Abstract
Bladder cancer is currently diagnosed using cystoscopy and cytology in patients with suspicious signs and symptoms. These tests are also used to monitor patients with a history of bladder cancer. The recurrence rate for bladder cancer is high, thus necessitating long-term follow-up. Urine cytology has high specificity but low sensitivity for low-grade bladder tumors. Recently, multiple noninvasive urine-based bladder cancer tests have been developed. Although several markers have been approved by the US Food and Drug Administration for bladder cancer surveillance, only a few are approved for detection of bladder cancer in high-risk patients.
Key words: Bladder cancer, Cystoscopy, Urine cytology, Tumor markers
Carcinoma of the urinary bladder, the fourth most common cancer in men and the ninth most common cancer in women, results in significant morbidity and mortality.1 Most patients with bladder cancer receive the diagnosis after they present with gross or microscopic hematuria. At initial diagnosis, approximately 70% of patients have bladder cancers that are confined to the epithelium or subepithelial connective tissue. These cancers can be managed with endoscopic resection and intravesical therapy. The recurrence rate for these tumors ranges from 50% to 70%, and 10% to 15% of cases progress to muscle invasion over a 5-year period.2,3 Recurrence may be seen locally and rarely in the upper urinary tract even after several years, necessitating lifelong surveillance.
The remaining 30% of patients have muscle-invasive cancer at initial diagnosis. Of this population, 50% have distant metastasis within 2 years, and 60% die within 5 years despite treatment.4
Cystoscopy aided by cytology is the mainstay for the diagnosis of bladder cancer. Current follow-up protocols after initial presentation typically include flexible cystoscopy and urine cytology every 3 months for 1 to 3 years, every 6 months for an additional 2 to 3 years, and then annually, assuming no recurrence.
Cystoscopy, a relatively short, minimally traumatic office procedure performed with local urethral anesthesia, identifies nearly all papillary and sessile lesions. Nevertheless, it is still invasive and a cause of discomfort and distress to the patient. In addition, cystoscopy may be inconclusive at times because of the grossly abnormal appearance of the bladder mucosa, especially in patients with an indwelling catheter or active inflammation. Although considered the gold standard for diagnosis, cystoscopy has a false-negative rate either from operator error or from small areas of carcinoma in situ (CIS), which may be difficult to detect.5,6
Emerging diagnostic techniques using intravesical 5-aminolevulinic acid combined with fluorescence cystoscopy have demonstrated that many malignant areas can be completely indiscernible in the eyes of even highly experienced cystoscopists. With this technology, both small papillary tumors and almost 33% more cases of CIS overlooked by cystoscopy were identified.7,8 Tumors that have been missed during resection account for at least part of the frequent recurrences after initial treatment. It is likely that cancer was present but not visible at the time of resection and became visible at follow-up when the tissue became morphologically abnormal enough to differentiate from adjacent normal urothelium. A multicenter study that involved several experienced and highly regarded bladder cancer specialists found that 37% of the biopsies performed on the basis of suspicious endoscopic findings had false-negative results.9
Urine cytology has a high sensitivity and specificity for the detection of high-grade urothelial carcinoma, but it lacks the sensitivity to detect low-grade tumors.10 The accuracy of urine cytology in predicting bladder cancer recurrence may vary widely among institutions.11 In a recent multi-institutional study that included 10 centers from 9 countries (4 continents), urine cytology was positive in 38% to 65% of patients with recurrent bladder cancer.11 Sensitivity for grade 3 recurrence was 33% to 95%, whereas sensitivity for tumor stage T2 and higher was 37% to 100%. In addition, results are not available immediately, and evaluation requires a highly trained cytopathologist, who may not be available in all areas. Hence, cytology is not ideal for screening for and surveillance of bladder cancer.
An accurate bladder tumor marker would be useful for both screening of high-risk populations and for monitoring of patients with a history of bladder cancer to help identify recurrence early and prevent disease progression. Because of the relatively low prevalence of bladder cancer in the general population, screening the whole population would not be cost effective.12–14 However, screening persons at high risk, such as those with exposure to known carcinogens (cigarette smoke, cyclophosphamide, pelvic radiation, aromatic amines) may be beneficial for early detection of bladder cancer. An accurate marker also has the potential to replace, delay, or complement cystoscopy in the monitoring of patients with bladder cancer. In addition, tumor markers may replace urine cytology. An ideal bladder cancer screening and monitoring test would be noninvasive, rapid, objective, easy to perform and interpret, and have high sensitivity and specificity.
Because urine comes into contact with bladder tumors, many tests of voided urine have been designed to detect molecules that may be associated with tumor growth or invasion. At present, the standard noninvasive bladder tumor test is voided urine cytology. A number of markers that take advantage of exfoliated cells in the urine for detection of cell-surface antigens, nuclear morphology, or gene expression have been studied in bladder cancer. Although most of them remain investigational and are undergoing preclinical evaluation, few have undergone clinical trials and have been approved for clinical use.
Markers widely studied in the clinical situation that are approved for the diagnosis of bladder cancer are discussed, followed by a description of those that are more investigational but highly likely to be introduced into clinical practice in the near future (Table 1). This review is limited to urothelial carcinoma, because this constitutes more than 90% of bladder cancer cases in Western countries.
Table 1.
Characteristics of Urine-Based Bladder Tumor Markers
| Testing | Sensitivity | Specificity | |||
|---|---|---|---|---|---|
| Test | Marker Detected | Assay Type | Situation | (%) | (%) |
| AccuDx15,112 | Fibrin-fibrinogen degradation | Sandwich immunoassay | Point-of-care | 52–81 | 75–86 |
| (Intracel, Rockville, MD) | product, fibrin, fibrinogen | ||||
| BLCA-465,66 | BLCA-4 transcription factor | ELISA | Specialized | 89–96 | 100 |
| laboratory | |||||
| BTA stat®17,113,114 | Complement factor H-related | Colorimetric | Point-of-care | 57–83 | 68–72 |
| (Polymedco, Cortlandt | protein | immunoreaction | |||
| Manor, NY) | |||||
| BTA TRAK®27,30,31 | Complement factor H | Sandwich | Specialized | 66–72 | 51–75 |
| (Polymedco, Cortlandt | immunoassay | laboratory | |||
| Manor, NY) | |||||
| Hyaluronic acid, | Hyaluronic acid, | Immunoassay | Specialized | 92–100 | 89–93 |
| hyaluronidase68,70 | hyaluronidase | laboratory | |||
| ImmunoCyt™34–36 | Mucins, high-molecular-weight | Immunofluorescence, | Specialized | 50–100 | 69–79 |
| (DiagnoCure, Quebec | carcinoembryonic | cytology | laboratory | ||
| City, Quebec, Canada) | antigen | ||||
| Lewis X antigen73 | Lewis X blood group antigen | Immunocytology with | Specialized | 80 | 86 |
| P12 monoclonal antibody | laboratory | ||||
| Microsatellite markers74,77 | Highly polymorphic DNA | PCR | Specialized | 72–97 | 80–100 |
| repeats | laboratory | ||||
| NMP2218–20,42–44,46–48 | Nuclear mitotic apparatus | Sandwich immunoassay | Specialized | 47–100 | 60–70 |
| laboratory | |||||
| Quanticyt™17,48 | Nuclear shape, DNA content | Feulgen stained specimen | Specialized | 45–59 | 71–93 |
| (Gentian Scientific Software, | image analysis by dual | laboratory | |||
| Niawer, The Netherlands) | parameter morphometry | ||||
| Survivin82,83 | Survivin antiapoptotic protein | BioDot system | Specialized | 64–100 | 87–93 |
| laboratory | |||||
| Telomerase21,115 | Human telomerase messenger | PCR | Specialized | 62–81 | 80–96 |
| RNA | laboratory | ||||
| UBC™ test62,116 | Cytokeratins 8 and 18 | 1-step immunoassay | Specialized | 66–82 | 83–90 |
| (IDL Biotech, Bromma, | laboratory | ||||
| Sweden) | |||||
| UroVysion™117 | Aneuploidy chromosome 3, 7 | Multitarget FISH | Specialized | 36–100 | 89–96 |
| (Vysis, Downers Grove, IL) | and 17 and loss of 9p21 locus | laboratory |
ELISA, enzyme-linked immunosorbent assay; BTA, bladder tumor antigen; PCR, polymerase chain reaction; NMP22, nuclear matrix protein 22; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; FISH, fluorescence in situ hybridization.
Commercially Available Bladder Tumor Markers
Bladder Tumor Antigen Tests
The term bladder tumor antigen (BTA) describes at least 3 distinct tests. The original BTA (Bard Diagnostics, Redmond, WA) had lower specificity and not superior sensitivity than urine cytology and was therefore taken off the market.15 Subsequently, the BTA stat® and BTA TRAK® (Polymedco, Cortlandt Manor, NY) tests were introduced. The BTA stat is a qualitative point-of-care test with an immediate result, whereas BTA TRAK is a quantitative test that requires trained personnel and a reference laboratory. These assays detect human complement factor H-related protein (as well as complement factor H), which is present in the urine of patients with bladder cancer.16 It is believed that complement factor H production by tumor cells may prevent tumor cell lysis by immune cells.
The overall sensitivity and specificity for the BTA stat test are 57% to 83%17–21 and 60% to 92%,18,22,23 respectively. The reported specificity must be assessed critically. Many of the studies excluded patients who had other commonly occurring genitourinary problems, such as renal stones, infection, and hematuria. In healthy persons without genitourinary signs or symptoms, the specificity is 97%, but in patients with benign genitourinary conditions the specificity is only 46%.23 Patients without bladder cancer but with other genitourinary conditions may have hematuria. The blood in the urine contains complement factor H, which can react with the antibody in the test and lead to a false-positive result.24,25
The BTA TRAK test is a quantitative sandwich immunoassay performed in a reference laboratory.26 The cutoff limit of human complement factor H-related protein to detect bladder cancer, recommended by the manufacturer, is 14 U/mL.27 When this cutoff is used, the reported overall sensitivity is 62% to 77%.27–32 As with the BTA stat test, benign genitourinary conditions, particularly hematuria, may yield false-positive results.27,28,30
Both tests have sensitivity comparable to that of cytology for high-grade tumors and better for low-grade tumors. These tests are approved by the US Food and Drug Administration (FDA) only for monitoring of bladder cancer, in combination with cystoscopy. They are not sufficiently accurate to be used for screening or diagnosis, particularly in patients with other genitourinary symptoms, because of their high false-positive rate.
ImmunoCyt
ImmunoCyt™ (DiagnoCure, Quebec City, Quebec, Canada) combines cytology with an immunofluorescence assay (immunocytochemistry).33 ImmunoCyt detects cellular markers for bladder cancer in exfoliated urothelial cells using 3 fluorescent monoclonal antibodies to pinpoint a high-molecular-weight form of carcinoembryonic antigen and 2 bladder tumor cell-associated mucins. Because the test requires the use of a fluorescence microscope by trained personnel, it is performed in a reference laboratory. ImmunoCyt has an overall sensitivity of 50% to 100%.34–36 Its specificity has been reported as 69% to 79%.34–36 Mian and coworkers35 found that 50% of patients with benign prostatic hyperplasia and 40% of patients with cystitis had false-positive ImmunoCyt test results. Another limitation of the test is the need for trained personnel to perform and interpret the result.36 This test may prove useful as an adjunct to cytology, but currently it requires further testing to define its role in the management of bladder cancer. In the United States, it is only approved for monitoring of patients with bladder cancer.
Nuclear Matrix Protein 22 Tests
The nuclear matrix protein 22 (NMP22) test detects a nuclear mitotic apparatus protein that is a component of the nuclear matrix. Nuclear matrix proteins make up the framework of a cell’s nucleus and play an important role in gene expression.37–39 NMP22 is a protein that localizes with the spindle poles during mitosis and thus regulates chromatid and daughter cell separation.40,41 There is a substantially higher level of NMP22 in the urine of patients with bladder cancer. However, because this protein is released from dead and dying urothelial cells, many benign conditions of the urinary tract, such as stones, infection, inflammation, and hematuria, and cystoscopy can cause a false-positive reading. Both a laboratory-based quantitative microplate enzyme immunoassay and a qualitative point-of-care test are available and are FDA-approved for use in bladder cancer surveillance. The latter is also approved for detection of bladder cancer in high-risk patients.
The sensitivity of the quantitative enzyme immunoassay (Matritech, Newton, MA) has ranged from 47% to 100%, most often falling between 60% and 70%,17,19,20,23,42–48 depending on the cutoff used. The specificity for the NMP22 test is 60% to 90%, again depending somewhat on the cutoff value used.17,19–21,23,28,30,42–44,49,50 Analysis of data shows that the NMP22 test is superior to cytology for detection of grade 1 and 2 bladder cancer but that it offers lower specificity.
Exclusion of the following 6 criteria may increase specificity of this test: benign inflammatory or infectious conditions, renal or bladder calculi, foreign body (stent or nephrostomy tube), bowel interposition, other genitourinary cancer, and instrumentation.19 When patients with these problems were excluded, specificity was increased to 99%.51
NMP22 has, however, not gained widespread use in routine urology practice for several reasons. First, reluctance may stem from the high false-positive rate and the absence of large-scale testing to confirm that NMP22 improves prediction of disease recurrence/progression in patients with stages Ta, T1, and/or CIS bladder cancer. In addition, the optimal NMP22 cut point remains controversial. Whereas the manufacturer recommends a value of 10 U/mL or greater as a positive test result, studies have suggested alternative threshold values between 3.6 and 13.7 U/mL, changing with the desired predicted endpoint (Figure 1) and the population characteristics (Figure 2).52,53
Figure 1.
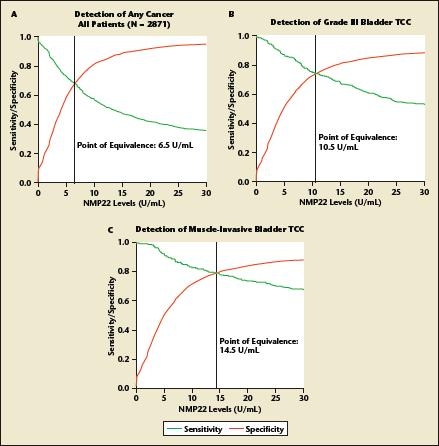
Variation of the optimal cutoff value for nuclear matrix protein 22 (NMP22) according to the predicted endpoint of interest. Curves of relative diagnostic sensitivity, specificity, and accuracy for detection of (A) any bladder transitional cell carcinoma (TCC); (B) grade 3 bladder transitional cell carcinoma; and (C) muscle-invasive bladder transitional cell carcinoma according to different diagnostic NMP22 cut point levels. Data from a multi-institutional study comprising 2871 patients who underwent office cystoscopy for monitoring for previous stage Ta, T1, and/or carcinoma in situ transitional cell carcinoma from 12 participating institutions. Reprinted from The Journal of Urology, Vol. 176, Shariat SF et al, “Variability in the performance of nuclear matrix protein 22 for the detection of bladder cancer,” pp. 919–926, Copyright American Urological Association 2006.
Figure 2.
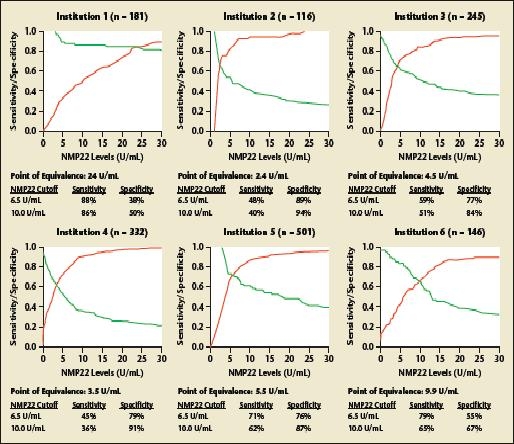
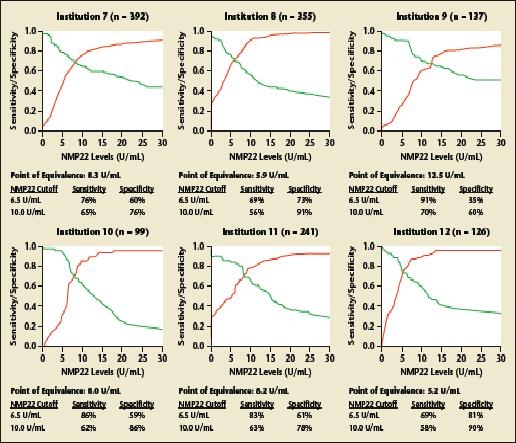
Variation of the optimal cutoff value for nuclear matrix protein 22 (NMP22) according to the patient population. Curves of relative diagnostic sensitivity, specificity, and accuracy for detection of any bladder transitional cell carcinoma according to different diagnostic NMP22 cutoff levels for each institution. Data from a multi-institutional study comprising 2871 patients who underwent office cystoscopy for monitoring for previous stage Ta, T1, and/or carcinoma in situ transitional cell carcinoma from 12 participating institutions. Reprinted from The Journal of Urology, Vol. 176, Shariat SF et al, “Variability in the performance of nuclear matrix protein 22 for the detection of bladder cancer,” pp. 919–926, Copyright American Urological Association 2006.
Shariat and colleagues54 have previously developed and internally validated highly accurate nomograms for prediction of disease recurrence and progression in patients with Ta, T1, and/or CIS urothelial carcinoma using data from 2871 patients from 12 centers, 9 countries, 4 continents (Figure 3). The nomograms incorporate urinary NMP22 levels, urinary cytology, patient age, and gender. Urinary levels of NMP22 improved the ability to predict bladder cancer recurrence and progression by a statistically and clinically significant margin. However, the investigators found important variability in the predictive accuracy of NMP22 between institutions. Therefore, in a follow-up study, they evaluated the variability of urinary levels of NMP22 for detecting cancer recurrence and progression.52 They found a substantial degree of heterogeneity in performance characteristics of NMP22 applied to different populations (area under the receiver operating characteristics curve for detection of bladder urothelial carcinoma across institutions ranged from 0.676 to 0.889).
Figure 3.
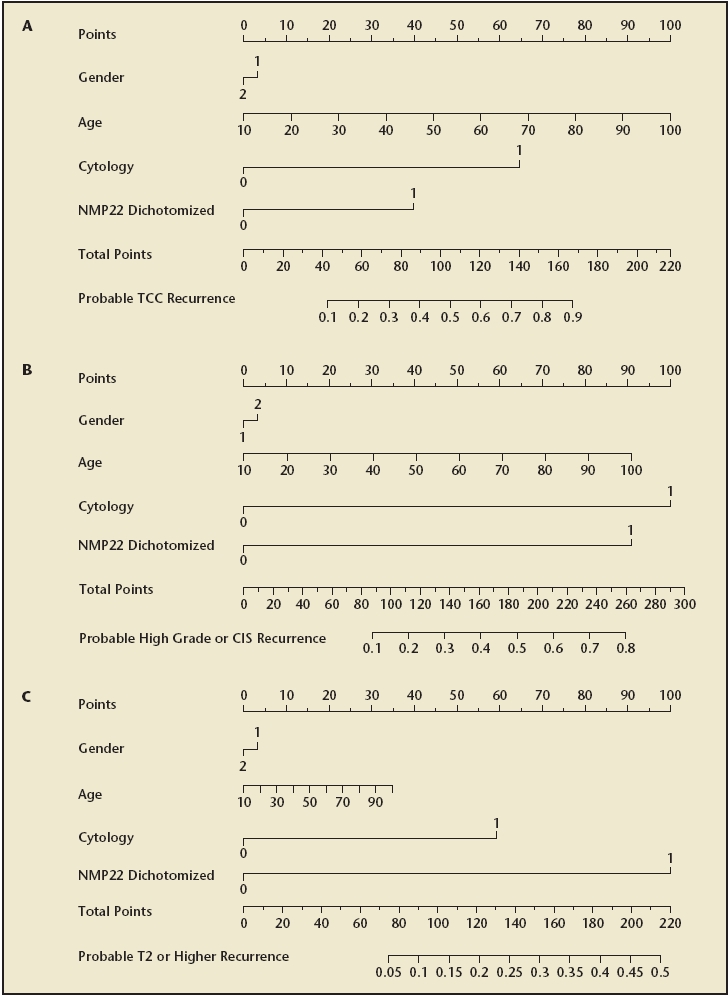
Nomograms for (A) recurence of any transitional cell carcinoma; (B) recurrence of grade 3 Ta or T1, or of carcinoma in situ (CIS); (C) recurrence of T2 or higher stage transitional cell carcinoma in 2681 patients who underwent office cystoscopy for detection of bladder cancer recurrence after treatment of stage Ta, T1, and/or CIS urothelial carcinoma of the urinary bladder. Reprinted from The Journal of Urology, Vol. 173, Shariat SF et al, “Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or CIS transitional cell carcinoma of the bladder,” pp. 1518–1525, Copyright American Urological Association 2005.
Recently, an in-office point-of-care test using NMP22 was introduced (NMP22® BladderChek®; Matritech). A multi-institutional trial revealed that the addition of the NMP22 BladderChek test to cystoscopy improves the detection rate of recurrent bladder cancer in patients with a history of bladder cancer.55 The NMP22 BladderChek test sensitivities were 50% and 90% for noninvasive and invasive cancer, respectively, with an overall sensitivity of 55.7%. In contrast, cytology performed poorly, with comparable sensitivities of 16.7% and 22.2% in noninvasive and invasive bladder cancers, respectively, with an overall sensitivity of 15.8%. Overall specificity was still higher for cytology at 99.2% compared with 85.7% for NMP22 BladderChek.
The NMP22 BladderChek test detected 8 of 9 cancers not detected by initial cystoscopy, including 7 that were considered aggressive. In contrast, urine cytology only detected 3 of the cancers missed by cystoscopy. The addition of the NMP22 BladderChek test to cystoscopy detected 94% of the cancers. Although this study was promising, concerns were raised regarding its findings. First, this study cites what is perhaps the lowest reported sensitivity for cytology. Unfortunately, cytologic assessment was not rigorously controlled or optimized. Moreover, with a sensitivity of 50%, use of the NMP22 BladderChek does not obviate the need for cystoscopy.
Finally, there is a question of the clinical value of a positive test result in the face of negative results on cystoscopy. The standard of care for patients with positive results on cytology in the face of negative cystoscopic findings is a trip to the operating room for bladder biopsies to exclude CIS. The 10% to 20% rate of false-positive results does not encourage one to take a patient to surgery, so many clinicians simply observe these patients. This raises patient and physician anxiety. One questions the value of a test that does not result in any further action or clear diagnostic information.
UroVysion
Multiple chromosomes, such as 1, 3, 4, 7, 8, 9, 11, and 17, are altered in urothelial tumors.56 These chromosomal alterations can be easily detected with fluorescence in situ hybridization (FISH) assay. UroVysion™ (Vysis, Downers Grove, IL) is a multitarget FISH assay that detects aneuploidy in chromosomes 3, 7, and 17 as well as loss of the 9p21 locus using a fluorescence microscope (Figure 4). This test has been approved by the FDA for both monitoring of patients with a history of bladder cancer and detection in patients with hematuria.
Figure 4.
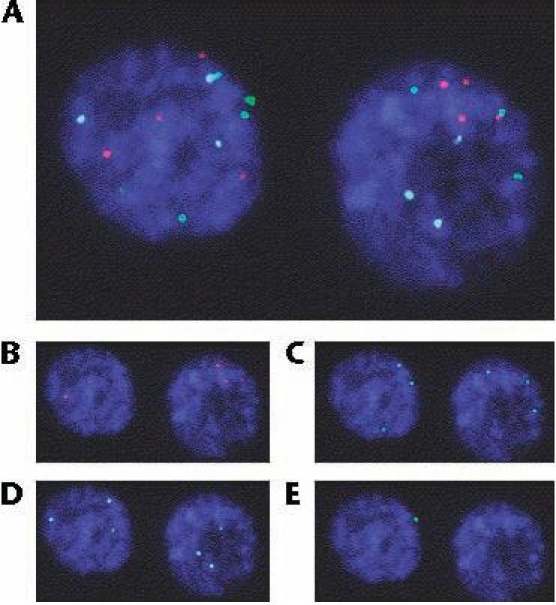
(A) Fluorescence in situ hybridization-positive nucleus; (B) chromosome 3, SpectrumRed; (C) chromosome 7, SpectrumGreen; (D) chromosome 17, SpectrumAqua; (E) locus 9p21, SpectrumGold. Reprinted from European Urology, Vol. 51, Moonen PM et al, “UroVysion compared with cytology and quantitative cytology in the surveillance of nonmuscle-invasive bladder cancer,” pp. 1275–1280, Copyright 2006, with permission from European Association of Urology.
Because such deoxyribonucleic acid (DNA) changes usually lead to morphologically abnormal cells, scanning the FISH slide focuses on the same cells as those considered during conventional cytology. Large or irregular nuclei or cells in clusters or those appearing otherwise suspicious are inspected for probe staining. Thus, FISH technology combines assessment of the morphologic changes of conventional cytology with molecular DNA changes. Each probe is a fluorescently labeled, single-stranded DNA fragment (nucleic acid sequence) complementary to specific target sequences of cellular DNA that are denatured to allow hybridization with the probe. Fluorescence microscopy allows visualization of the hybridized, labeled probe. The kit contains a mixture of unlabeled blocking DNA to suppress sequences contained within the target loci that are common to other chromosomes. Currently, no uniform criteria for a positive UroVysion assay exist. A minimum of 25 morphologically abnormal cells is viewed. At our institution, UroVysion assay is considered positive when 4 or more cells with multiple chromosomal gains of 3, 7, or 17 or 12 or more cells with loss of both copies of locus 9p21 exist.
In all published comparative studies, FISH outperforms cytology across all stages and grades of urothelial carcinoma.57–60 Although overall sensitivity of cytology was 48% and of FISH was 74%, the greatest advantage of FISH was in the detection of high-grade urothelial carcinoma, including CIS (67% vs 100% for cytology vs FISH, respectively). Cumulative data from comparative studies showed that the sensitivity of cytology compared with FISH was 19% versus 58% for grade 1, 50% versus 77% for grade 2, and 71% versus 96% for grade 3. Similar findings occurred by stage: sensitivity of cytology compared with FISH was 35% versus 64% for Ta, 66% versus 83% for T1, and 76% versus 94% for invasive carcinoma.
Despite these promising data, there are limitations to this test. Regardless of its high sensitivity, FISH depends on the presence of adequate numbers of exfoliated abnormal cells in the urine specimen. Thus, small urine volume, low tumor burden, or tumor cells that do not exfoliate will preclude the fulfillment of positivity criteria. Bladder washing can increase the number of cells available for inspection with FISH, increasing diagnostic yield. The usefulness of FISH for the detection of a nested variant urothelial carcinoma (the rare tumor completely covered by normal urothelium that prevents cellular shedding) and tumor in a diverticulum (which theoretically limits the cells shed into the voided specimen) remains to be determined.
Finally, FISH does not detect diploid cells without 9p21 deletions. There are other issues, such as high cost and the fact that this is not a point-of-care test, that limit its clinical usefulness. The poor positive predictive value leads to false-positive results. However, some researchers have argued that false-positive findings may indicate premalignant changes preceding the discovery of recurrent malignancy (what has been termed anticipatory positive results). Skacel and colleagues58 studied UroVysion in 120 patients. Nine patients had a falsepositive test result. However, 8 of those 9 patients (89%) had a positive bladder biopsy within 12 months of the UroVysion test. The ninth patient had a positive bladder biopsy at 15 months of follow-up. Unfortunately, one cannot justify a preemptive intervention, such as intravesical therapy, on the basis of an increased likelihood for recurrence, especially considering the baseline high recurrence rate of bladder tumors.
Bladder Cancer Tumor Markers Under Investigation
Urinary Bladder Cancer Test
IDL Biotech (Bromma, Sweden) recently developed UBC™, a point-of-care test that qualitatively measures cytokeratins 8 and 18 in the urine,61 and UBC™ enzyme-linked immunosorbent assay (ELISA), which is a 2-hour sandwich test.62 Cytokeratins are intermediate filament proteins that are characteristic of epithelial cells. A dark line on the test strip indicates a positive result.63 Mian and coworkers62 performed UBC tests on the urine of 180 patients and reported an overall sensitivity of 66% and specificity of 90%. This test requires investigation in multicenter trials.
BLCA-1 and BLCA-4
BLCA-1 is a nuclear transcription factor present in the tumor area of the bladder but not in adjacent benign tissue or nonmalignant bladder. BLCA-1 levels are increased in bladder cancer and with higher tumor stage. BLCA-4 is present in both the tumor and adjacent benign areas of the bladder but not in benign bladders.64 BLCA-4 is measured in the urine using ELISA, and its sensitivity ranges between 89% and 96%, with a specificity of 100% for bladder cancer (Figure 5).65,66 BLCA-1 and BLCA-4 seem to be promising markers for bladder cancer, with a high sensitivity and specificity. Randomized trials are needed to further study their usefulness on a larger scale.
Figure 5.
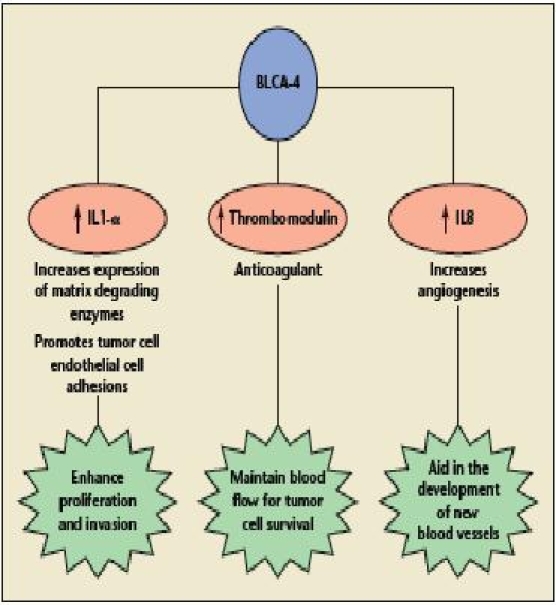
Model of potential BLCA-4 action. IL, interleukin. Reprinted with permission from Myers-Irvin JM et al.119
Hyaluronic Acid and Hyaluronidase
Hyaluronic acid (HA) is a nonsulfated glycosaminoglycan found in normal tissue and tissue fluids. When present in tumor tissues, it promotes metastasis and may interfere with immune surveillance.67 At a cutoff value of 100 ng/mL, urine HA had 92% sensitivity and 93% specificity for detecting bladder cancer.68
Hyaluronidase (HAase), an endoglycosidase, degrades HA into small fragments that promote angiogenesis.69 Hyaluronidase secretion by bladder tumor cells correlates with invasive potential. Pham and coworkers70 analyzed 139 urine specimens and detected a 5- to 8-fold elevation of hyaluronidase in the urine of patients with grade 2 or 3 bladder cancer. However, its widespread applicability may be somewhat limited; the accuracy of this test for detecting low-grade tumor is poor and may even be less than that of routine voided urine cytology. Further refinement in the assay technique and evaluation in larger clinical trials would help to define the clinical applicability of this marker.
Lewis X Antigen
Lewis-related antigens are cell-surface molecules divided into 4 subclasses, of which only the Lewis X group is associated with bladder cancer.63 The Lewis X antigen is expressed in epithelium from urothelial carcinoma, regardless of the tumor grade or stage.71 Overall, the sensitivity is approximately 80% with a specificity of 86%.72,73 The sensitivity increases to 95% when 2 consecutive urine samples are examined. Testing on more heterogeneous populations of patients is needed to determine the true specificity.
Microsatellite Analysis
Microsatellites are highly polymorphic DNA repeats (1 to 4 base pairs each) found throughout the human genome.74 These microsatellites can undergo mutations, leading to loss of heterogeneity and/or microsatellite instability, and thus can be used as markers of neoplasia. The most common genetic change in bladder cancer is loss of heterogeneity in chromosome 9.63 Chromosomes 4p, 8p, 9p, 11p, and 17p also often display loss of heterogeneity in patients with bladder cancer.75,76
Several studies have been conducted analyzing voided urine with 17 to 20 microsatellite markers.74,77 The overall sensitivity from these studies was 72% to 97%, and overall specificity was 80% to 100%. Although these studies demonstrated high sensitivity and specificity, all involved small numbers of patients, particularly in the control groups. In addition, with the currently available techniques, there is a need for expensive equipment and trained personnel.
Quanticyt
Bladder wash flow cytometry detecting aneuploid cells has initially yielded promising results. Later studies demonstrated that sensitivity was only marginally better than that of urine cytology, with slightly decreased specificity.78,79 Quanticyt™ (Gentian Scientific Software, Niawier, The Netherlands) is an automated karyometric image analysis system that evaluates nuclear shape and DNA content of exfoliated cells obtained from bladder washings. It then is able to assign a low-, intermediate-, or high-risk “score” for bladder cancer.80 Overall sensitivity of the Quanticyt system is 45% to 69% for detection of bladder cancer.17,48,80 The overall specificity is 70% to 93%.17,48,80 The test requires catheterization for a bladder wash specimen, because a relatively large number of cells are needed. The test is unlikely to become widely available because it requires technical expertise and expensive equipment.
Soluble Fas
The Fas signaling pathway is a primary mediator of apoptosis in various physiologic processes, such as tumor cell death mediated by cytotoxic T cells and natural killer cells, and maintenance of immune privileged sites, such as the eye, testis, brain, and maternal-fetal interface. Using a conventional ELISA assay, Svatek and associates81 showed that higher urinary levels of soluble Fas (sFas) are an independent predictor of bladder cancer recurrence and progression to invasive tumor stage, after controlling for the effects of cytology, NMP22, and patient age. This association remained significant in patients with a normal cytology assay result.
Although the overall performances for the detection of bladder cancer of urine sFas and NMP22 were not significantly different from each other, at sensitivity values above 75%, sFas had a consistently higher specificity than NMP22 (Figure 6). This is an important difference, because high sensitivity is desired to detect cancer, but poor specificity can lead to many false-positive findings with resultant unnecessary workups, patient morbidity, anxiety, and cost. However, these findings can only be considered preliminary. Before use as a bladder cancer marker, the sample acquisition protocols and the sFas assay need to be refined and standardized. In addition, before introduction to patient care, the findings of this study need to be confirmed in large, prospective, collaborative phase II/III trials.
Figure 6.
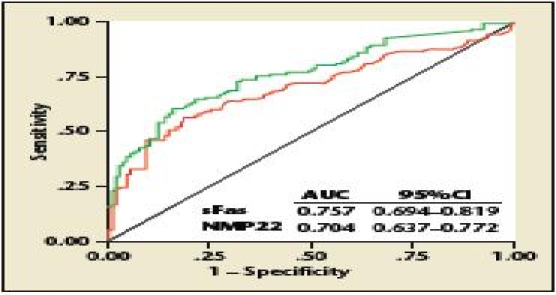
Receiver operating curves of soluble Fas (sFas) and nuclear matrix protein 22 (NMP22) for prediction of bladder cancer presence. AUC, area under the curve. From Svatek RS et al.81 Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Survivin
Survivin is a novel member of the inhibitor of apoptosis gene family that counteracts cell death, controls mitotic progression, and induces changes in gene expression that are associated with tumor cell invasiveness.82 Characteristically, survivin messenger ribonucleic acid (mRNA) is selectively expressed during embryonic and fetal development, becomes undetectable or expressed at low levels in most differentiated normal adult tissues, and is overexpressed in human cancers.
Survivin levels in urine are detected using a Bio-dot immunoassay incorporating a rabbit polyclonal antisurvivin antibody. Urinary levels of survivin gene activation, both at the protein and the mRNA level, are associated with bladder cancer presence, higher tumor grade, and advanced pathologic stage.83–85
In the largest study performed in bladder cancer, higher levels of survivin were found to correlate with increased risk of bladder cancer and higher-grade tumors but not with tumor invasion.84 In this study, survivin sensitivity was 64% with a specificity of 93%. After controlling for the effects of age, urine cytology, and urine NMP22, urinary survivin was independently associated with bladder presence and high-grade urothelial carcinoma.84 Another study reported that the survivin mRNA (obtained from bladder washings) copy number correlated with recurrence-free survival.85
The performance of the assay used in these studies does not comply with validation criteria appropriate for analytic techniques. Further studies are necessary to generate receiver operating characteristics analyses, which could then be used to establish biologic and clinically relevant cut points. Until then, this assay remains experimental, requiring further validation of its sensitivity, sensibility, reliability, and accuracy, as well as innovation and standardization.
Telomerase
Telomeres are repetitive sequences that cap the terminal ends of eukaryotic chromosomes. During each cell division, telomeres decrease in size. This leads to chromosomal instability and, eventually, to cell death.86 Telomerase is a ribonucleoprotein enzyme that helps to synthesize telomeres and thus maintain chromosomal ends.87–89 In normal somatic tissue, cells do not produce telomerase. Malignant neoplasms, including bladder cancer,90 have been shown to produce telomerase and thus to regenerate telomeres and prevent cell death.91
The standard technique to measure telomerase activity is the telomeric repeat amplification protocol assay (TRAP assay).91 Another telomerase-based assay involves detection of the catalytic subunit of telomerase, hTERT, using polymerase chain reaction (PCR). Because specialized equipment and trained personnel are needed, the testing must be done in a reference laboratory. When compared with TRAP, hTERT PCR has higher sensitivity than TRAP assay, ranging between 75% and 100%.92,93 The overall sensitivity of telomerase testing for detection of bladder cancer is between 7% and 100%, with the results of most studies between 70% and 86%.21,42,94–101 Examining the population studied or the technique of sample collection may explain the wide range of sensitivity.
The overall specificity of telomerase for bladder cancer is between 24% and 90%, with the results of most studies in the 60% to 70% range.21,42,94,96–102 However, because many bladder cancer patients have other urologic and nonurologic comorbidities, the clinical applicability of the telomerase assay could be limited. Another possible limitation of this test is the potential for inactivation of the telomerase enzyme in urine, leading to extremely low sensitivity (7% in 1 study).103 Considering the need for trained personnel in a reference laboratory and the wide range of results from different studies, telomerase assays are not useful in their current form for detection and monitoring of bladder cancer.
Conclusions
Noninvasive bladder cancer tests have many potential applications, and each should be assessed individually. These tests may eventually be used to screen patients in high-risk groups, to help diagnose or even predict recurrence, and to decrease the necessity of invasive testing. In analyzing the potential for bladder cancer tests as screening tools, an interesting comparison is the use of serum prostate-specific antigen (PSA) in prostate cancer. Patients can be screened annually with a PSA blood test and a digital rectal examination. In screening studies using the cut point of 4 ng/mL, the positive predictive value of PSA ranged from 17% to 38%, and the sensitivity ranged from 27% to 80%.104–109 The sensitivity for many of the bladder tumor markers is at least this high. The prevalence of bladder cancer is less than that for prostate cancer; therefore, widespread screening would lead to many false-positive tests and unnecessary, costly, and invasive workups. Screening only patients at high risk for bladder cancer could potentially produce similar results as screening with PSA.
Another potential use for bladder tumor markers is in the diagnosis of bladder cancer, either as an adjunct to or a replacement for current standard tests. Most of the comparative studies have shown that noninvasive tumor markers (BTA stat, BTA TRAK, NMP22, telomerase, Quanticyt, and UroVysion) have sensitivity for bladder cancer equal to or greater than that with cytology, even in high-grade cancers. Many other markers (ImmunoCyt, AccuDx [Intracel, Rockville, MD], HA-HAase, survivin, UBC ELISA, UBC Rapid, Lewis X antigen, microsatellite analysis) also have demonstrated high sensitivity for bladder cancer. None of these tests, however, meets all of the criteria of an ideal tumor marker. Some of the newer tests are close but require automation of the testing process to decrease the time and expense, or need additional testing on heterogeneous populations of patients to determine their accuracy. At this stage, the physician can choose either one of the currently available bladder tumor markers (eg, BTA stat, BTA TRAK, ImmunoCyt, NMP22, UroVysion) or cytology as adjunctive tests to cystoscopy in the follow-up of patients with bladder cancer.
Another potential role for bladder tumor markers is to decrease the necessity of invasive testing to improve patient comfort and decrease cost. It would be reasonable to consider decreasing the number of cystoscopies performed by alternating the endoscopic examination with a bladder tumor marker test. This could be done in patients with a history of low-grade, stage Ta urothelial carcinoma who are at low risk of progression.110 If the marker misses a low-grade recurrence, it would be picked up by the next cystoscopy with little risk of progression. On the other hand, cystoscopy is performed in the office with local anesthesia and a small-caliber flexible endoscope, which is minimally invasive in men and even less invasive in women.
The psychological impact of testing patients with markers versus a visual inspection also should be considered. Will the patient be able to sleep at night if he or she knows the physician did not “see” any cancer? Will patients accept the use of a marker? Vriesema and colleagues111 found that patients chose flexible cystoscopy over a bladder tumor marker when the sensitivity of the marker was less than 90%. These markers are intended to provide an accurate, less invasive alternative. However, it is possible that if the marker does not have a high specificity, it could, in fact, lead to more invasive tests. If the patient had a “false-positive” marker test result (positive for marker, but negative on cystoscopy), will this lead the clinician to search for the cancer before it can be called a false-positive? It may lead to upper urinary tract imaging or endoscopy to rule out disease in those areas. Therefore, the full evaluation of patients with “false-positive” test results should be considered in research settings to determine the answer to this question: When is a false-positive a real false-positive? Much progress in developing bladder cancer markers has been made in the last 5 years. With more research on these and other markers not reviewed in this article, the role for bladder cancer markers will be further defined. Until then, cystoscopy and urinary cytology still represent the gold standard for diagnosis of bladder cancer.
Main Points.
An accurate bladder tumor marker would be useful for both screening of high-risk populations and for monitoring of patients with a history of bladder cancer to help identify recurrence early and prevent disease progression.
Most comparative studies have shown that noninvasive tumor markers have sensitivity for bladder cancer equal to or greater than that with cytology, even in high-grade cancers. However, none meets all of the criteria of an ideal tumor marker.
At this stage, the physician can choose either one of the currently available bladder tumor markers (eg, BTA stat, BTA TRAK, ImmunoCyt, nuclear matrix protein 22, UroVysion) or cytology as adjunctive tests to cystoscopy in the follow-up of patients with bladder cancer.
It would be reasonable to consider decreasing the number of cystoscopies performed by alternating the endoscopic examination with a bladder tumor marker test.
The psychological impact of testing patients with markers versus a visual inspection also should be considered.
With more research, the role for bladder cancer markers will be further defined. Until then, cystoscopy and urinary cytology still represent the gold standard for diagnosis of bladder cancer.
Another potential role for bladder tumor markers is to decrease the necessity of invasive testing to improve patient comfort and decrease cost. It would be reasonable to consider decreasing the number of cystoscopies performed by alternating the endoscopic examination with a bladder tumor marker test. This could be done in patients with a history of low-grade, stage Ta urothelial carcinoma who are at low risk of progression.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Prout GR, Jr, Barton BA, Griffin PP, Friedell GH. Treated history of noninvasive grade 1 transitional cell carcinoma. The National Bladder Cancer Group. J Urol. 1992;148:1413–1419. doi: 10.1016/s0022-5347(17)36924-0. [DOI] [PubMed] [Google Scholar]
- 3.Pagano F, Bassi P, Galetti TP, et al. Results of contemporary radical cystectomy for invasive bladder cancer: a clinicopathological study with an emphasis on the inadequacy of the tumor, nodes and metastases classification. J Urol. 1991;145:45–50. doi: 10.1016/s0022-5347(17)38244-7. [DOI] [PubMed] [Google Scholar]
- 4.Stein JP, Grossfeld GD, Ginsberg DA, et al. Prognostic markers in bladder cancer: a contemporary review of the literature. J Urol. 1998;160:645–659. doi: 10.1016/S0022-5347(01)62747-2. [DOI] [PubMed] [Google Scholar]
- 5.van der Poel HG, Debruyne FM. Can biological markers replace cystoscopy? An update. Curr Opin Urol. 2001;11:503–509. doi: 10.1097/00042307-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Herr HW. The natural history of a T1 bladder cancer: life-long tumour diathesis. BJU Int. 1999;84:1102–1103. doi: 10.1046/j.1464-410x.1999.00370.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidbauer J, Witjes F, Schmeller N, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004;171:135–138. doi: 10.1097/01.ju.0000100480.70769.0e. [DOI] [PubMed] [Google Scholar]
- 8.Jichlinski P, Guillou L, Karlsen SJ, et al. Hexyl aminolevulinate fluorescence cystoscopy: new diagnostic tool for photodiagnosis of superficial bladder cancer—a multicenter study. J Urol. 2003;170:226–229. doi: 10.1097/01.ju.0000060782.52358.04. [DOI] [PubMed] [Google Scholar]
- 9.Riedl CR, Daniltchenko D, Koenig F, et al. Fluorescence endoscopy with 5-aminolevulinic acid reduces early recurrence rate in superficial bladder cancer. J Urol. 2001;165:1121–1123. [PubMed] [Google Scholar]
- 10.Wiener HG, Vooijs GP. van’t Hof-Grootenboer B. Accuracy of urinary cytology in the diagnosis of primary and recurrent bladder cancer. Acta Cytol. 1993;37:163–169. [PubMed] [Google Scholar]
- 11.Karakiewicz PI, Benayoun S, Zippe C, et al. Institutional variability in the accuracy of urinary cytology for predicting recurrence of transitional cell carcinoma of the bladder. BJU Int. 2006;97:997–1001. doi: 10.1111/j.1464-410X.2006.06036.x. [DOI] [PubMed] [Google Scholar]
- 12.Lokeshwar V, Soloway M. Current bladder tumor tests: does their projected utility fulfill clinical necessity? J Urol. 2001;165:1067–1077. [PubMed] [Google Scholar]
- 13.Svatek RS, Sagalowsky AI, Lotan Y. Economic impact of screening for bladder cancer using bladder tumor markers: a decision analysis. Urol Oncol. 2006;24:338–343. doi: 10.1016/j.urolonc.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Lotan Y, Svatek RS, Sagalowsky AI. Should we screen for bladder cancer in a high-risk population? A cost per life-year saved analysis. Cancer. 2006;107:982–990. doi: 10.1002/cncr.22084. [DOI] [PubMed] [Google Scholar]
- 15.Johnston B, Morales A, Emerson L, Lundie M. Rapid detection of bladder cancer: a comparative study of point of care tests. J Urol. 1997;158:2098–2101. doi: 10.1016/s0022-5347(01)68166-7. [DOI] [PubMed] [Google Scholar]
- 16.Kinders R, Jones T, Root R, et al. Complement factor H or a related protein is a marker for transitional cell cancer of the bladder. Clin Cancer Res. 1998;4:2511–2520. [PubMed] [Google Scholar]
- 17.Wiener HG, Mian C, Haitel A, et al. Can urine bound diagnostic tests replace cystoscopy in the management of bladder cancer? J Urol. 1998;159:1876–1880. doi: 10.1016/S0022-5347(01)63184-7. [DOI] [PubMed] [Google Scholar]
- 18.Takashi M, Schenck U, Kissel K, et al. Use of diagnostic categories in urinary cytology in comparison with the bladder tumour antigen (BTA) test in bladder cancer patients. Int Urol Nephrol. 1999;31:189–196. doi: 10.1023/a:1007124724817. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Zippe CD, Pandrangi L, et al. Exclusion criteria enhance the specificity and positive predictive value of NMP22 and BTA stat. J Urol. 1999;162:53–57. doi: 10.1097/00005392-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez Banos JL, Martin Garcia B, Hernandez Rodriguez R, et al. Usefulness of BTA Stat test (Bard) in the diagnosis of bladder cancer. Preliminary results and comparison with cytology and cystoscopy. Arch Esp Urol. 1998;51:778–782. [PubMed] [Google Scholar]
- 21.Landman J, Chang Y, Kavaler E, et al. Sensitivity and specificity of NMP-22, telomerase, and BTA in the detection of human bladder cancer. Urology. 1998;52:398–402. doi: 10.1016/s0090-4295(98)00219-2. [DOI] [PubMed] [Google Scholar]
- 22.Leyh H, Mazeman E. Bard BTA test compared with voided urine cytology in the diagnosis of recurrent bladder cancer. Eur Urol. 1997;32:425–428. [PubMed] [Google Scholar]
- 23.Leyh H, Marberger M, Conort P, et al. Comparison of the BTA stat test with voided urine cytology and bladder wash cytology in the diagnosis and monitoring of bladder cancer. Eur Urol. 1999;35:52–56. doi: 10.1159/000019819. [DOI] [PubMed] [Google Scholar]
- 24.Nasuti JF, Gomella LG, Ismial M, Bibbo M. Utility of the BTA stat test kit for bladder cancer screening. Diagn Cytopathol. 1999;21:27–29. doi: 10.1002/(sici)1097-0339(199907)21:1<27::aid-dc8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Oge O, Kozaci D, Gemalmaz H. The BTA stat test is nonspecific for hematuria: an experimental hematuria model. J Urol. 2002;167:1318–1319. doi: 10.1016/s0022-5347(05)65290-1. discussion 1319–1320. [DOI] [PubMed] [Google Scholar]
- 26.Malkowicz SB. The application of human complement factor H-related protein (BTA TRAK) in monitoring patients with bladder cancer. Urol Clin North Am. 2000;27:63–73. ix. doi: 10.1016/s0094-0143(05)70235-4. [DOI] [PubMed] [Google Scholar]
- 27.Thomas L, Leyh H, Marberger M, et al. Multicenter trial of the quantitative BTA TRAK assay in the detection of bladder cancer. Clin Chem. 1999;45:472–477. [PubMed] [Google Scholar]
- 28.Mahnert B, Tauber S, Kriegmair M, et al. BTATRAK—a useful diagnostic tool in urinary bladder cancer? Anticancer Res. 1999;19:2615–2619. [PubMed] [Google Scholar]
- 29.Irani J, Desgrandchamps F, Millet C, et al. BTA stat and BTA TRAK: a comparative evaluation of urine testing for the diagnosis of transitional cell carcinoma of the bladder. Eur Urol. 1999;35:89–92. doi: 10.1159/000019824. [DOI] [PubMed] [Google Scholar]
- 30.Heicappell R, Wettig IC, Schostak M, et al. Quantitative detection of human complement factor H-related protein in transitional cell carcinoma of the urinary bladder. Eur Urol. 1999;35:81–87. doi: 10.1159/000019822. [DOI] [PubMed] [Google Scholar]
- 31.Ellis WJ, Blumenstein BA, Ishak LM, Enfield DL. Clinical evaluation of the BTA TRAK assay and comparison to voided urine cytology and the Bard BTA test in patients with recurrent bladder tumors. Urology. 1997;50:882–887. doi: 10.1016/s0090-4295(97)00508-6. [DOI] [PubMed] [Google Scholar]
- 32.Abbate I, D’Introno A, Cardo G, et al. Comparison of nuclear matrix protein 22 and bladder tumor antigen in urine of patients with bladder cancer. Anticancer Res. 1998;18:3803–3805. [PubMed] [Google Scholar]
- 33.Fradet Y, Lockhard C. Performance characteristics of a new monoclonal antibody test for bladder cancer: ImmunoCyt. Can J Urol. 1997;4:400–405. [PubMed] [Google Scholar]
- 34.Olsson H, Zackrisson B. ImmunoCyt a useful method in the follow-up protocol for patients with urinary bladder carcinoma. Scand J Urol Nephrol. 2001;35:280–282. doi: 10.1080/003655901750425846. [DOI] [PubMed] [Google Scholar]
- 35.Mian C, Pycha A, Wiener H, et al. Immunocyt: a new tool for detecting transitional cell cancer of the urinary tract. J Urol. 1999;161:1486–1489. doi: 10.1016/s0022-5347(05)68934-3. [DOI] [PubMed] [Google Scholar]
- 36.Vriesema JL, Atsma F, Kiemeney LA, et al. Diagnostic efficacy of the ImmunoCyt test to detect superficial bladder cancer recurrence. Urology. 2001;58:367–371. doi: 10.1016/s0090-4295(01)01217-1. [DOI] [PubMed] [Google Scholar]
- 37.Gordon JN, Shu WP, Schlussel RN, et al. Altered extracellular matrices influence cellular processes and nuclear matrix organisations of overlying human bladder urothelial cells. Cancer Res. 1993;53:4971–4977. [PubMed] [Google Scholar]
- 38.Berezney R, Coffey DS. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974;60:1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- 39.Pardoll DM, Vogelstein B, Coffey DS. A fixed site of DNA replication in eukaryotic cells. Cell. 1980;19:527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- 40.Compton DA, Cleveland DW. NuMA is required for the proper completion of mitosis. J Cell Biol. 1993;120:947–957. doi: 10.1083/jcb.120.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shelfo SW, Soloway MS. The role of nuclear matrix protein 22 in the detection of persistent or recurrent transitional-cell cancer of the bladder. World J Urol. 1997;15:107–111. doi: 10.1007/BF02201981. [DOI] [PubMed] [Google Scholar]
- 42.Ramakumar S, Bhuiyan J, Besse JA, et al. Comparison of screening methods in the detection of bladder cancer. J Urol. 1999;161:388–394. [PubMed] [Google Scholar]
- 43.Miyanaga N, Akaza H, Tsukamoto T, et al. Urinary nuclear matrix protein 22 as a new marker for the screening of urothelial cancer in patients with microscopic hematuria. Int J Urol. 1999;6:173–177. doi: 10.1046/j.1442-2042.1999.06437.x. [DOI] [PubMed] [Google Scholar]
- 44.Hughes JH, Katz RL, Rodriguez-Villanueva J, et al. Urinary nuclear matrix protein 22 (NMP22): a diagnostic adjunct to urine cytologic examination for the detection of recurrent transitional-cell carcinoma of the bladder. Diagn Cytopathol. 1999;20:285–290. doi: 10.1002/(sici)1097-0339(199905)20:5<285::aid-dc7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 45.Zippe C, Pandrangi L, Potts JM, et al. NMP22: a sensitive, cost-effective test in patients at risk for bladder cancer. Anticancer Res. 1999;19:2621–2623. [PubMed] [Google Scholar]
- 46.Del Nero A, Esposito N, Curro A, et al. Evaluation of urinary level of NMP22 as a diagnostic marker for stage pTa-pT1 bladder cancer: comparison with urinary cytology and BTA test. Eur Urol. 1999;35:93–97. doi: 10.1159/000019825. [DOI] [PubMed] [Google Scholar]
- 47.Serretta V, Lo Presti, Vasile P, et al. Urinary NMP22 for the detection of recurrence after transurethral resection of transitional cell carcinoma of the bladder: experience on 137 patients. Urology. 1998;52:793–796. doi: 10.1016/s0090-4295(98)00332-x. [DOI] [PubMed] [Google Scholar]
- 48.Witjes JA, van der Poel HG, van Balken MR, et al. Urinary NMP22 and karyometry in the diagnosis and follow-up of patients with superficial bladder cancer. Eur Urol. 1998;33:387–391. doi: 10.1159/000019621. [DOI] [PubMed] [Google Scholar]
- 49.Stampfer D, Carpinito G, Rodriguez-Villanueva J, et al. Evaluation of NMP22 in the detection of transitional cell carcinoma of the bladder. J Urol. 1998;159:394–398. doi: 10.1016/s0022-5347(01)63930-2. [DOI] [PubMed] [Google Scholar]
- 50.Soloway MS, Briggman V, Carpinito GA, et al. Use of new tumor marker, urinary NMP22, in detection of occult or rapidly recurring transitional cell carcinoma of the urinary tract following surgical treatment. J Urol. 1996;156:363–367. doi: 10.1097/00005392-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Ponsky LE, Sharma S, Pandrangi L, et al. Screening and monitoring for bladder cancer: refining the use of NMP22. J Urol. 2001;166:75–78. [PubMed] [Google Scholar]
- 52.Shariat SF, Marberger MJ, Lotan Y, et al. Variability in the performance of nuclear matrix protein 22 for the detection of bladder cancer. J Urol. 2006;176:919–926. doi: 10.1016/j.juro.2006.04.017. discussion 926. [DOI] [PubMed] [Google Scholar]
- 53.Shariat SF, Casella R, Wians FH, Jr, et al. Risk stratification for bladder tumor recurrence, stage and grade by urinary nuclear matrix protein 22 and cytology. Eur Urol. 2004;45:304–313. doi: 10.1016/j.eururo.2003.10.020. author reply 313. [DOI] [PubMed] [Google Scholar]
- 54.Shariat SF, Zippe C, Ludecke G, et al. Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or CIS transitional cell carcinoma of the bladder. J Urol. 2005;173:1518–1525. doi: 10.1097/01.ju.0000154696.48217.75. [DOI] [PubMed] [Google Scholar]
- 55.Grossman HB, Soloway M, Messing E, et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295:299–305. doi: 10.1001/jama.295.3.299. [DOI] [PubMed] [Google Scholar]
- 56.Junker K, Boerner D, Schulze W, et al. Analysis of genetic alterations in normal bladder urothelium. Urology. 2003;62:1134–1138. doi: 10.1016/s0090-4295(03)00692-7. [DOI] [PubMed] [Google Scholar]
- 57.Halling KC. Vysis UroVysion for the detection of urothelial carcinoma. Expert Rev Mol Diagn. 2003;3:507–519. doi: 10.1586/14737159.3.4.507. [erratum in Expert Rev Mol Diagn. 2004;4:266] [DOI] [PubMed] [Google Scholar]
- 58.Skacel M, Fahmy M, Brainard JA, et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J Urol. 2003;169:2101–2105. doi: 10.1097/01.ju.0000066842.45464.cc. [DOI] [PubMed] [Google Scholar]
- 59.Friedrich MG, Hellstern A, Toma MI, et al. Are false-positive urine markers for the detection of bladder carcinoma really wrong or do they predict tumor recurrence? Eur Urol. 2003;43:146–150. doi: 10.1016/s0302-2838(02)00555-9. discussion 150–151. [DOI] [PubMed] [Google Scholar]
- 60.Halling KC, King W, Sokolova IA, et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J Urol. 2002;167:2001–2006. [PubMed] [Google Scholar]
- 61.Heicappell R, Schostak M, Muller M, Miller K. Evaluation of urinary bladder cancer antigen as a marker for diagnosis of transitional cell carcinoma of the urinary bladder. Scand J Clin Lab Invest. 2000;60:275–282. doi: 10.1080/003655100750046431. [DOI] [PubMed] [Google Scholar]
- 62.Mian C, Lodde M, Haitel A, et al. Comparison of two qualitative assays, the UBC rapid test and the BTA stat test, in the diagnosis of urothelial cell carcinoma of the bladder. Urology. 2000;56:228–231. doi: 10.1016/s0090-4295(00)00664-6. [DOI] [PubMed] [Google Scholar]
- 63.Saad A, Hanbury DC, McNicholas TA, et al. The early detection and diagnosis of bladder cancer: a critical review of the options. Eur Urol. 2001;39:619–633. doi: 10.1159/000052519. [DOI] [PubMed] [Google Scholar]
- 64.Konety BR, Nguyen TS, Dhir R, et al. Detection of bladder cancer using a novel nuclear matrix protein, BLCA-4. Clin Cancer Res. 2000;6:2618–2625. [PubMed] [Google Scholar]
- 65.Van Le TS, Myers J, Konety BR, et al. Functional characterization of the bladder cancer marker, BLCA-4. Clin Cancer Res. 2004;10:1384–1391. doi: 10.1158/1078-0432.ccr-0455-03. [DOI] [PubMed] [Google Scholar]
- 66.Van Le TS, Miller R, Barder T, et al. Highly specific urine-based marker of bladder cancer. Urology. 2005;66:1256–1260. doi: 10.1016/j.urology.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 67.Knudson W. Tumor-associated hyaluronan. Providing an extracellular matrix that facilitates invasion. Am J Pathol. 1996;148:1721–1726. [PMC free article] [PubMed] [Google Scholar]
- 68.Lokeshwar VB, Obek C, Soloway MS, Block NL. Tumor-associated hyaluronic acid: a new sensitive and specific urine marker for bladder cancer. Cancer Res. 1997;57:773–777. [erratum in Cancer Res. 1998;58:3191] [PubMed] [Google Scholar]
- 69.Lokeshwar VB, Block NL. HA-HAase urine test. A sensitive and specific method for detecting bladder cancer and evaluating its grade. Urol Clin North Am. 2000;27:53–61. doi: 10.1016/s0094-0143(05)70234-2. [DOI] [PubMed] [Google Scholar]
- 70.Pham HT, Block NL, Lokeshwar VB. Tumor-derived hyaluronidase: a diagnostic urine marker for high-grade bladder cancer. Cancer Res. 1997;57:778–783. [erratum in Cancer Res. 1997;57:1622] [PubMed] [Google Scholar]
- 71.Decenzo JM, Howard P, Irish CE. Antigenic deletion and prognosis of patients with stage A transitional cell bladder carcinoma. J Urol. 1975;114:874–878. doi: 10.1016/s0022-5347(17)67163-5. [DOI] [PubMed] [Google Scholar]
- 72.Golijanin D, Sherman Y, Shapiro A, Pode D. Detection of bladder tumors by immunostaining of the Lewis X antigen in cells from voided urine. Urology. 1995;46:173–177. doi: 10.1016/s0090-4295(99)80189-7. [DOI] [PubMed] [Google Scholar]
- 73.Pode D, Golijanin D, Sherman Y, et al. Immunostaining of Lewis X in cells from voided urine, cytopathology and ultrasound for noninvasive detection of bladder tumors. J Urol. 1998;159:389–392. doi: 10.1016/s0022-5347(01)63928-4. [DOI] [PubMed] [Google Scholar]
- 74.van Rhijn BW, Lurkin I, Kirkels WJ, et al. Microsatellite analysis—DNA test in urine competes with cystoscopy in follow-up of superficial bladder carcinoma: a phase II trial. Cancer. 2001;92:768–775. doi: 10.1002/1097-0142(20010815)92:4<768::aid-cncr1381>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 75.Czerniak B, Chaturvedi V, Li L, et al. Superim-posed histologic and genetic mapping of chromosome 9 in progression of human urinary bladder neoplasia: implications for a genetic model of multistep urothelial carcinogenesis and early detection of urinary bladder cancer. Oncogene. 1999;18:1185–1196. doi: 10.1038/sj.onc.1202385. [DOI] [PubMed] [Google Scholar]
- 76.Knowles MA, Elder PA, Williamson M, et al. Allelotype of human bladder cancer. Cancer Res. 1994;54:531–538. [PubMed] [Google Scholar]
- 77.von Knobloch R, Hegele A, Brandt H, et al. Serum DNA and urine DNA alterations of urinary transitional cell bladder carcinoma detected by fluorescent microsatellite analysis. Int J Cancer. 2001;94:67–72. doi: 10.1002/ijc.1434. [DOI] [PubMed] [Google Scholar]
- 78.Gregoire M, Fradet Y, Meyer F, et al. Diagnostic accuracy of urinary cytology, and deoxyribonucleic acid flow cytometry and cytology on bladder washings during followup for bladder tumors. J Urol. 1997;157:1660–1664. [PubMed] [Google Scholar]
- 79.Cajulis RS, Haines GK, 3rd, Frias-Hidvegi D, et al. Cytology, flow cytometry, image analysis, and interphase cytogenetics by fluorescence in situ hybridization in the diagnosis of transitional cell carcinoma in bladder washes: a comparative study. Diagn Cytopathol. 1995;13:214–223. doi: 10.1002/dc.2840130307. discussion 224. [DOI] [PubMed] [Google Scholar]
- 80.van der Poel HG, van Balken MR, Schamhart DH, et al. Bladder wash cytology, quantitative cytology, and the qualitative BTA test in patients with superficial bladder cancer. Urology. 1998;51:44–50. doi: 10.1016/s0090-4295(97)00496-2. [DOI] [PubMed] [Google Scholar]
- 81.Svatek RS, Herman MP, Lotan Y, et al. Soluble Fas—a promising novel urinary marker for the detection of recurrent superficial bladder cancer. Cancer. 2006;106:1701–1707. doi: 10.1002/cncr.21795. [DOI] [PubMed] [Google Scholar]
- 82.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 83.Smith SD, Wheeler MA, Plescia J, et al. Urine detection of survivin and diagnosis of bladder cancer. JAMA. 2001;285:324–328. doi: 10.1001/jama.285.3.324. [DOI] [PubMed] [Google Scholar]
- 84.Shariat SF, Casella R, Khoddami SM, et al. Urine detection of survivin is a sensitive marker for the noninvasive diagnosis of bladder cancer. J Urol. 2004;171(2 pt 1):626–630. doi: 10.1097/01.ju.0000107826.78479.90. [DOI] [PubMed] [Google Scholar]
- 85.Schultz IJ, Kiemeney LA, Karthaus HF, et al. Survivin mRNA copy number in bladder washings predicts tumor recurrence in patients with superficial urothelial cell carcinomas. Clin Chem. 2004;50:1425–1428. doi: 10.1373/clinchem.2004.032003. [DOI] [PubMed] [Google Scholar]
- 86.Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 87.Blackburn EH. Telomeres and their synthesis. Science. 1990;249:489–490. doi: 10.1126/science.2200120. [DOI] [PubMed] [Google Scholar]
- 88.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 89.Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 90.Ito H, Kyo S, Kanaya T, et al. Expression of human telomerase subunits and correlation with telomerase activity in urothelial cancer. Clin Cancer Res. 1998;4:1603–1608. [PubMed] [Google Scholar]
- 91.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 92.De Kok JB, Schalken JA, Aalders TW, et al. Quantitative measurement of telomerase reverse transcriptase (hTERT) mRNA in urothelial cell carcinomas. Int J Cancer. 2000;87:217–220. [PubMed] [Google Scholar]
- 93.Melissourgos N, Kastrinakis NG, Davilas I, et al. Detection of human telomerase reverse transcriptase mRNA in urine of patients with bladder cancer: evaluation of an emerging tumor marker. Urology. 2003;62:362–367. doi: 10.1016/s0090-4295(03)00254-1. [DOI] [PubMed] [Google Scholar]
- 94.Wu X, Kakehi Y, Takahashi T, et al. Telomerase activity in urine after transurethral resection of superficial bladder cancer and early recurrence. Int J Urol. 2000;7:210–217. doi: 10.1046/j.1442-2042.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 95.Cheng CW, Chueh SC, Chern HD. Diagnosis of bladder cancer using telomerase activity in voided urine. J Formos Med Assoc. 2000;99:920–925. [PubMed] [Google Scholar]
- 96.Cassel A, Rahat MA, Lahat N, et al. Telomerase activity and cytokeratin 20 as markers for the detection and followup of transitional cell carcinoma: an unfulfilled promise. J Urol. 2001;166:841–844. [PubMed] [Google Scholar]
- 97.Kavaler E, Landman J, Chang Y, et al. Detecting human bladder carcinoma cells in voided urine samples by assaying for the presence of telomerase activity. Cancer. 1998;82:708–714. doi: 10.1002/(sici)1097-0142(19980215)82:4<708::aid-cncr14>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 98.Lee DH, Yang SC, Hong SJ, et al. Telomerase: a potential marker of bladder transitional cell carcinoma in bladder washes. Clin Cancer Res. 1998;4:535–538. [PubMed] [Google Scholar]
- 99.Dalbagni G, Han W, Zhang ZF, et al. Evaluation of the telomeric repeat amplification protocol (TRAP) assay for telomerase as a diagnostic modality in recurrent bladder cancer. Clin Cancer Res. 1997;3:1593–1598. [PubMed] [Google Scholar]
- 100.Yokota K, Kanda K, Inoue Y, et al. Semi-quantitative analysis of telomerase activity in exfoliated human urothelial cells and bladder transitional cell carcinoma. Br J Urol. 1998;82:727–732. doi: 10.1046/j.1464-410x.1998.00827.x. [DOI] [PubMed] [Google Scholar]
- 101.Mayfield MP, Shah T, Flannigan GM, et al. Telomerase activity in malignant and benign bladder conditions. Int J Mol Med. 1998;1:835–840. doi: 10.3892/ijmm.1.5.835. [DOI] [PubMed] [Google Scholar]
- 102.Lee MY, Tsou MH, Cheng MH, et al. Clinical application of NMP22 and urinary cytology in patients with hematuria or a history of urothelial carcinoma. World J Urol. 2000;18:401–405. doi: 10.1007/s003450000124. [DOI] [PubMed] [Google Scholar]
- 103.Muller M, Krause H, Heicappell R, et al. Comparison of human telomerase RNA and telomerase activity in urine for diagnosis of bladder cancer. Clin Cancer Res. 1998;4:1949–1954. [PubMed] [Google Scholar]
- 104.Crawford ED, DeAntoni EP, Etzioni R, et al. Serum prostate-specific antigen and digital rectal examination for early detection of prostate cancer in a national community-based program. The Prostate Cancer Education Council. Urology. 1996;47:863–869. doi: 10.1016/s0090-4295(96)00061-1. [DOI] [PubMed] [Google Scholar]
- 105.Crawford ED, Leewansangtong S, Goktas S, et al. Efficiency of prostate-specific antigen and digital rectal examination in screening, using 4.0 ng/ml and age-specific reference range as a cutoff for abnormal values. Prostate. 1999;38:296–302. doi: 10.1002/(sici)1097-0045(19990301)38:4<296::aid-pros5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 106.Gustafsson O, Mansour E, Norming U, et al. Prostate-specific antigen (PSA), PSA density and age-adjusted PSA reference values in screening for prostate cancer—a study of a randomly selected population of 2,400 men. Scand J Urol Nephrol. 1998;32:373–377. doi: 10.1080/003655998750015124. [DOI] [PubMed] [Google Scholar]
- 107.Filella X, Molina R, Ballesta AM, et al. Value of PSA (prostate-specific antigen) in the detection of prostate cancer in patients with urological symptoms. Results of a multicentre study. Eur J Cancer. 1996;32A:1125–1128. doi: 10.1016/0959-8049(96)00092-5. [DOI] [PubMed] [Google Scholar]
- 108.Ito K, Kubota Y, Suzuki K, et al. Correlation of prostate-specific antigen before prostate cancer detection and clinicopathologic features: evaluation of mass screening populations. Urology. 2000;55:705–709. doi: 10.1016/s0090-4295(99)00568-3. [DOI] [PubMed] [Google Scholar]
- 109.Kihara T, Ito F, Kobayashi C, et al. Utility of prostate specific antigen (PSA) density in detecting prostate cancer in men showing gray zone serum PSA levels. Hinyokika Kiyo. 1997;43:13–18. [PubMed] [Google Scholar]
- 110.Soloway MS. Bladder tumor markers, intravesical therapy and systemic chemotherapy. J Urol. 2001;166:488–489. [PubMed] [Google Scholar]
- 111.Vriesema JL, Poucki MH, Kiemeney LA, Witjes JA. Patient opinion of urinary tests versus flexible urethrocystoscopy in follow-up examination for superficial bladder cancer: a utility analysis. Urology. 2000;56:793–797. doi: 10.1016/s0090-4295(00)00777-9. [DOI] [PubMed] [Google Scholar]
- 112.Schmetter BS, Habicht KK, Lamm DL, et al. A multicenter trial evaluation of the fibrin/fibrinogen degradation products test for detection and monitoring of bladder cancer. J Urol. 1997;158:801–805. doi: 10.1097/00005392-199709000-00029. [DOI] [PubMed] [Google Scholar]
- 113.Pode D, Shapiro A, Wald M, et al. Noninvasive detection of bladder cancer with the BTA stat test. J Urol. 1999;161:443–446. [PubMed] [Google Scholar]
- 114.Sarosdy MF, Hudson MA, Ellis WJ, et al. Improved detection of recurrent bladder cancer using the Bard BTA stat Test. Urology. 1997;50:349–353. doi: 10.1016/s0090-4295(97)00292-6. [DOI] [PubMed] [Google Scholar]
- 115.Yoshida K, Sugino T, Tahara H, et al. Telomerase activity in bladder carcinoma and its implication for noninvasive diagnosis by detection of exfoliated cancer cells in urine. Cancer. 1997;79:362–369. doi: 10.1002/(sici)1097-0142(19970115)79:2<362::aid-cncr20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 116.Sumi S, Arai K, Kitahara S, Yoshida KI. Preliminary report of the clinical performance of a new urinary bladder cancer antigen test: comparison to voided urine cytology in the detection of transitional cell carcinoma of the bladder. Clin Chim Acta. 2000;296:111–120. doi: 10.1016/s0009-8981(00)00208-4. [DOI] [PubMed] [Google Scholar]
- 117.Sarosdy MF, Schellhammer P, Bokinsky G, et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. J Urol. 2002;168:1950–1954. doi: 10.1016/S0022-5347(05)64270-X. [DOI] [PubMed] [Google Scholar]
- 118.Moonen PM, Merkx GF, Peelen P, et al. UroVysion compared with cytology and quantitative cytology in the surveillance of non-muscle-invasive bladder cancer. Eur Urol. 2007;51:1275–1280. doi: 10.1016/j.eururo.2006.10.044. discussion 1280. [DOI] [PubMed] [Google Scholar]
- 119.Myers-Irvin JM, Van Le TS, Getzenberg RH. Mechanistic analysis of the role of BLCA-4 in bladder cancer pathobiology. Cancer Res. 2005;65:7145–7150. doi: 10.1158/0008-5472.CAN-05-1142. [DOI] [PubMed] [Google Scholar]


