Abstract
The QT interval is the electrocardiographic manifestation of ventricular depolarization and repolarization. Drug-induced long QT syndrome is characterized by acquired, corrected QT (QTc) interval prolongation that is associated with increased risk of torsade de pointes. Every physician must recognize if the drugs he or she prescribes prolongs the QTc interval, especially if the drug is prescribed for a chronic condition in older patients who are on polypharmacy. The evolution of alpha-blockers for the treatment of benign prostatic hyperplasia has allowed the development of drugs that are easier to administer and better tolerated. Because alpha-blockers generally have equivalent efficacy, this class of drugs is typically differentiated by safety and side effects. Studies suggest that alpha-blockers may vary in regard to their effect on the QT interval, and, therefore, on their predisposition to cause potentially life-threatening ventricular arrhythmias.
Key words: Drug-induced long QT syndrome, Torsade de pointes, Benign prostatic hyperplasia, Alpha-blockers
A drug’s effect on the QT interval is an important component of its safety profile. This article will discuss why and how the QT interval is measured, risk factors for QT interval prolongation, how assessment of the QT interval has affected modern drug development, commonly prescribed drugs for urologic indications that cause corrected QT (QTc) interval prolongation, and how the clinician should interpret a warning from the US Food and Drug Administration (FDA). This article will also consider the selection of alpha-blockers for benign prostatic hyperplasia (BPH) therapy from the perspectives of an electrophysiologist and a urologist.
Why Do We Measure the QT Interval?
Drug-induced long QT syndrome (LQTS) is characterized by acquired QTc prolongation that is associated with increased risk of torsade de pointes (TdP). Symptoms may include palpitations, syncope, and seizurelike activity. Episodes of TdP are usually self-limited, but they may degenerate to ventricular fibrillation and result in sudden cardiac death. Some pharmacologic agents prolong QTc interval in a dose-dependent manner, and others do so independent of dose.1
The QT interval is the electrocardiographic (ECG) manifestation of ventricular depolarization and repolarization. Other ECG variables have been investigated as predictors of TdP. QT dispersion (the difference between QT maximum and minimum intervals), postulated as a more direct measure of heterogeneity of repolarization, depends primarily on T-wave morphology and does not accurately predict drug-induced TdP.2,3 Tpeak-Tend measurement, suggested as a measure of transmural dispersion of repolarization, needs prospective validation.4 Manifest T-wave alternans (beat-to-beat amplitude or polarity alternation), a harbinger of instability in congenital LQTS, is rare in acquired LQTS and may not have the same implications.5 Microvolt T-wave alternans predicts susceptibility to ventricular arrhythmias.6 Its predictive value for drug-induced TdP is unclear. Despite a lack of specificity, QT interval prolongation is the most useful clinical variable to predict risk of TdP.
How Do We Measure the QT Interval?
The QT interval on the ECG remains somewhat mystical, and it is often misunderstood and underappreciated by the thousands of clinicians who routinely order ECGs and read them on a daily basis. The QT interval is ascertained because it identifies patients who are at a higher risk of developing life-threatening polymorphic ventricular tachycardia or TdP. The QT interval represents the duration of the action potential in the myocardial cell, which includes cellular depolarization and repolarization (Figure 1).
Figure 1.
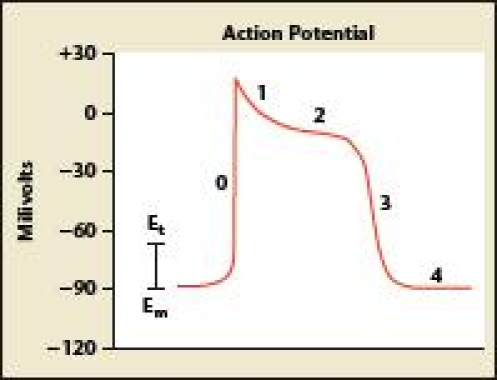
The QT interval represents the duration of the action potential in the myocardial cell, including cellular depolarization and repolarization. Adapted with permission from Arnsdorf M and Lee P.29
Measuring the QT interval is not an exact science. The relationship between the duration of cellular action potentials and the QT interval recorded at the body surface is complex, making it difficult to measure this phenomenon with precision. First, there is inherent imprecision in identifying the end of the T wave. Significant variation among some ECG leads, both in the onset of the QRS complex and at the end of the T wave, provides different QT values. Technical factors such as paper speed and sensitivity influence QT measurements, with higher paper speed leading to shorter interval values and higher sensitivity resulting in QT prolongation.7,8 On a surface ECG, the QT interval is measured from the QRS segment onset to T-wave termination. QT interval should be measured manually when there is a particular need for accuracy. The longest QT intervals are generally found in the precordial leads (V1 to V6), with V3 and V4 the most reliable for assessing QT prolongation (Figure 2).
Figure 2.
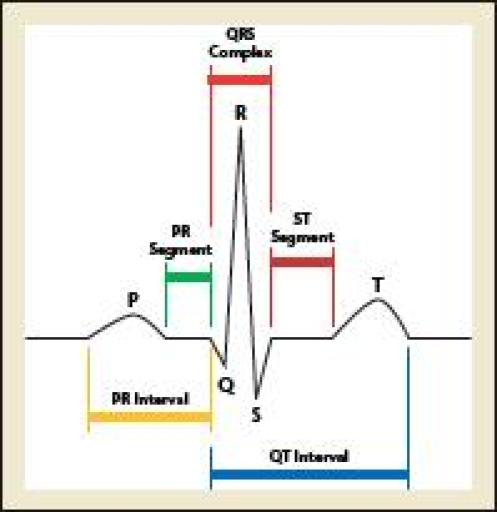
The longest QT intervals are generally found in the precordial leads (V1–V6), with V3 and V4 most reliable for assessing QT prolongation.
QT intervals may vary according to diurnal fashion, electrolyte imbalance, autonomic fluctuations, and ECG acquisition technique. The QT interval becomes prolonged with bradycardia and shortens with tachycardia, a finding that led to the development of a rate correction, or QTc interval, calculated as the longest measured QT interval in milliseconds divided by the square root of the RR interval. A QTc of less than 440 milliseconds is considered normal, and intervals of 440 to 460 milliseconds in men and 440 to 470 milliseconds in women are considered borderline. QTc intervals above these values are abnormal and considered prolonged. It is difficult to accurately measure QTc intervals in patients with irregular heart rates, especially those with atrial fibrillation and conduction abnormalities, such as a right or left bundle branch block that prolong the QRS intervals. In the presence of atrial fibrillation, some physicians manually measure the longest and shortest QT interval and take an average, whereas others measure 10 intervals and take an average.
Who Is at Risk for QT Interval Prolongation?
Myocardial repolarization is primarily mediated by potassium ion efflux. Nearly all drugs that prolong QTc interval block potassium efflux via the rapid component of the delayed rectifier (repolarizing) current (Ikr). Many patients with marked QTc interval prolongation never develop TdP, and others develop it with minimal prolongation. Fortunately, most patients treated with QT-prolonging medications never develop TdP. Several risk factors predispose patients to drug-induced LQTS and TdP. In a review of 249 TdP incidents due to noncardiac medications, virtually all patients had 1 risk factor, and 71% had multiple risk factors.9 The most common risk factor was female sex; 71% of the study subjects were women. Other common risk factors included hypokalemia, structural heart disease, the coadministration of multiple QT-prolonging drugs or agents interfering with metabolism, greater-than-average drug dosage, baseline QTc interval of at least 450 milliseconds, familial congenital LQTS, and prior drug-induced TdP. Hepatic impairment, bradycardia, and atrioventricular block also increase TdP risk.10
Subclinical mutations in genes causing congenital LQTS have been found in patients with drug-induced QT interval prolongation and ventricular arrhythmias.11–13 Yang and colleagues14 found mutations or polymorphisms in one of the long QT genes in 10% to 15% of patients with drug-related TdP. One mutation in the cardiac sodium channel gene SCN5A has been identified primarily in African Americans (13.2%).15 Patients with congenital LQTS generally do not have arrhythmias in the absence of provocation. Variable redundancy of repolarizing currents may explain why some patients with mutations do not develop QT interval prolongation or TdP until exogenous insults further limit repolarization.16
Assessment of the QT Interval in Modern Drug Development
An unfortunate characteristic of some non-antiarrhythmic pharmaceuticals is their propensity to delay cardiac repolarization as measured by the QT interval of a surface ECG. Although the degree of QT interval prolongation is understood to be an imperfect biomarker for arrhythmia risk, regulatory agencies around the globe have nevertheless begun to employ intense oversight over QT interval prolongation for non-antiarrhythmic drugs during the marketing application process. Indeed, prolongation of the QT interval by noncardiac drugs continues to be the most common source of regulatory nonapprovals.17 In May 2005, a guidance document from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) was published that discussed many aspects of QT interval clinical investigations, including study design and data analysis issues, and the impact of study results on drug labeling and future risk management. This document, entitled E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non- Antiarrhythmic Drugs,18 continues today to be the most important regulatory document on this subject for pharmaceutical firms soliciting marketing approval of new drugs.
Nevertheless, although the publication of this guidance did much to assuage the heightened anxiety in the drug development industry regarding the regulatory requirement to describe arrhythmic risk, many study design and analysis issues continue to plague those who must implement such research. The scope of this article precludes a full description and exploration of these unanswered questions, but they have been examined elsewhere.19 Suffice it to say that as in most areas of drug research, the sponsor must consult heavily with specialists who understand this discipline thoroughly and who are current in their knowledge of the scientific and regulatory controversies.
In addition to the design and analysis issues associated with these studies, the subsequent use of the results to predict risk itself becomes formidable. For instance, debate exists among cardiologists regarding the point at which a QT interval delay begins to predict meaningful clinical jeopardy. It is generally believed that drugs that prolong the mean QT interval by 5 milliseconds or less do not create an arrhythmic threat; however, that threshold may be as high as 10 milliseconds or beyond. Although minor delays in repolarization cannot be construed in any way as a hazard for healthy individuals, patients with predisposing circumstances, such as electrolyte abnormalities, congestive heart failure, and impaired drug clearance, may be unfortunately affected after the addition of such an incremental risk.
Finally, sponsors of QT interval studies must be prepared for the significant research costs associated with these investigations. Although the final expense of such a study will be the result of a multifactorial formula, outside costs will normally range from $1 million to $3 million, making such explorations one of the most expensive nonpivotal studies in a drug development program.
The assessment of the effect of drugs on cardiac repolarization continues to be a rapidly evolving and elaborate endeavor. Fortunately, the birth and maturation of an ambitious subindustry specializing in this distinct research has brought welcome assistance to clinical development groups facing this challenging undertaking.
Commonly Prescribed Drugs for Urologic Indications That Cause QTc Interval Prolongation
Because of the potentially life-threatening consequences of QT interval prolongation, the FDA has mandated that all new drugs under development undergo critical assessment of their effects on the QTc interval. It is important to recognize that drugs approved by the FDA prior to 2005 did not undergo a rigorous assessment of QTc interval prolongation. In some cases where postapproval follow-up suggested increased cardiovascular complications, the FDA has required the pharmaceutical sponsors to conduct postmarketing studies specifically examining QTc interval prolongation.
Every physician must recognize if the drugs he or she prescribes promotes QTc interval prolongation, especially if the drug is prescribed for a chronic condition in older patients who are on polypharmacy. A complete list of drugs that cause QTc interval prolongation is available from the Arizona Center for Education and Research on Therapeutics (CERT) at http://www.qtdrugs.org/medical-pros/drug-lists/drug-lists.cfm. The drugs on this list commonly prescribed by urologists include alfuzosin (Uroxatral®, sanofi-aventis, Bridgewater, NJ), ciprofloxacin (Cipro®, Bayer Health-Care Pharmaceuticals, Wayne, NJ), imipramine (Tofranil-PM®, Mallinckrodt, St. Louis, MO), levofloxacin (Levaquin ®, Ortho-McNeil-Janssen Pharmaceuticals, Titusville, NJ), tolterodine (Detrol LA®, Pfizer Inc, New York, NY), trimethoprim/sulfa (Bactrim®, Roche, Nutley, NJ; Septra®, Monarch Pharmaceuticals, Inc., Bristol, TN), and vardenafil (Levitra®, Bayer HealthCare Pharmaceuticals) (Table 1). The level of evidence for causing TdP is scored from 1 to 4, with level 1 having the greatest risk. Alfuzosin, levofloxacin, and vardenafil are categorized as level 2, which is defined as drugs that in some reports may be associated with TdP but at this time lack substantial evidence for causing TdP.
Table 1.
Urology Drugs That Cause Corrected QT Interval Prolongation
|
How Should the Clinician Interpret an FDA Warning for an Increase in the QT Interval?
Part of the thought process that goes into the decision to prescribe a medication or device for a patient includes performance of an accurate risk:benefit assessment. There is no absolute definition of what constitutes abnormal incremental QTc prolongation caused by a pharmaceutical agent. The ICH E14 guidelines define an incremental QTc prolongation of more than 5 milliseconds as abnormal.18 A warning that a medical therapy can lead to a prolongation of the QT interval should be of concern to clinicians. Particular caution is advised in treating patients who are predisposed to TdP, including those who have conditions that will prolong QTc, such as hypokalemia and hypomagnesemia; those with underlying cardiac abnormalities, such as heart failure, hypothyroidism, or familial long QT syndrome; or those who are taking other drugs that are known to prolong the QT interval.
Many men with BPH are taking commonly prescribed medications such as thiazide or loop diuretics that predispose to hypokalemia or hypomagnesemia; drugs that predispose to TdP, such as clarithromycin or amiodarone; or drugs with possible risk of TdP, including vardenafil, venlafaxine, nicardipine, or levofloxacin.20 Other pharmaceuticals may interfere with the metabolism of drugs that normally cause minimal QT interval prolongation by inhibiting pathways such as CYP3A4, leading to an exaggeration of QT prolongation and a greater predisposition to TdP.
With safety being the primary concern in patient care, particularly when treating quality-of-life conditions such as BPH, physicians in the complex and fast-paced world of clinical practice should gravitate to the use of agents with the best safety profile. This approach is particularly important with agents that prolong the QT interval or predispose to life-threatening arrhythmias, which usually occur with no warning. Many physicians who prescribe agents for BPH do not have immediate access to ECG monitoring equipment to check baseline and on-treatment QTc interval, so it is prudent to avoid agents that affect cardiac repolarization if other alternatives are available.
The QTc Interval and Selection of Alpha-Blockers for BPH Therapy: An Electrophysiologist’s Perspective
Before prescribing any medication, the risks, benefits, and alternatives should be carefully considered. Several issues must be clarified in order to put into perspective how QT interval data should influence the selection of alpha-blockers for BPH. For example,
To what degree does the drug prolong the QT interval, and is the QT interval prolongation routine? Small, inconsistent changes in QTc interval may be clinically insignificant.
Has the drug been implicated in TdP? Some drugs, like amiodarone, routinely prolong the QT interval but are quite unlikely to cause TdP.21 Arizona CERT stratifies drugs into 4 classes: drugs with risk of TdP, drugs with possible risk of TdP, drugs to be avoided by congenital long QT patients, and drugs unlikely to cause TdP.
If QT interval prolongation is a risk, is my patient likely to develop TdP? Women are at higher risk for developing TdP. Therefore, the risk of TdP is lower in the BPH population. A personal and family history of cardiac disease or hepatic impairment should be solicited. Hepatic impairment may also be identified through baseline blood tests. The QT interval, QTc interval, and the presence of bradycardia and/or atrioventricular block are easily assessed on 12-lead ECG.
Is the patient taking other QTinterval- prolonging drugs or agents that interfere with drug metabolism? This issue can be assessed by checking the Arizona CERT list. It is also imperative that clinicians be aware of metabolic drug-drug interactions before prescribing any new therapy.1
Alfuzosin (Uroxatral), an alpha1-blocker widely used for BPH, produces a mild, dose-dependent QT interval prolongation. Alfuzosin was approved by the FDA in 2003, and had been used extensively in Europe for several years before that.22,23 Arizona CERT lists alfuzosin among the drugs that have been associated (in some reports) with QT interval prolongation and/or TdP but currently lack substantial evidence for causing TdP. Some investigators report no significant effect on the QT interval with the recommended 10 mg once daily sustained-release preparation.24 Coadministration with potent CYP3A4 inhibitors (cytochrome P450 system liver metabolism), such as ketoconazole, itraconazole, and ritonavir, increases alfuzosin blood levels and is contraindicated.25 It seems prudent to avoid this agent in patients with congenital or acquired LQTS. Alfuzosin may delay cardiac repolarization by increasing the sodium current.26 It is unclear whether patients with QT interval prolongation due to increased sodium channel activity (LQTS 3) or predisposing mutations (African Americans) have an especially high risk.
There are no definitive comparative data that alfuzosin is more risky than other alpha-blockers currently in use for BPH. However, TdP may degenerate to ventricular fibrillation and result in sudden cardiac death. Therefore, it is not prudent to recommend an agent with proarrhythmic potential (albeit low or unknown) as a first-line choice in patients at risk if alternatives are available.
If an agent that prolongs the QT interval is prescribed, patients should be counseled about proarrhythmic risk. Symptoms such as new-onset palpitations, syncope, or seizurelike activity require immediate medical attention and cessation of the offending pharmacotherapy.1
The QTc Interval and Selection of Alpha-Blockers for BPH Therapy: A Urologist’s Perspective
Over the past 25 years, the evolution of alpha-blockers for the treatment of BPH has allowed the development of drugs that are easier to administer and better tolerated.23 Terazosin and doxazosin are long-acting alpha1-blockers that are dosed once daily. The introduction of these longer acting drugs diminished side effects and increased the convenience of drug administration. The primary advantage of tamsulosin over terazosin and doxazosin is the ability to administer an effective dose without dose titration.23 Although tamsulosin was associated with a higher risk of ejaculatory problems, it readily became the market leader due to its ease of administration and lack of effect on blood pressure. Alfuzosin SR is the most recent alpha1-blocker to enter the US marketplace. The primary advantage of alfuzosin SR over tamsulosin is the lack of ejaculatory problems.23
Due to concerns regarding postmarketing cardiovascular events, the FDA required the pharmaceutical sponsor of alfuzosin to conduct QTc interval studies to determine the proarrhythmic potential of this agent. These studies suggested that alfuzosin prolonged the QTc interval. The labeling information was amended to include a warning related to QTc effects.25 There have been no studies demonstrating that tamsulosin, doxazosin, or terazosin does not prolong the QTc interval. It is unlikely that these studies will ever be performed because generic terazosin and doxazosin are currently available and tamsulosin will likely lose its patent exclusivity in the near future.
Silodosin (Watson Pharmaceuticals, Inc., Corona, CA) is a novel alpha-blocker in development for the treatment of BPH. In anticipation of the marketing application for silodosin during the new regulatory and research climate, a double-blind, randomized, parallel-group QT trial was conducted in 186 subjects to define the potential impact of this drug on cardiac repolarization. The expected clinical dose (8 mg once daily) and a supratherapeutic dose (24 mg) were investigated, along with placebo and moxifloxacin, an antibiotic known to cause small increases in the QT interval (a positive control). Results from this investigation indicated that neither dose of silodosin caused any positive delay on cardiac repolarization (0.6 and −2.5 milliseconds, respectively), whereas the placebo and the positive control demonstrated expected changes (−0.8 and 3.2 milliseconds, respectively). 27 Like tamsulosin, silodosin causes ejaculatory dysfunction in a small subset of men.28 The fact that tamsulosin remains the most commonly prescribed alpha-blocker in 2008 suggests that ejaculatory issues are not perceived to be a significant clinical problem by the prescribing community.
The individual physician prescribing any drug cannot simply ignore a warning related to QTc interval prolongation. It is incumbent upon the prescribing physician to understand the implications of QTc interval prolongation and to recognize any coexisting conditions or drug interactions that may increase the clinical significance of the QTc interval prolongation. Because alpha-blockers generally have equivalent efficacy, this class of drugs is typically differentiated by safety and side effects. The primary disadvantage of tamsulosin and, now, silodosin is the effect on ejaculatory function. The primary advantage of silodosin is its proven lack of effect on QTc interval prolongation. The primary disadvantage of alfuzosin is the fact it has a prolongation effect on the QTc interval. If silodosin is approved by the FDA, the decision to prescribe either silodosin or alfuzosin must balance ejaculatory dysfunction and QTc interval prolongation. It may be reasonable to offer silodosin as first-line therapy especially in men on polypharmacy or those who have significant cardiovascular comorbidity.
Main Points.
QT prolongation is the most useful clinical variable to predict risk of torsade de pointes (TdP). Episodes of TdP are usually self-limited, but they may degenerate to ventricular fibrillation and result in sudden cardiac death.
On a surface electrocardiogram, the QT interval is measured from the QRS segment onset to T-wave termination; it should be measured manually when there is a particular need for accuracy.
Several risk factors predispose patients to drug-induced long QT syndrome and TdP, including female sex, hypokalemia, structural heart disease, and the coadministration of multiple QT-prolonging drugs or agents interfering with their metabolism.
Prolongation of the QT interval by noncardiac drugs continues to be the most common source of regulatory nonapprovals.
Alfuzosin, an alpha1-blocker widely used for benign prostatic hyperplasia, produces a mild, dose-dependent QT interval prolongation.
The primary advantage of silodosin is its proven lack of effect on QTc interval prolongation.
References
- 1.Gupta A, Lawrence AT, Krishnan K, et al. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J. 2007;153:891–899. doi: 10.1016/j.ahj.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Hii JT, Wyse DG, Gillis AM, et al. Precordial QT interval dispersion as a marker of torsade de pointes. Disparate effects of class Ia antiarrhythmic drugs and amiodarone. Circulation. 1992;86:1376–1382. doi: 10.1161/01.cir.86.5.1376. [DOI] [PubMed] [Google Scholar]
- 3.Shah RR. Drug-induced QT dispersion: does it predict the risk of torsade de pointes? J Electrocardiol. 2005;38:10–18. doi: 10.1016/j.jelectrocard.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Antzelevitch C. Role of transmural dispersion of repolarization in the genesis of drug-induced torsades de pointes. Heart Rhythm. 2005;2(2 suppl):S9–S15. doi: 10.1016/j.hrthm.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trohman RG, Sahu J. Drug-induced torsade de pointes. Circulation. 1999;99:E7. doi: 10.1161/01.cir.99.16.e7. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum DS, Jackson LE, Smith JM, et al. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 7.Sylvén JC, Horacek BM, Spencer CA, et al. QT interval variability on the body surface. J Electrocardiol. 1984;17:179–188. doi: 10.1016/s0022-0736(84)81093-6. [DOI] [PubMed] [Google Scholar]
- 8.Kautzner J. QT interval measurements. Card Electrophysiol Rev. 2002;6:273–277. doi: 10.1023/a:1016389227576. [DOI] [PubMed] [Google Scholar]
- 9.Zeltser D, Justo D, Halkin A, et al. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 2003;82:282–290. doi: 10.1097/01.md.0000085057.63483.9b. [DOI] [PubMed] [Google Scholar]
- 10.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–1372.. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napolitano C, Schwartz PJ, Brown AM, et al. Evidence for a cardiac ion channel mutation underlying drug-induced QT prolongation and life-threatening arrhythmias. J Cardiovasc Electrophysiol. 2000;11:691–696. doi: 10.1111/j.1540-8167.2000.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 12.Abbott GW, Sesti F, Splawski I, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 13.Makita N, Horie M, Nakamura T, et al. Drug-induced long-QT syndrome associated with a subclinical SCN5A mutation. Circulation. 2002;106:1269–1274. doi: 10.1161/01.cir.0000027139.42087.b6. [DOI] [PubMed] [Google Scholar]
- 14.Yang P, Kanki H, Drolet B, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105:1943–1948. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]
- 15.Splawski I, Timothy KW, Tateyama M, et al. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 16.Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 17.Darpö B. Detection and reporting of drug-induced proarrhythmias: room for improvement. Europace. 2007;9(suppl 4):iv23–iv36. doi: 10.1093/europace/eum168. [DOI] [PubMed] [Google Scholar]
- 18.Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; International Conference on Harmonisation; 2005; [Accessed April 22, 2008.]. US Food and Drug Administration Web site. http://www.fda.gov/CbER/gdlns/iche14qtc.htm. [PubMed] [Google Scholar]
- 19.Morganroth J. Cardiac repolarization and the safety of new drugs defined by electrocardiography. Clin Pharmacol Ther. 2007;81:108–113. doi: 10.1038/sj.clpt.6100010. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed April 22, 2008];Welcome to the Arizona CERT. Arizona Center for Education and Research on Therapeutics Web site. http://www.qtdrugs.org/index.cfm.
- 21.Vassallo P, Trohman RG. Prescribing amiodarone: an evidence-based review of clinical indications. JAMA. 2007;298:1312–1322. doi: 10.1001/jama.298.11.1312. [DOI] [PubMed] [Google Scholar]
- 22.Roehrborn CG, McConnell JD, Barry MJ, et al. for the AUA BPH Guideline Update Panel, authors. [Accessed April 3, 2008];Guideline on the management of benign prostatic hyperplasia. American Urological Association Web site. http://www.auanet.org/guidelines/bph.cfm.
- 23.Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007;9:181–190. [PMC free article] [PubMed] [Google Scholar]
- 24.Extramiana F, Maison-Blanche P, Cabanis MJ, et al. Clinical assessment of drug-induced QT prolongation in association with heart rate changes. Clin Pharmacol Ther. 2005;77:247–258. doi: 10.1016/j.clpt.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Alfuzosin [package insert] Sanofi-Synthelabo, Inc: Bridgewater, NJ; 2003. [Google Scholar]
- 26.Lacerda AE, Kuryshev YA, Chen Y, et al. Alfuzosin delays cardiac repolarization by a novel mechanism. J Pharmacol Exp Ther. 2008;324:427–433. doi: 10.1124/jpet.107.128405. [DOI] [PubMed] [Google Scholar]
- 27.Lepor H, Hoel G, Hill L, Volinn W. Double-blind, randomized, parallel-group study to define electrocardiographic effects of silodosin, a uroselective-adrenergic antagonist; Abstract presented at: American Urological Association Annual Meeting; May 17–22, 2008; Orlando, FL. [Google Scholar]
- 28.Khurana N, Srivastava A. Search for new α1a-adrenoceptor- selective antagonist for treating lower urinary tract symptoms associated with benign prostatic hyperplasia. Indian J Urol. 2007;23:215–216. [PMC free article] [PubMed] [Google Scholar]
- 29.Arnsdorf M, Lee P. [Accessed April 22, 2008];Myocardial action potential and action of antiarrhythmic drugs. UpToDate Web site. https://www.uptodate.com/home/index.html.


