between census tract maps and morbidity data] Cad Saude Publica. 1999;15:53–61. doi: 10.1590/s0102-311x1999000100006. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RM, May RM. Age-related changes in the rate of disease transmission: implications for the design of vaccination programmes. J Hygiene. 1985;94:365–436. doi: 10.1017/s002217240006160x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling using Stata. College Station, Texas: Stata Press; 2005. [Google Scholar]
- 15.Pinho JR, Sumita LM, Moreira RC, et al. Duality of patterns in hepatitis A epidemiology: a study involving two socioeconomically distinct populations in Campinas, Sao Paulo State, Brazil. Rev Inst Med Trop Sao Paulo. 1998;40:105–6. doi: 10.1590/s0036-46651998000200007. [DOI] [PubMed] [Google Scholar]
- 16.Queiroz DA, Cardoso DD, Martelli CM, et al. Risk factors and prevalence of antibodies against hepatitis A virus (HAV) in children from day-care centers, in Goiania, Brazil. Rev Inst Med Trop Sao Paulo. 1995;37:427–33. doi: 10.1590/s0036-46651995000500008. [DOI] [PubMed] [Google Scholar]
- 17.Almeida LM, Werneck GL, Cairncross S, Coeli CM, Costa MC, Coletty PE. The epidemiology of hepatitis A in Rio de Janeiro: environmental and domestic risk factors. Epidemiol Infect. 2001;127:327–33. doi: 10.1017/s0950268801005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministerio da Saude. Guia de Vigilância Epidemiológica. In: Saúde SdVe., editor. Brasília: Ministério da Saúde; 2005. [Google Scholar]
- 19.Bell BP, Kruszon-Moran D, Shapiro CN, Lambert SB, McQuillan GM, Margolis HS. Hepatitis A virus infection in the United States: serologic results from the Third National Health and Nutrition Examination Survey. Vaccine. 2005;23:5798–806. doi: 10.1016/j.vaccine.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 20.Saback FL, Palmer TE, Sabino RR, et al. Infection with hepatitis A and TT viruses and socioeconomic status in Rio de Janeiro, Brazil. Scand J Infect Dis. 2001;33:121–25. doi: 10.1080/003655401750065508. [DOI] [PubMed] [Google Scholar]
- 21.Hepatitis A vaccine recommendations. Pediatrics. 2007;120:189–99. doi: 10.1542/peds.2007-1088. [DOI] [PubMed] [Google Scholar]
- 22.WHO. Vaccine Introduction Guidelines. Adding a vaccine to National Immunization programme: Geneva: Department of Immunization, Vaccines and Biologicals, 2005. Available at: www.who.int/vaccines-documents/