Abstract
Androgen deprivation therapy (ADT) and bone metastases are the most important risk factors for developing skeletal complications (eg, bone loss, pathologic fractures) in prostate cancer (PC) patients with locally advanced and metastatic disease. Bisphosphonates, which inhibit excessive osteoclast activity caused by ADT and bone metastases, have proven to be safe and effective in preventing skeletal complications and presently are the standard of care in patients with metastatic disease. Bisphosphonates should be considered for use in all PC patients with locally advanced disease initiating ADT for an intended duration of at least 1 year, especially those with a low baseline bone mineral density.
Key words: Prostate cancer, Bone loss, Bone metastasis, Skeletal-related events, Bisphosphonates, Androgen deprivation therapy
In 2006, approximately 235,000 men received a diagnosis of prostate cancer (PC).1 Although prostate-specific antigen (PSA) screening has improved detection of early-stage, low-risk PC, many men still are diagnosed with advanced or metastatic disease.2 Early detection and contemporary treatment strategies (eg, administering androgen deprivation therapy [ADT] early in the course of the disease) have been associated with increased survival rates. Approximately 90% of PC patients with localized, low-grade disease survive more than 10 years, whereas patients with bone metastases survive a median of 3 years.3,4 Therefore, the long-term effects of PC and its treatment, such as diminished skeletal integrity, present a challenge to clinicians.
Patients diagnosed with PC often present with several risk factors for skeletal complications (eg, bone loss, fractures).5,6 For example, the results of 1 study show that approximately 75% of locally advanced PC patients receiving ADT for 12 months or less had 2 or more osteoporosis-related risk factors other than ADT (eg, low testosterone levels, smoking history, insufficient vitamin D or dairy intake, age ≥ 65 years, lack of exercise) at baseline.6 A common risk factor for skeletal complications in PC patients is hypogonadism caused by ADT. Among men with locally advanced PC, up to 20% of those with intermediate risk and 50% of those with high risk receive primary ADT.5,7
In patients with metastatic disease, a major risk factor for skeletal complications is bone metastases. As many as 90% of PC patients will develop bone metastases during the course of their disease.5 Therefore, both PC patients with locally advanced disease who are receiving ADT and those with metastatic disease are considered at high risk for developing skeletal-related events (SREs), such as debilitating bone pain, pathologic fractures, and spinal cord compression.
Besides lifestyle modifications (eg, smoking cessation, regular exercise) and an increase in intake of calcium and vitamin D, bisphosphonate therapy should be considered for PC patients who are at high risk for skeletal complications. Bisphosphonates, available in oral and intravenous (IV) formulations, are potent inhibitors of osteoclast-mediated bone resorption. During bone resorption, bisphosphonates accumulate in the bone matrix at sites of active bone turnover and are internalized by osteoclasts, where they inhibit osteoclast activity by inducing apoptosis and/or inhibiting maturation.8
In 1995, the US Food and Drug Administration (FDA) approved the IV use of bisphosphonate pamidronate to treat bone lesions in patients with multiple myeloma and metastatic breast cancer.9 Currently, zoledronic acid, the most potent commercially available bisphosphonate, is the only FDA-approved bisphosphonate for the treatment of bone metastases in PC patients (Table 1).5,10–16 Although no bisphosphonate has received FDA approval for the prevention or treatment of ADT-related bone loss, several studies have evaluated the use of bisphosphonates for this indication (Tables 1 and 2).6,10–23 Recent studies have evaluated bisphosphonates for the prevention and/or treatment of ADT- or bone metastases-related skeletal complications in PC patients with locally advanced or metastatic disease.
Table 1.
Food and Drug Administration-Approved Indications for Bisphosphonates
| Bisphosphonate | Indications |
|---|---|
| Alendronate10 |
|
| Clodronate5* |
|
| Etidronate11 |
|
| Ibandronate12 |
|
| Pamidronate13 |
|
| Risedronate14 |
|
| Tiludronate15 |
|
| Zoledronic acid16,17 |
|
Not commercially available in the United States.
Table 2.
Clinical Trials Evaluating Bisphosphonates for the Prevention of Bone Loss in Prostate Cancer Patients Without Bone Metastases Receiving ADT*
| Study/ | Annualized Mean Percentage Change in BMD‡ |
|||
|---|---|---|---|---|
| Regimen† | LS | TH | FN | Comments and AEs |
| Greenspan 200718 | ||||
| Alendronate | 3.7§ | 0.7§ | 1.6§ |
|
| 70 mg PO q 1 wk | (P < .001)∥ | (P < .031)∥ | (P = .008)∥ | |
| × 12 mo (n = 56) | ||||
| vs | ||||
| placebo | − 1.4§ | 0.7§ | − 0.7§ | |
| (n = 56) | (P < .045)∥ | (P < .052)∥ | (P = .081)∥ | |
| ADT duration | ||||
| ≥ 6 mo at entry | ||||
| Smith 200122 | ||||
| Pamidronate | No change | No change | No change |
|
| 60 mg IV q 12 wk | ||||
| × 48 wk | ||||
| (n = 21) | ||||
| vs | ||||
| control group | − 3.3 | − 1.8 | No change | |
| (n = 22) | (P < .001)¶ | (P = .005)¶ | (P = .56)¶ | |
| ADT initiation | ||||
| at entry | ||||
| Casey 200620 | ||||
| Zoledronic acid | 3.3 (n = 68) | 0.9 (n = 66) | 1.8 (n = 66) |
|
| 4 mg IV q 3 mo | ||||
| × 1 y | ||||
| (n = 68) | ||||
| vs | ||||
| control group | − 1.5 (n = 71) | − 2.0 (n = 69) | − 1.7 (n = 72) | |
| (n = 72) | (P = .0005)¶ | (P = .0012)¶ | (P = .0001)¶ | |
| ADT initiation | ||||
| at entry | ||||
| Israeli 20066 | ||||
| Zoledronic acid | 4.7 | 1.6 | NR |
|
| 4 mg IV q 3 mo | ||||
| × 48 wk | ||||
| (n = 106) | ||||
| vs | ||||
| placebo | − 2 | − 2.1 | NR | |
| (n = 109) | (P < .0001)¶ | (P < .0001)¶ | ||
| ADT duration | ||||
| ≤ 12 mo at entry | ||||
| Ryan 200619 | ||||
| Zoledronic acid | 4.6 | 1.4 | 1.3 |
|
| 4 mg IV q 3 mo | ||||
| × 1 y | ||||
| (n = 50) | ||||
| vs | ||||
| placebo | − 2.1 | − 2.4 | − 2.4 | |
| (n = 51) | (P < .0001)¶ | (P < .0001)¶ | (P = .0004)¶ | |
| ADT duration | ||||
| ≤ 12 mo at entry | ||||
| Smith 200323 | ||||
| Zoledronic acid | 5.6 | 1.1 | 1.2 |
|
| 4 mg IV q 3 mo | ||||
| × 1 y | ||||
| (n = 42) | ||||
| vs | ||||
| placebo | − 2.2 | − 2.8 | − 2.1 | |
| (n = 37) | (P < .001)¶ | (P < .001)¶ | (P < .001)¶ | |
| ADT initiation | ||||
| at entry | ||||
| Michaelson 200721 | ||||
| Zoledronic acid | 4 | 0.7 | 2 |
|
| 4 mg IV | ||||
| × 1 dose in 12 mo | ||||
| (n = 22) | ||||
| vs | ||||
| placebo | − 3.1 | − 1.9 | − 0.1 | |
| (n = 22) | (P < .001)¶ | (P = .004)¶ | (P = .06)¶ | |
| ADT duration | ||||
| ≥ 12 mo at entry | ||||
ADT consisted of LHRH agonist, LHRH agonist ± antiandrogen, or orchidectomy.
Patients (n) assessable for efficacy analysis.
BMD measured by dual-energy x-ray absorptiometry, unless otherwise specified.
Interim, 12-month results.
P value provided for within-group comparison of baseline and 12-month BMD.
P value provided for between-group comparison.
ADT, androgen deprivation therapy; AE, adverse event; BMD, bone mineral density; FN, femoral neck; IV, intravenous; LHRH, luteinizing hormone-releasing hormone; LS, lumbar spine; NR, not reported; ONJ, osteonecrosis of the jaw; TH, total hip.
Skeletal Complications in Patients With Locally Advanced PC
Pathophysiology and Consequences
Skeletal complications in patients with locally advanced PC result primarily from ADT-induced bone loss. Historically, ADT was reserved for patients with metastatic disease, but the most recent National Comprehensive Cancer Network guidelines for PC recommend ADT with or without radiation therapy as initial therapy for patients with locally advanced disease.7,24 ADT is also commonly used to decrease gland volume before radiation therapy in PC patients with larger prostate glands. ADT, through either medical (ie, administering luteinizing hormone-releasing hormone agonists ± an antiandrogen) or surgical (ie, bilateral orchidectomy) castration, significantly decreases endogenous levels of testosterone (up to 95%) and estrogen (up to 80%), which are essential mediators of bone resorption.5
Estrogen is well known for its pivotal role in maintaining bone health in men. Estrogen decreases osteoclast formation, activity, and lifespan and possibly increases osteoblast formation, proliferation, differentiation, and function.25 Normal fluctuations in estrogen levels help maintain the delicate balance between bone resorption and bone formation, a process known as bone remodeling. Testosterone may indirectly or directly influence the bone-remodeling process. The conversion of testosterone to estrogen via aromatization indirectly affects bone health by increasing estrogen levels.25 Testosterone has also been shown to increase the lifespan of osteoclasts and osteoblasts directly by inhibiting their apoptosis and may stimulate osteoblast proliferation.
Deficiencies in testosterone and estrogen, such as those induced by ADT, shift the balance of bone remodeling toward increased bone resorption by stimulating osteoclast activity, decreasing osteoclast apoptosis, and increasing osteoblast apoptosis.25 Consequently, PC patients with locally advanced disease receiving ADT experience annual reductions in bone mineral density (BMD) of 1.9% to 4.6% in the lumbar spine (LS), 1.8% to 2.8% in the total hip (TH), and 1.1% to 4% in the femoral neck (FN). These rates exceed by 5- to 10-fold the annual rates of bone loss observed in healthy, age-matched controls and PC patients who are not receiving ADT.5,19,22,23,26–29 Based on the results of a study evaluating bone loss in patients receiving acute (mean duration of therapy, 2.9 months) or chronic (mean duration, 33.2 months) ADT, Greenspan and colleagues28 concluded that bone loss is most significant during the first 12 months of ADT.
Although prospective studies evaluating the relationship between BMD and fracture rates have not been performed in nonmetastatic PC patients receiving ADT, the results of several studies demonstrate that these patients are 21% to 37% more likely to experience a fracture than are patients not receiving ADT.30–32 Furthermore, PC patients receiving ADT are more likely to require fracture-related hospitalizations (4.9% vs 2.2%, P < .001).30
Bisphosphonate Therapy for ADT-Related Skeletal Complications
Efficacy. Several studies have evaluated bisphosphonates, including oral alendronate, IV pamidronate, and IV zoledronic acid, for the prevention and treatment of ADT-related bone loss in PC patients with locally advanced disease (Table 2).6,18–23 Oral bisphosphonates have been proven effective for preventing further bone loss in osteoporotic men.33 The results of a recent 2-year, double-blind, randomized, placebo-controlled study by Greenspan and colleagues18 demonstrated that oral alendronate (70 mg) administered once weekly was more effective than placebo in preventing bone loss and increasing BMD in patients with locally advanced PC receiving ADT. Further studies confirming these results are warranted before oral bisphosphonates can be recommended for the prevention or treatment of ADT-related bone loss.
In a randomized, open-label, controlled study, Smith and colleagues22 demonstrated that pamidronate 60 mg IV administered every 12 weeks prevented bone loss in PC patients initiating ADT. Although no significant change in LS or TH BMD measurements from baseline was observed in patients receiving pamidronate at 48 weeks, patients receiving placebo showed significant decreases in LS and TH BMD measurements. The authors concluded that pamidronate maintains BMD in locally advanced PC patients who are receiving ADT.22
The results of 4 randomized, controlled studies indicate that zoledronic acid (4 mg IV every 3 months) not only prevents further bone loss but also increases BMD of the LS (3.3% to 5.6%), TH (0.9% to 1.6%), and FN (1.2% to 1.8%) when initiated either concurrently or within the first year of ADT initiation.6,19,20,23 Patients in the control group of these studies experienced net losses in BMD of the LS (−1.5% to −2.2%), TH (−2.0% to −2.8%), and FN (−1.7% to −2.4%).6,19,20,23 Interestingly, Israeli and colleagues6 demonstrated that patients with a low baseline BMD had greater increases in LS BMD than patients with a normal baseline BMD (5.8% vs 4.4%)—suggesting an inverse relationship between baseline BMD and magnitude of response to zoledronic acid.
A more recent randomized, open-label study evaluating the effects of a single IV dose of zoledronic acid (4 mg) on BMD at 12 months in 44 PC patients receiving ADT showed a similar increase in LS (4%) BMD, but a slightly lower increase in TH (0.7%) BMD compared with those observed in the studies evaluating zoledronic acid 4 mg every 3 months; patients receiving placebo showed decreases in both LS (−3.1%) and TH (−1.9%).21 Further study is required to determine the feasibility of administering zoledronic acid less frequently (eg, every 12 months vs every 3 months).
Which bisphosphonate and which regimen will produce the most favorable outcomes and whether bisphosphonate therapy should be used routinely in all patients with locally advanced PC initiating or during the first year of ADT are unknown. Nonetheless, patients being considered for ADT should be evaluated for baseline osteoporotic risk factors, including low BMD. The results of recent studies suggest that zoledronic acid not only prevents bone loss but also reverses existing bone loss in PC patients receiving ADT. Although patients with either normal or low baseline BMD responded to zoledronic acid in these trials, patients with low baseline BMD experienced maximum benefit when zoledronic acid therapy was initiated during the first 12 months of ADT.6,19,20,23 Furthermore, evidence supports the effectiveness of bisphosphonates in otherwise healthy men with osteoporosis.33 Therefore, bisphosphonate therapy should be strongly considered for patients with baseline osteopenia or osteoporosis who are initiating or receiving ADT.24
Fractures are the most clinically relevant potential consequence of ADT-induced bone loss, but whether bisphosphonate therapy actually prevents fragility fractures in PC patients receiving ADT remains unknown.34 Fractures typically occur in patients who have received more than 12 months of ADT.35 Studies evaluating the effects of zoledronic acid on BMD have not assessed outcomes beyond 12 months of therapy and were not designed to detect differences in 12-month fracture rates between the bisphosphonate and control groups; therefore, detecting a clinically meaningful or significant difference in the 1-year fracture rate would be unlikely. 6,19–23 Because BMD is inversely related to fracture risk in men with PC, maintaining or increasing BMD in PC patients receiving ADT is likely to lower the risk and incidence of fragility fractures.34 Nevertheless, studies evaluating bisphosphonates for preventing fractures in PC patients receiving ADT are needed.
Safety. Generally, oral and IV bisphosphonate therapies are well tolerated in patients with locally advanced PC receiving ADT (Table 2). The most common adverse events (AEs) observed in men receiving alendronate in male osteoporosis studies are gastrointestinal related, including gastroesophageal reflux, dyspepsia, and diarrhea.10
In recent randomized, controlled studies evaluating the IV bisphosphonates (ie, pamidronate, zoledronic acid) in men with locally advanced PC, the most common AEs were an acute-phase reaction or influenza-like illness (14% to 15%), hot flashes (23% to 58%), fatigue (10% to 38%), arthralgias (13% to 22%), and fever (10% to 11.5%).6,19,20,22,23 The 6 clinical trials evaluating use of IV bisphosphonate in PC patients with locally advanced disease summarized in this article reported only 2 cases of notable increases in serum creatinine (SCr) levels and no cases of osteonecrosis of the jaw (ONJ).6,19–23 ONJ is a poorly understood and uncommon event that has been reported in cancer patients receiving complex treatment regimens, including radiation therapy, chemotherapy, and/or corticosteroids, along with an IV bisphosphonate.36,37 ONJ has been reported more frequently in patients with cancer types other than PC.37
Furthermore, cancer patients who have undergone invasive dental procedures (eg, tooth extraction) are at greater risk of developing ONJ. Therefore, preventative dentistry before and during bisphosphonate therapy should be considered. Referral to a dental professional is important in suspected cases of ONJ. Prospective clinical trials to further characterize ONJ and evaluate its recognition, prevention, and management in cancer patients receiving IV bisphosphonates are ongoing.
Skeletal Complications in PC Patients With Metastatic Disease
Pathophysiology and Consequences
The molecular and cellular characteristics of PC cells as well as the bone microenvironment and the accessibility of PC cells to the systemic vasculature make bone the most common site for PC metastases.8,38 The mechanism by which PC cells metastasize to the bone is a complex, multistep process highly influenced by various growth factors, proteolytic enzymes, and cell adhesion molecules.8,39 This process begins with angiogenesis, which in turn allows detached PC cells to invade the vasculature and deposit into capillaries of the bone, where they depart from the vasculature, invade the marrow stroma, and eventually adhere to and colonize the bone matrix (Figure 1).8,39,40
Figure 1.
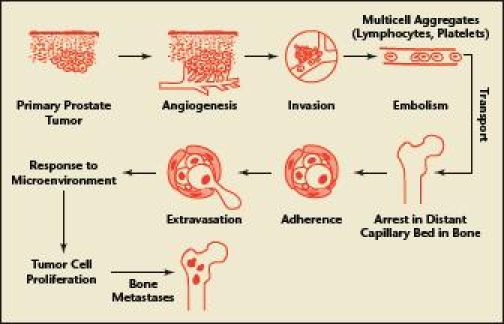
Steps required for prostate cancer to metastasize to bone. Reprinted with permission from The Endocrine Society and Guise and Mundy.40 Copyright 1998, Endocrine Reviews.
Once established within the bone microenvironment, PC cells secrete growth factors that stimulate osteoclast and osteoblast activity, interrupting bone homeostasis.8 Increased osteoclast activity and osteolysis trigger the release of bone-derived growth factors and cytokines that, in turn, stimulate PC cell survival and proliferation.8 Increased osteolysis naturally causes a paracrine stimulation of osteoblasts to repair bone. PC cells also secrete factors, such as bone morphogenetic protein 6 (BMP-6), that further encourage osteoblast activity and are believed to contribute to the osteoblastic phenotype of PC bone metastases.39 This process is cyclical; osteoblasts further stimulate PC cell proliferation.39
Osteoblastic lesions produce new bone growth that is often misplaced and poorly mineralized, resulting in new bone tissue with reduced integrity.8,39 Bone metastases often lead to debilitating bone pain and other skeletal complications, such as impaired mobility, spinal cord compression, pathologic fractures, and, sometimes, hypercalcemia.39 In a clinical trial evaluating zoledronic acid therapy for the prevention of SREs, approximately 50% of control patients with hormone-refractory PC and bone metastases experienced a skeletal complication, such as requiring a change in antineoplastic therapy to treat bone pain (7%), requiring radiation therapy to relieve bone pain or prevent fracture or nerve compression (33%), spinal cord compression (8%), or pathologic fractures (25%).4,41,42
Bisphosphonate Therapy for the Treatment of Bone Metastases-Related Skeletal Complications
Efficacy. Although PC bone metastases are typically osteoblastic in nature, osteoclast-mediated bone destruction is believed to contribute significantly to bone morbidity in PC patients with bone metastases; therefore, bisphosphonate therapy to inhibit osteoclast-mediated osteolysis is a rational treatment option.5,24 Clodronate, pamidronate, and zoledronic acid have been evaluated for the prevention of SREs in patients with bone metastases in recent clinical trials (Table 3).41–45 The use of bisphosphonates has typically been reserved for patients with bone metastases after ADT failure, but one recent study evaluated clodronate (not commercially available in the United States) in patients with hormone-sensitive metastatic disease.23,43,46 In this double-blind, randomized, placebo-controlled trial, Dearnaley and colleagues43 evaluated oral clodronate (2080 mg/day) administered to patients initiating or responding to ADT. Patients receiving clodronate had slightly longer, albeit statistically insignificant, bone progression (ie, symptomatic skeletal-disease progression)-free survival and overall survival times compared with patients receiving placebo. Furthermore, patients receiving clodronate were significantly less likely to experience worsening of their World Health Organization performance status (P = .008).
Table 3.
Clinical Trials Evaluating Bisphosphonates for the Prevention of Skeletal Complications in Prostate Cancer Patients With Bone Metastases
| Study/Regimen* | Primary Endpoint | Comments and AEs |
|---|---|---|
| Dearnaley 200343 | ||
| Clodronate | BPFS time† |
|
| 2080 mg PO q d | ||
| × 3 y | ||
| (n = 155) | ||
| vs | ||
| placebo | ||
| (n = 156) | ||
| Ernst 200344 | ||
| Clodronate | Reduction in pain and/or analgesic use§ |
|
| 1500 mg IV | ||
| q 3 wk | ||
| (n = 104) | ||
| vs | ||
| placebo | ||
| (n = 105) | ||
| Saad 2002,41 200442 | ||
| Zoledronic acid | Proportion of patients having at least 1 SRE∥ |
15-month analysis
|
| 4 mg IV q 3 wk | ||
| × 20 | ||
| (n = 214) | ||
| vs | ||
| zoledronic acid | ||
| 8/4 mg¶ IV q 3 wk | ||
| × 20 | ||
| (n = 221) | ||
| vs | ||
| placebo | ||
| (n = 208) | ||
| Small 200345 | ||
| Pamidronate | Reduction in pain and/or analgesic use** |
|
| 90 mg IV q 3 wk | ||
| × 9 | ||
| (n = 169) | ||
| vs | ||
| placebo | ||
| (n = 181) |
Patients (n) evaluable for efficacy analysis.
Defined as either time to development of symptomatic bone metastases requiring intervention or PC-related death; does not include asymptomatic disease progression (eg, asymptomatic vertebral fracture) often included in the definition of skeletal-related event.
Most common AEs included gastrointestinal problems, increased lactate dehydrogenase levels, cardiovascular problems, joint pain.
Pain reduction defined as PPI of 0 or reduction by 2 points in PPI scale; a decrease in analgesic use defined as 50% reduction.
Defined as pathologic bone fracture, spinal cord compression, bone surgery, radiation therapy of bone, change in antineoplastic therapy to treat bone pain.
To reduce renal toxicities, zoledronic acid dose decreased to 4 mg for all patients per protocol amendment.
Defined as number of SREs divided by years at risk.
Pain measured using a self-administered numeric rating scale and categorized as “least,” “average,” or “worst” pain at each study visit; analgesic use self-recorded daily and assigned oral morphine equivalents.
Defined as radiation therapy of bone for pain relief, radiation therapy of bone to prevent fracture or spinal cord compression, pathologic fracture, spinal cord compression, bone surgery, hypercalcemia, or a need for a spinal orthotic brace.
AE, adverse event; BPFS, bone progression-free survival; CI, confidence interval; HR, hazard ratio; IV, intravenous; ONJ, osteonecrosis of the jaw; PFS, progression-free survival; PPI, present pain index; QOL, quality of life; RR, relative risk; SRE, skeletal-related event; WHO, World Health Organization.
Whether the use of a more potent IV bisphosphonate (eg, zoledronic acid) would be more effective in this patient population is unknown.43 The National Cancer Institute is currently conducting a phase III, randomized study evaluating zoledronic acid in PC patients with bone metastases undergoing ADT, to further define the role of bisphosphonates in these patients.46
Three randomized, placebo-controlled studies have recently evaluated clodronate, pamidronate, and zoledronic acid in patients with hormone-refractory PC with bone metastases; only zoledronic acid significantly reduced the incidence of skeletal complications (ie, SREs, bone pain) in this patient population. 41,42,44,45 For the National Cancer Institute of Canada Clinical Trials Group, Ernst and colleagues compared IV clodronate with placebo for pain palliation in men with symptomatic bone metastases and observed no differences between groups in primary or secondary outcome measures.44 Small and colleagues45 also reported no differences in pain scores, analgesic use, and SRE rates between patients with symptomatic bone metastases receiving IV pamidronate or placebo.
In a 24-month study evaluating zoledronic acid 4 mg or 8 mg infused over 5 to 15 minutes (because of renal toxicity, midstudy protocol amendments increased the infusion time to 15 minutes from 5 minutes and reduced the dose to 4 mg from 8 mg in all patients receiving zoledronic acid) every 3 weeks for the prevention of SREs, Saad and colleagues41,42 observed a statistically significant difference in patients who experienced at least 1 SRE at both the 15-month interim and the 24-month final analysis between patients receiving zoledronic acid 4 mg and placebo (33.2% vs 44.2%, P = .021). Additionally, patients in the placebo group experienced an SRE approximately 160 days earlier than patients in the zoledronic acid 4 mg group. Finally, patients receiving placebo were more likely to experience a pathologic fracture.41,42
Small and colleagues45 observed a 50% decrease in urinary markers of osteoclast activity in patients receiving pamidronate, whereas a decrease of 70% was observed by Saad and colleagues41 in patients receiving zoledronic acid. This difference may indicate a dissimilarity in the ability of these 2 bisphosphonates to inhibit osteoclast activity and explain the improved efficacy of zoledronic acid in preventing skeletal complications in patients with bone metastases.9 Zoledronic acid has been shown to significantly decrease the risk of skeletal complications in PC patients with bone metastases. Consequently, current PC treatment algorithms support the use of zoledronic acid to prevent skeletal complications.24
Safety. In general, oral and IV bisphosphonates are well tolerated in PC patients with bone metastases (Table 3).41–45 Dearnaley and colleagues,43 however, observed a higher incidence of AEs and AE-related dose modifications in patients receiving oral clodronate compared with placebo. The most common AEs reported in this trial included gastrointestinal problems and increased lactate dehydrogenase levels.43
In a clinical trial assessing PC patients with bone metastases, the incidence of AEs did not differ between patients receiving pamidronate or placebo.45 In clinical trials evaluating zoledronic acid, however, anemia, fatigue, fever, lower-limb edema, and myalgia occurred in at least 5% more patients receiving zoledronic acid compared with placebo.41,42
In recent studies of patients receiving IV pamidronate or IV clodronate, changes in baseline SCr levels were not reported.44,45 In contrast, approximately 3% of patients receiving zoledronic acid 4 mg or 8/4 mg experienced grade-3 increases in SCr levels.41,42 The rate of SCr-level increases in patients receiving the 15-minute infusion of zoledronic acid 4 mg, however, was similar to that in patients receiving placebo. This finding is similar to that of other studies and indicates that renal impairment in patients receiving IV bisphosphonates is most likely related to the dose and infusion rate.41 Although ONJ has been reported in PC patients receiving zoledronic acid 4 mg administered every 4 to 6 weeks, no cases of ONJ were reported in these clinical trials.36,41,42,44,45 Prospective clinical trials are planned to further evaluate the incidence and risk factors for ONJ in cancer patients receiving IV bisphosphonates. 37
Conclusions
Skeletal complications, such as bone loss, fractures, bone pain, and spinal cord compression, are a major cause of morbidity in patients with locally advanced or metastatic PC. Bisphosphonates have proved to be safe and effective for preventing or treating skeletal complications secondary to ADT or bone metastases.6,18–23,41,42 Currently available evidence is most ample in supporting the use of zoledronic acid in both patients with locally advanced disease receiving ADT and patients with bone metastases.
Bisphosphonate therapy is the standard of care for reducing skeletal complications in patients with bone metastases, in whom the benefits of improved BMD outweigh the risks of AEs.24 Evidence supporting the use of bisphosphonates in patients with locally advanced disease receiving ADT is mounting, and recommendations for their use in this population are most likely forthcoming. Several unanswered questions regarding bisphosphonate use in patients with locally advanced disease warrant further study.
First, the feasibility of administering bisphosphonate therapy to all patients receiving ADT is unknown. In a study that completed a subanalysis of the effects of zoledronic acid in patients stratified according to baseline T score (low baseline T score [< −1 and < −2] vs normal baseline T score [> −1]), zoledronic acid was more effective in preventing and reversing bone loss in patients with low baseline T scores.6 Because most PC patients receive several years of ADT, which places them at risk for ongoing bone loss, and the results of clinical trials show patients with normal baseline BMD (T score ≥ −1.0) benefit from zoledronic acid therapy, administering zoledronic acid to prevent bone loss in PC patients receiving ADT with normal baseline BMD seems logical.6
Second, the cost-effectiveness of prescribing bisphosphonate therapy in all PC patients is unknown. Studies evaluating which patients should receive bisphosphonates, from both clinical and economic perspectives, need to be completed.
Third, although under investigation, neither the appropriate time to initiate nor the duration of bisphosphonate therapy has been clearly established. Most studies have reported only 12-month results with bisphosphonate therapy in patients initiating or having received 12 months or less of ADT.6,19–23 Therefore, additional studies evaluating other potential initiation times (eg, when decreases in baseline BMD are evident), as well as longer follow-up (eg, 5–10 years), are needed to determine the best time to initiate as well as the long-term effects of bisphosphonates.
Although evidence to date suggests that the optimal regimen for preventing bone loss in patients with locally advanced disease receiving ADT is zoledronic acid 4 mg administered every 3 months, results from a recent study by Michaelson and colleagues21 suggest that a single annual dose of zoledronic acid may be effective in this population; thus, studies confirming these results and evaluating alternative dosages, including head-to-head evaluations of various regimens, are warranted.6,19–21,23 Some clinicians believe that initiating bisphosphonate therapy before the first dose of ADT is administered is optimal, but there is no evidence from randomized, controlled trials to support this strategy.
Fourth, the difference in fracture rates between patients receiving bisphosphonate therapy and control groups has not been adequately assessed in clinical trials. The Radiation Therapy Oncology Group is conducting a large, ongoing, randomized, double-blind, placebo-controlled study assessing the safety and efficacy of zoledronic acid for the prevention of bone loss and associated fractures in patients receiving radiation therapy and long-term ADT (luteinizing hormone-releasing hormone agonists) for high-grade and/or locally advanced PC.47 Finally, whether bisphosphonates prevent the development of bone metastases is unknown. Preliminary evidence is promising, however, and the results of ongoing studies are eagerly awaited.48
Main Points.
Zoledronic acid is the most potent commercially available bisphosphonate and the only US Food and Drug Administration-approved bisphosphonate for the treatment of bone metastases in prostate cancer (PC) patients.
PC patients receiving androgen deprivation therapy (ADT) are more likely to require fracture-related hospitalizations than those who are not.
Bisphosphonate therapy should be strongly considered for patients with baseline osteopenia or osteoporosis who are initiating or receiving ADT.
Generally, oral and intravenous (IV) bisphosphonate therapies are well tolerated in patients with locally advanced PC receiving ADT.
Zoledronic acid has been shown to significantly decrease the risk of skeletal complications in PC patients with bone metastases.
Longer infusion times for IV bisphosphonates minimize effects on renal function.
Acknowledgments
The author thanks Syntaxx Communications, Inc., specifically Stephanie Butler, PharmD, who provided manuscript development and medical writing services, and Alison Shore, MS, who provided editorial services, on behalf of Novartis Oncology.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson RA. Prostate cancer trends in the era of prostate-specific antigen: an update of incidence, mortality, and clinical factors from the SEER database. Urol Clin North Am. 2002;29:173–181. doi: 10.1016/s0094-0143(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Lubeck DP, Meng MV, et al. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saad F. The role of bisphosphonates in the management of prostate cancer. Curr Oncol Rep. 2006;8:221–227. doi: 10.1007/s11912-006-0023-7. [DOI] [PubMed] [Google Scholar]
- 5.Higano CS. Understanding treatments for bone loss and bone metastases in patients with prostate cancer: a practical review and guide for the clinician. Urol Clin North Am. 2004;31:331–352. doi: 10.1016/j.ucl.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Israeli RS, Rosenberg S, Saltzstein D, et al. The effect of zoledronic acid on bone mineral density in patients undergoing androgen-deprivation therapy. Clin Genitourin Cancer. 2007;5:271–277. doi: 10.3816/CGC.2007.n.003. [DOI] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Grossfeld GD, Lubeck DP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipton A. Pathophysiology of bone metastases: how this knowledge may lead to therapeutic intervention. J Support Oncol. 2004;2:205–213. [PubMed] [Google Scholar]
- 9.Michaelson MD, Smith MR. Bisphosphonates for treatment and prevention of bone metastases. J Clin Oncol. 2005;23:8219–8224. doi: 10.1200/JCO.2005.02.9579. [DOI] [PubMed] [Google Scholar]
- 10.Fosamax [package insert] Whitehouse Station, NJ: Merck & Co, Inc; 2007. [Google Scholar]
- 11.Didronel [package insert] Cincinnati, OH: Procter & Gamble Pharmaceuticals; 2006. [Google Scholar]
- 12.Boniva [package insert] Nutley, NJ: Roche Pharmaceuticals; 2006. [Google Scholar]
- 13.Aredia [package insert] East Hanover, NJ: Novartis Pharmaceutical Corporation; 2007. [Google Scholar]
- 14.Actonel [package insert] Bridgewater, NJ: Sanofi-Aventis US LLC; 2006. [Google Scholar]
- 15.Skelid [package insert] Bridgewater, NJ: sanofiaventis; 2006. [Google Scholar]
- 16.Zometa [package insert] East Hanover, NJ: Novartis Pharmaceutical Corporation; 2007. [Google Scholar]
- 17.Reclast [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007. [Google Scholar]
- 18.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer. Ann Intern Med. 2007;146:416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 19.Ryan CW, Huo D, Demers LM, et al. the Zometa US05 Investigators, authors. Zoledronic acid initiated during the first year of androgen-deprivation therapy increases bone mineral density in prostate cancer patients. J Urol. 2006;176:972–978. doi: 10.1016/j.juro.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 20.Casey R, Love W, Mendoza C, et al. Zoledronic acid reduces bone loss in men with prostate cancer undergoing androgen deprivation therapy; Presented at: 2006 Multidisciplinary Prostate Cancer Symposium; February 24–26, 2006; San Francisco, CA. Abstract 184. [Google Scholar]
- 21.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 23.Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 24.The NCCN, authors. Prostate Cancer Clinical Practice Guidelines in Oncology. 2007. [Accessed February 5, 2008]. © 2008 National Comprehensive Cancer Network, Inc. http://www.nccn.org.
- 25.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 26.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–2367. [PubMed] [Google Scholar]
- 27.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 28.Greenspan SL, Coates P, Sereika SM, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–6417. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 29.Mittan D, Lee S, Miller E, et al. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab. 2002;87:3656–3661. doi: 10.1210/jcem.87.8.8782. [DOI] [PubMed] [Google Scholar]
- 30.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 31.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 32.Smith MR, Boyce SP, Moyneur E, et al. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–139. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 33.Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 35.Oefelein MG, Ricchuiti V, Conrad W, et al. Skeletal fracture associated with androgen suppression induced osteoporosis: the clinical incidence and risk factors for patients with prostate cancer. J Urol. 2001;166:1724–1728. doi: 10.1016/s0022-5347(05)65661-3. [DOI] [PubMed] [Google Scholar]
- 36.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk-factors. J Clin Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 37.Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2:7–14. doi: 10.1200/jop.2006.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 39.Saad F, Clarke N, Colombel M. Natural history and treatment of bone complications in prostate cancer. Eur Urol. 2006;49:429–440. doi: 10.1016/j.eururo.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 40.Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19:18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 41.Saad F, Gleason DM, Murray R, et al. for the Zoledronic Acid Prostate Cancer Study Group, authors. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 42.Saad F, Gleason DM, Murray R, et al. for the Zoledronic Acid Prostate Cancer Study Group, authors. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 43.Dearnaley DP, Sydes MR, Mason MD the MRC PR05 Collaborators, authors. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial) J Natl Cancer Inst. 2003;95:1300–1311. doi: 10.1093/jnci/djg038. [DOI] [PubMed] [Google Scholar]
- 44.Ernst DS, Tannock I, Venner P, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21:3335–3342. doi: 10.1200/JCO.2003.03.042. [DOI] [PubMed] [Google Scholar]
- 45.Small EJ, Smith MR, Seaman JJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277–4284. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 46.National Cancer Institute, authors. [Accessed February 5, 2008];Featured Clinical Trials. Preventing bone fractures in prostate cancer patients. http://www.cancer.gov/clinicaltrials/ft-CALGB-90202/print?page=&keyword=
- 47.Radiation Therapy Oncology Group, authors. Active protocols. A phase III, double-blind, placebo-controlled study to evaluate the efficacy of Zometa for the prevention of osteoporosis and associated fractures in patients receiving radiation therapy and long term LHRH agonists for high-grade and/or locally advanced prostate cancer (RTOG 0518) [Accessed February 5, 2008]. http://www.rtog.org/members/protocols/0518/0518.pdf.
- 48.Saad F. Clinical benefit of zoledronic acid for the prevention of skeletal complications in advanced prostate cancer. Clin Prostate Cancer. 2005;4:31–37. doi: 10.3816/cgc.2005.n.009. [DOI] [PubMed] [Google Scholar]


