Abstract
Recent studies support a link between stress and the increased consumption of palatable foods. However, there has been a noted lack of genetic models to examine predisposing factors of overweight, obesity, and binge eating, particularly the role that stress sensitivity might play in the development of these conditions. We have examined the effects of chronic stress exposure on macronutrient choice preferences in a genetic mouse model of stress sensitivity (corticotropin-releasing factor receptor-2 deficient mice). Mice were provided with high fat, high protein, and high carbohydrate diets during exposure to chronic variable stress (CVS). Mice given free access to these diets during CVS selected a greater proportion of their calories in the form of the high fat diet compared to non-stressed mice. Apparent genotypic differences in high protein and high carbohydrate preferences were also diminished during the stress exposure. Stress-sensitive mice showed reduced weight gain and caloric efficiency during CVS, indicating a role for this phenotype in energy balance. When the preferred high fat diet was provided under limited access, stress-sensitive mice showed an increase in high fat consumption during CVS that was not observed in wild type mice, indicating a potential role for stress-sensitivity in stress-induced bingeing. These studies support an involvement of stress pathways in macronutrient selection where stress selectively elevates the intake of a preferred high fat diet. Based on the alterations in caloric efficiency, increases in stress sensitivity may further predispose an organism toward altered energy balance in times of stress.
Keywords: Chronic variable stress, stress sensitivity, macronutrient, obesity, energy utilization
Introduction
The current epidemic of obesity and its related health problems has made the examination of influences on feeding behavior of paramount importance. While the causes of the recent escalation of obesity are complex, the increasing availability of highly palatable and calorically-dense foods rich in fat and carbohydrates has played a key role (1). The increasing stress of daily life has been associated with an increased motivation for such foods (2–4). An elevated snesitivity to stress has also been linked to both obesity and binge eating disorder (5, 6). Recently, in agreement with this clinical literature, a model has been proposed in which the intake of energy-dense foods attenuates the negative effects associated with a chronic stress (7). The current study was designed to determine how chronic stress affects macronutrient preferences to gain insight into the contributions of stress and a stress-sensitive phenotype on palatable feeding, binge-eating, and the metabolic syndrome.
Differences in stress sensitivity have also been shown to influence human feeding behaviors. In a laboratory setting, females with a greater cortisol response consumed more calories following the stress than those with lower cortisol levels (2). Further, in this study, caloric intake was positively correlated with negative mood following the stress. The stress system, specifically hyperactivity of the hypothalamic-pituitary-adrenal (HPA) stress axis, has also been associated with binge eating disorder in humans (5, 6). Interestingly, individuals who decrease their overall caloric intake during stress still show an increased preference for highly palatable foods (3). While these and other studies have focused on overall caloric intake and general food categories (snack-type foods vs. meal-type foods), the preference for specific macronutrients during stress exposure is less clear.
Examination of the effects of stress or stress hormones on macronutrient preference in animal studies has yielded conflicting results. Administration of exogenous stressors has differing effects on preferences, with larger glucocorticoid doses appearing to promote fat consumption and relatively lower doses affecting carbohydrate consumption (8, 9), with background strain and age also playing a role in the relationship between stress and diet choice (10). A number of studies focusing specifically on fat intake have also shown that elevated glucocorticoids lead to increased fat consumption when offered as the only preferred diet (11–13). Appropriately, fat intake decreases anxiety-like behaviors and facilitates stress recovery (13–16). Studies of binge eating in rats also show that prior stress exposure may be a necessary trigger to promote the overconsumption of palatable foods (17, 18). These studies support an important link between stress pathways and dietary preferences that may be important for the predisposing effects of stress toward obesity and binge-eating in individuals with heightened sensitivity.
Based on these connections between stress responsivity and stress-induced eating, we have utilized a mouse model that displays maladaptive stress responses and increased sensitivity to evaluate the effects of chronic stress on macronutrient selection. These mice, deficient for corticotropin-releasing factor receptor-2 (CRFR2), show an exaggerated HPA response to stress, delayed stress recovery, and increased maladaptive coping responses in multiple behavioral paradigms (19). Further, we have previously reported that these mice also display alterations in markers of peripheral energy utilization and storage, allowing us to examine the effects of whole organism stress sensitivity on macronutrient preference during chronic stress (20, 21). In these studies, we hypothesized that the proportion of calories consumed by the mice from a high fat diet would be increased by stress, and that these effects would be exaggerated in our mouse model of stress sensitivity. Further, we hypothesized that the specific intake of the highly preferred high fat diet during limited access would show an even greater genotypic response to stress.
Materials and Methods
Unlimited macronutrient choice preference
CRFR2-deficient (KO) mice and wild type (WT) littermates were generated on a mixed C57Bl/6:129J background by heterozygous crosses as previously described (19). Male mice (WT n = 20; KO n = 19) were individually housed under a 12:12 light/dark cycle (lights on 0700 h), with food and water available ad libitum. Mice were provided with pre-weighed pellets of high fat, high protein, and high carbohydrate diets (Research Diets, Inc., New Brunswick, NJ) for 16 days. High fat diet (4.73 kcal/g) contained 44.9% fat, 35.1% carbohydrate, and 20% protein by kcal. High protein diet (4.29 kcal/g) contained 29.5% fat, 30.5% carbohydrate, and 40% protein, and high carbohydrate diet (3.85 kcal/g) contained 10% fat, 70% carbohydrate, and 20% protein. See Table 1 for detailed diet composition. All diets contained an equal amount of vitamins and minerals and were each designed to be nutritionally complete and as similar in texture and appearance as possible.
Table 1.
Composition of diets.
| % kcal | High Fat | High Protein | High Carbohydrate |
|---|---|---|---|
| Casein | 19.7 | 39.4 | 19.7 |
| L-cystine | 0.3 | 0.6 | 0.3 |
| Corn Strach | 7.2 | 7.5 | 31.1 |
| Maltodextrin | 9.9 | 4.9 | 3.5 |
| Surcose | 17 | 17 | 34.5 |
| Soybean Oil | 5.5 | 5.5 | 5.5 |
| Lard | 39.4 | 24 | 4.4 |
| Vitamin Mix | 1 | 1 | 1 |
Food intake and body weight were measured on alternate days (at approximately 1600 h) throughout the study. Mice were given one week of access to all diets to become familiar with them before measurements began. At that point, pre-weighed food pellets were placed on the floor of the cage and were weighed and changed every two days. The amount of food given was enough to ensure that mice did not run out of any diet. Following the initial studies, the experiment was repeated in a second set if animals (n = 4–6) in order to collect individual fat pads and measure plasma leptin levels. All studies were done according to experimental protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee, and all procedures were conducted in accordance with institutional guidelines.
Chronic Variable Stress
To examine the effects of stress on macronutrient choice preference we employed chronic variable stress (CVS), an ethologically relevant stress model designed to be resistant to habituation. Mice (WT n = 11; KO n = 12) experienced one stressor per day for 17 days. Stressors were designed to be unpredictable, occurring during both the light and dark cycle. Seven individual stressors were used, with the order of stressors changed each week (see Table 2). The stressors were 24 h of constant light, 5 min isoflurane anesthesia, being housed in another male’s soiled cage for 24 h, multiple cage changes (4–5 X) with varying intervals in a single day, 15 min of physical restraint, 24 h of novel noise using a white noise generator (Brookstone, Merrimack, NH), and a novel object placed in the cage overnight (glass tube or marbles). Mice were sacrificed 24 hrs following the final stressor. A control group (WT n = 9; KO n = 7) did not experience CVS but otherwise received identical handling and treatment throughout the study period.
Table 2.
Order of stressors.
| Stressor | Day of study |
|---|---|
| 24 hr Light | 0,13 |
| 5 min anesthesia am/pm | 1,7,14 |
| Housed in another male’s cage | 2,10,15 |
| Multiple cage changes | 3,11 |
| 15 min restraint am/pm | 0, 4, 8, 17(final time point) |
| Novel object | 6, 9, 16 |
| Novel noise | 5, 12, 16 |
Physiology
To assess alterations in circulating glucose levels due to stress and dietary changes, tail blood was taken to measure fed serum glucose using the OneTouch Ultra glucose meter (Johnson & Johnson, Milpitas, CA) in the afternoon on the day prior to sacrifice.
Plasma corticosterone was compared throughout CVS to ensure a continued stress response. Tail blood was drawn immediately prior to and following the 15 min restraint to determine the effects of CVS on basal and stress-induced corticosterone levels. CVS groups were bled on day 0, day 8, and day 17 of stress. Restraint was conducted between 1000 and 1100. Control mice were not restrained or bled to eliminate any effects of stress on their diet consumption or body weight, as we have previously reported genotypic responses to an acute restraint (19). Blood was centrifuged for 10 min at 5000 rpm at 4°C, and plasma collected and stored at −80°C. Corticosterone levels were determined using a commercially available radioimmune assay kit (MP Biomedicals, Orangeburg, NY). Two microliters of plasma were used per sample, and all samples were run in duplicate. The sensitivity of the assay was 7.7 ng/ml, and the intra- and interassay coefficients of variance were 7.3% and 6.9% respectively.
Plasma leptin was analyzed from trunk blood taken on the day of sacrifice, with plasma separated as for the corticosterone assay. Leptin levels were determined by radioimmune assay (Linco Research, St. Charles, MO). Fifty microliters of plasma were used per sample, and all samples were run in duplicate. The sensitivity of the assay was 0.2 ng/ml, and the intra- and interassay coefficients of variance were 7.2% and 7.9% respectively.
To examine relative amounts of body fat following stress and macronutrient choice preference, decapitated carcasses were frozen on dry ice and transported to the University of Pennsylvania Mouse Phenotyping Core for body fat analysis. Carcasses were thawed to room temperature, weighed, dried overnight in a 60°C oven, and weighed again. Carcasses were then dissolved in a 2:1 mixture of EtOH:KOH overnight in a 60°C oven, mixed with 50% EtOH, and incubated with 1M MgCl2 on ice for 10 min. Samples were centrifuged at 15,000 rpm for 30 min and supernatant removed to determine fat content. Individual fat pads (reproductive, renal, mesenteric, and brown fat) were also dissected from separate set of identically treated mice (n = 4 – 6 per group) and weighed to examine differences in fat distribution.
Biochemical analysis
Brown adipose tissue (BAT) was homogenized and protein extracted and separated by SDS-PAGE followed by Western blotting as previously described (21). To examine possible changes in non-shivering thermogenesis, membranes were probed for levels of uncoupling protein (UCP1; Calbiochem, La Jolla, CA) and normalized to -actin (Sigma, St. Louis, MO). Analysis was conducted as previously described (22).
Limited diet access
As the amount of high fat diet consumed during ad libitum access was approximately 95% of total calories for both genotypes, we developed a limited access model wherein mice were provided with the preferred high fat diet for only one hour per day in order to better assess possible genotypic differences in stress-induced consumption when the high fat diet was not the main source of calories. Male mice (n = 4–6 per group) were individually housed with food pellets placed on the floor. A pre-weighed amount of standard chow (Purina Lab Diet, St. Louis, MO) was available at all times. House chow contains 4.00 kcal/g consisting of 28% protein, 12.1% fat, and 59.8% carbohydrate. For one hour each day (1430 –1530 hrs), each mouse was provided a single pellet of pre-weighed HF diet in the home cage. Diet intake and 24 hr chow intake were measured. Mice were provided three days to become familiar with the diet and feeding schedule prior to the start of the study. CVS was initiated on the day prior to the first intake measurement, as described above, and continued for 13 days.
Data analysis and statistics
All data are presented as average ± SEM and were analyzed using StatView SE+ (Abacus Concepts, Berkeley, CA). For the macronutrient choice preference experiment, caloric intake was normalized to mean body weight to correct for individual weight differences. Daily measures of food intake, weight gain, and caloric efficiency were analyzed using a repeated measures ANOVA with genotype and CVS treatment as the independent variables. Where time was a significant factor, post hoc testing was conducted at individual time points were using a two-way ANOVA for genotype and stress treatment. Plasma corticosterone was analyzed using a two-way ANOVA for genotype and time point, and separate repeated-measures ANOVAs were run for 0 and 15 min time points with genotype as the independent variable. Total caloric intake, overall weight gain, overall caloric efficiency, serum glucose, plasma leptin, fat pad weight, and carcass composition data were analyzed using a two-way ANOVA with Fisher’s PLSD post hoc test to compare the effects of genotype and CVS treatment. UCP1 protein levels were analyzed using a student’s t-test to compare treatment groups within each blot. Limited access intake was analyzed using a repeated-measures ANOVA for daily intake and two-way ANOVA for total intake with genotype and CVS treatment as the independent variables. Two-way ANOVAs were performed on individual time points where repeated measures analysis revealed a significant change over time.
Results
Stress effects on caloric intake and macronutrient choice
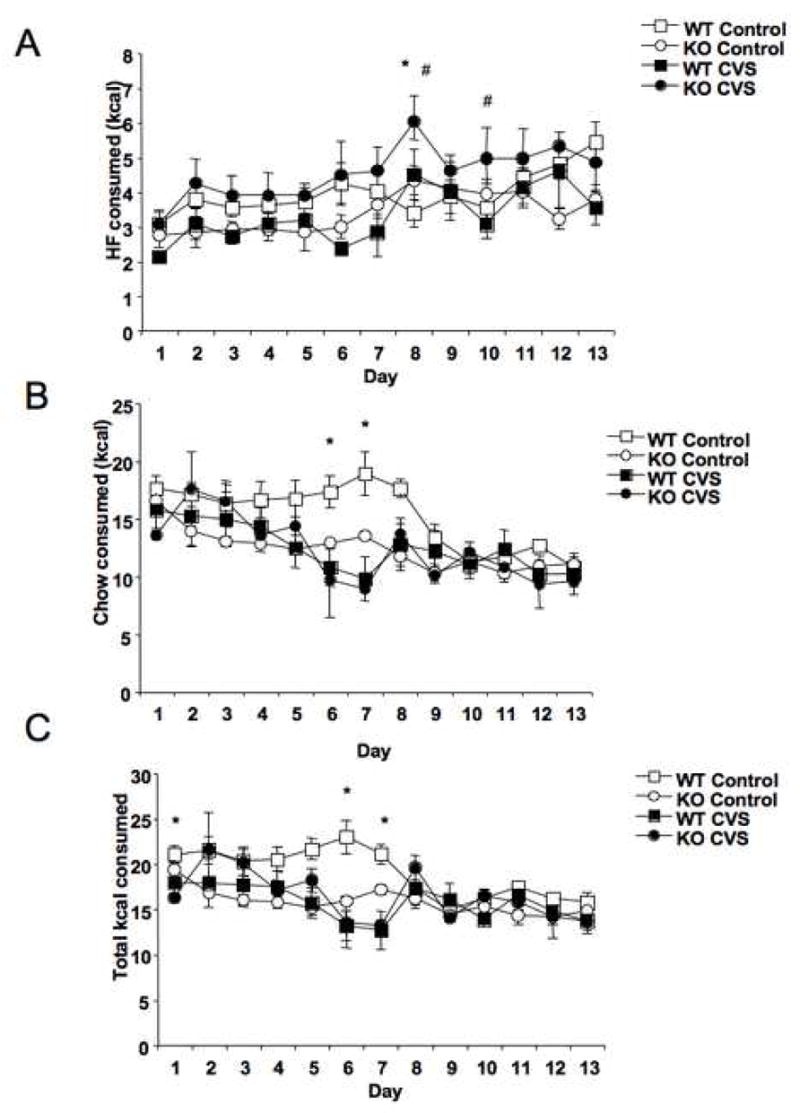
In order to determine how stress affects macronutrient preference, mice were presented with 16 days of diet choice under basal conditions or during CVS. Total caloric intake normalized to mean body weight was not different between genotypes, but was affected by stress treatment (F = 32.1, P < 0.0001), with mice consuming more total calories if subjected to CVS (Fig. 1A). Analysis of total caloric intake over the course of the study revealed that caloric intake decreased over time (F = 29.3, P < 0.0001; Fig. 1B) and was increased by CVS (F = 32.0, P < 0.0001). There was also a significant interaction between time and stress, with stress-exposed mice showing a greater decrease in total caloric intake (F = 12.9, P < 0.0001). To correct for the differences in overall caloric intake, macronutrient consumption was analyzed as a percentage of total calories. Overall intake of high fat diet was increased by CVS but not affected by genotype (F = 61.3, P < 0.0001; Fig 1A). There were also a significant effects of stress (F = 27.3, P < 0.0001) and time (F = 4.2, P < 0.001), with daily fat intake decreasing by about 5% over the course of the study, as well as an interaction between time and stress (F = 2.5, P < 0.05), with CVS mice showing a lesser change in daily fat intake compared to controls (Fig. 1C). Analysis of high protein intake also revealed a significant effect of stress (F = 13.0, P = 0.001), with control mice consuming more high protein diet than stressed mice (Fig. 1A). A main effect of stress on relative protein consumption was also observed (F = 12.9, P = 0.001; Fig. 1D). Analysis of carbohydrate intake revealed a significant effect of stress on overall high carbohydrate diet consumption (Fig. 1A), with mice undergoing CVS consuming less of the high carbohydrate diet (F = 12.1, P < 0.01). Proportional daily intake of the high carbohydrate diet increased over time (F = 4.9, P < 0.0001) and was affected by stress (F = 10.4, P < 0.01), with CVS leading to decreased relative consumption of high carbohydrate diet (Fig. 1E).
Fig. 1.
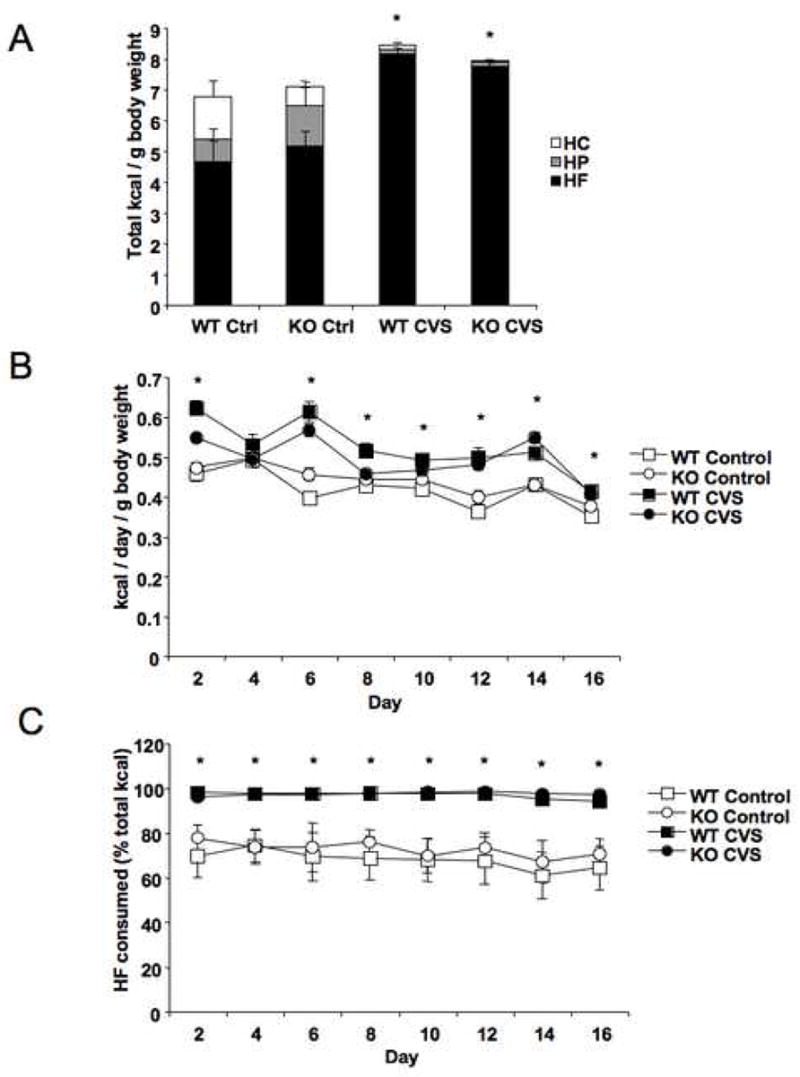
Macronutrient preferences are altered by chronic variable stress (CVS) in both wild type (WT) and CRFR2-deficient (KO) mice. (A) CVS resulted in an increase in overall caloric intake as well as a decrease in calories consumed as high protein (HP) and high carbohydrate (HC) diets and an increase in calories consumed as high fat (HF) diet relative to non-stressed control groups (Ctrl). *P < 0.05 compared to control. (B) There was a main effect of CVS revealed by repeated measures ANOVA, resulting in increased caloric intake in both genotypes (F = 61.0, P < 0.0001). There was also an effect of time where daily caloric intake decreased over the course of the study (F = 40.9, P < 0.0001). *P < 0.05 post hoc effect, CVS compared to control. (C) Analysis of HF diet intake as a percent of total calories by repeated measures ANOVA revealed a significant effect of CVS to increase HF intake (F = 27.3, P < 0.0001) and an effect of time to decrease HF intake (F = 4.2, P < 0.001). *P < 0.05 post hoc effect, CVS compared to control. (D) During CVS, HP diet consumption as a percent of total calories was decreased (F = 12.9, P = 0.001). (E) Consumption of HC diet as a percent of total calories was decreased by CVS treatment as revealed by repeated measures ANOVA (F = 10.4, P < 0.01). There was also an effect of time where HC intake increased over time (F = 4.9, P < 0.0001). *P < 0.05 post hoc effect, CVS compared to control, #P < 0.05 post hoc effect, KO compared to WT.
Effects on caloric efficiency
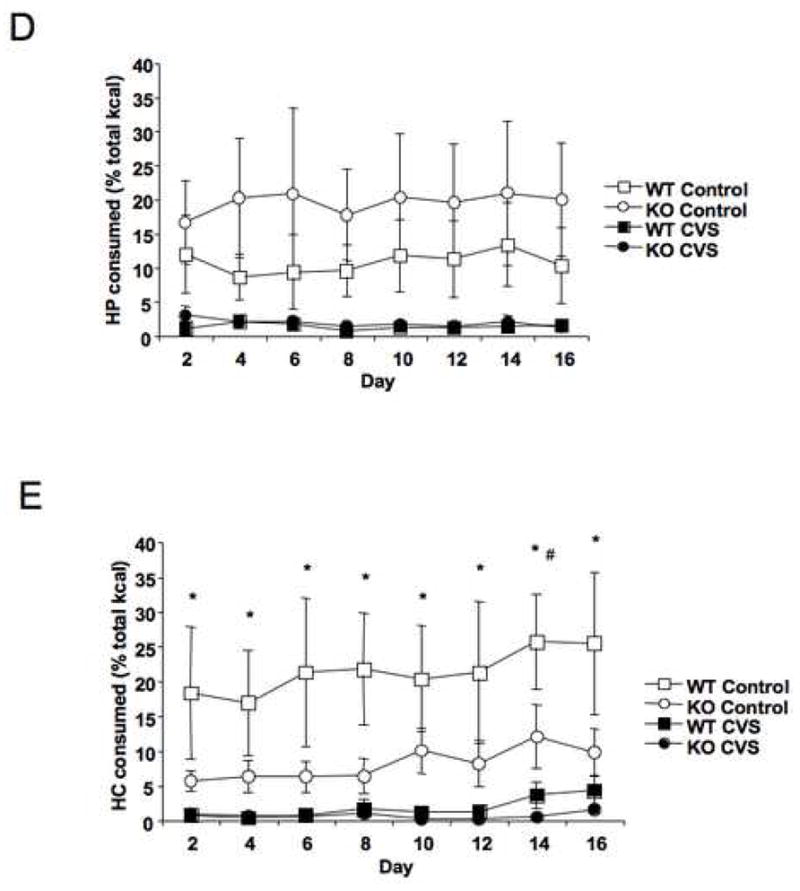
Mice were weighed on alternate days to investigate the role of stress on energy utilization. The data revealed a significant effect of stress (F = 7.3, P < 0.05) and an interaction between time and stress (F = 6.4, P < 0.0001) and between time, genotype, and stress (F = 3.9, P < 0.001) on percent weight gain (Fig. 2A). Mice that experienced CVS gained significantly less as percent of start weight compared to control mice (F = 8.7, P < 0.01), and post hoc analysis revealed this difference to be most apparent in the stress-sensitive KO mice (F = 5.2, P < 0.05). Caloric efficiency was calculated by dividing weight gain during a given period by the number of calories consumed during that period. Similar to weight gain, there were significant changes in caloric efficiency over the course of the study (F = 14.4, P < 0.0001), a main effect of stress (F = 28.8, P < 0.0001), an interaction between genotype and stress (F = 4.5, P < 0.05), and an interaction between time and stress (F = 12.3, P < 0.0001; Fig. 2B). CVS treatment led to a significant decrease in overall caloric efficiency (F = 19.5, P < 0.0001), and as with weight gain post hoc analysis showed that this decrease was exaggerated in the KO mice (F = 4.7, P < 0.05; Fig. 2C).
Fig. 2.
Weight gain and caloric efficiency were reduced by chronic variable stress (CVS) primarily in stress-sensitive CRFR2-deficient (KO) mice. (A) Proportional weight gain since day 0 for wild type (WT) and KO mice. Repeated measures analysis revealed a main effect of CVS (F = 6.8, P < 0.05) that was primarily due to reduced weight gain in the KO mice during CVS. Post hoc testing of individual time points revealed a significant interaction between genotype and CVS treatment (P < 0.05) on days 10, 12, and 16. *P < 0.05 post hoc effect, CVS compared to control. (B) CVS led to significant changes in caloric efficiency as revealed by repeated measures ANOVA (F = 28.8, P < 0.001), which also revealed an effect of time (F = 14.4, P < 0.0001). *P < 0.05 post hoc effect, CVS compared to control. (C) Overall caloric efficiency was significantly reduced following CVS in both genotypes (P < 0.0001). This reduction was greater in KO mice. *P < 0.001 compared to KO control group.
Plasma corticosterone physiology
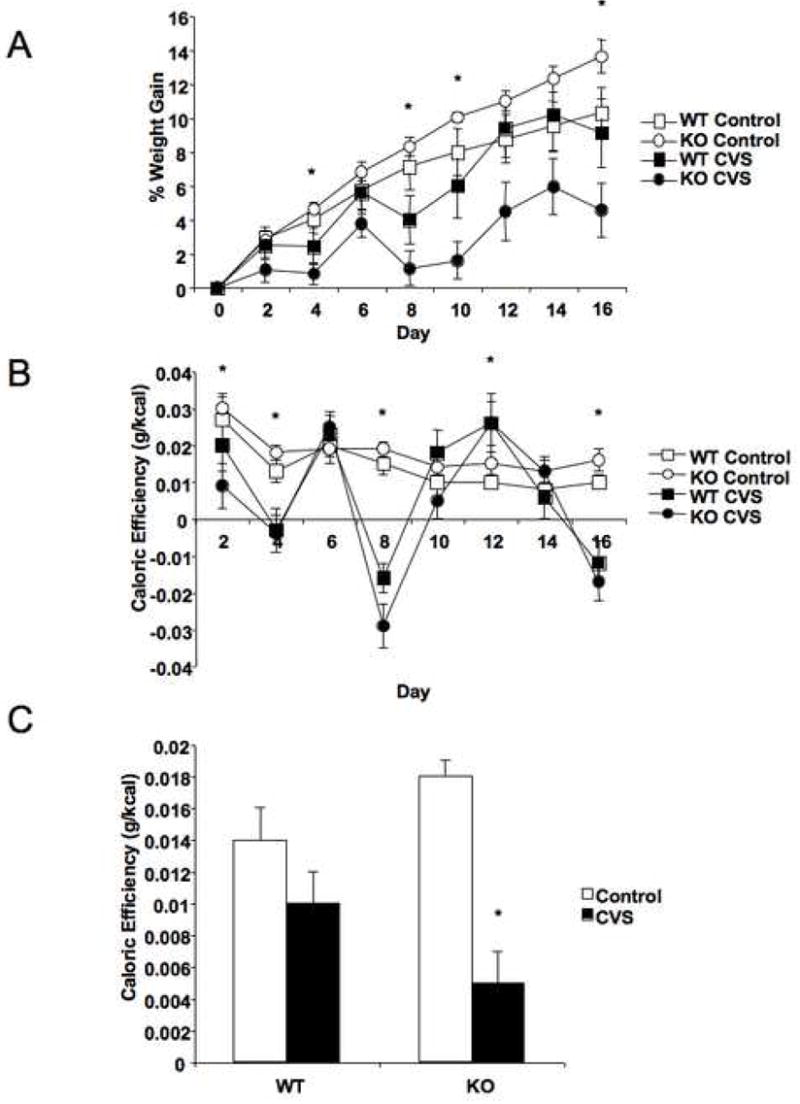
Plasma corticosterone was compared weekly throughout CVS to ensure a continued stress response in the CVS exposed mice (Fig. 3A). Both groups showed a significant elevation in plasma corticosterone following 15 min restraint relative to baseline at all points in the study (F = 37.1, P < 0.001 at day 0; F = 62.9, P < 0.0001 at day 8; F = 79.0, P < 0.0001 at day 17). Repeated measures ANOVA revealed that basal corticosterone levels remained stable over the course of the study. Stress-induced levels, however, fell over the course of the experiment (F = 4.3, P < 0.05), with KO corticosterone levels following stress falling to half their original levels. Stress-induced WT corticosterone levels remained unchanged. The rise in corticosterone was not different between genotypes at day 0 or day 8, but there was an effect of genotype on corticosterone rise on day 17 (F = 10.6, P < 0.01), with WT mice displaying a greater rise than KO mice.
Fig. 3.
Chronic variable stress (CVS) and diet altered circulating levels of corticosterone and glucose, but not leptin. (A) CRFR2-deficient (KO) mice showed a reduction in the plasma corticosterone rise following a restraint stress during prolonged macronutrient choice preference (P < 0.05 Day 17 vs. Day 0), while stress-induced corticosterone levels remain elevated in wild type (WT) mice. Both KO and WT mice showed significant elevations in corticosterone following a 15 min restraint stress (Stress) compared to levels at the time of stress initiation (Basal; P < 0.0001). By day 17, KO mice showed a significantly reduced stress-induced rise in corticosterone relative to WT levels (*P < 0.01). Day 0 represents the day prior to initiation of CVS. (B) Fed serum glucose levels in WT and KO mice were measured in the afternoon one day prior to sacrifice, and were significantly affected by both genotype (P < 0.05) and CVS (P < 0.01), where CVS reduced WT glucose levels to that of KO mice. *P < 0.05 compared to WT. (C) No significant differences were observed in plasma leptin levels between either genotypic or stress treatment groups following 2.5 weeks of macronutrient choice preference and CVS.
Blood glucose, leptin, and body fat
Fed glucose levels were measured in the afternoon on the day prior to sacrifice. There were main effects of both genotype (F = 2.3, P < 0.05) and stress (F = 9.2, P < 0.01) on average serum glucose (Fig. 3B). Post hoc analysis showed that under baseline conditions, WT mice showed higher levels of serum glucose (F = 4.5, P = 0.05), and that CVS treatment significantly decreased WT glucose to KO levels (F = 6.4, P < 0.05). No further decrease was detected in KO mice with CVS.
Plasma leptin levels were assessed from blood samples taken at sacrifice. No significant differences were observed between groups (Fig. 3C). Body fat content was analyzed at the end of the study to determine changes in energy storage (Fig. 4A). There were no significant effects of either genotype or stress treatment on overall body fat percentage, although the data suggest that KO mice may display reduced fat stores following CVS, as we have previously reported (21). Individual reproductive, renal, and mesenteric fat pads were dissected and weighed, as was brown fat (Fig. 4B). No stress-induced differences were observed in any of these fat depots. However, there was a genotypic difference in relative weight of the mesenteric fat pad, with KO mice showing an increased proportion of mesenteric fat (F = 6.3, P < 0.05).
Fig. 4.
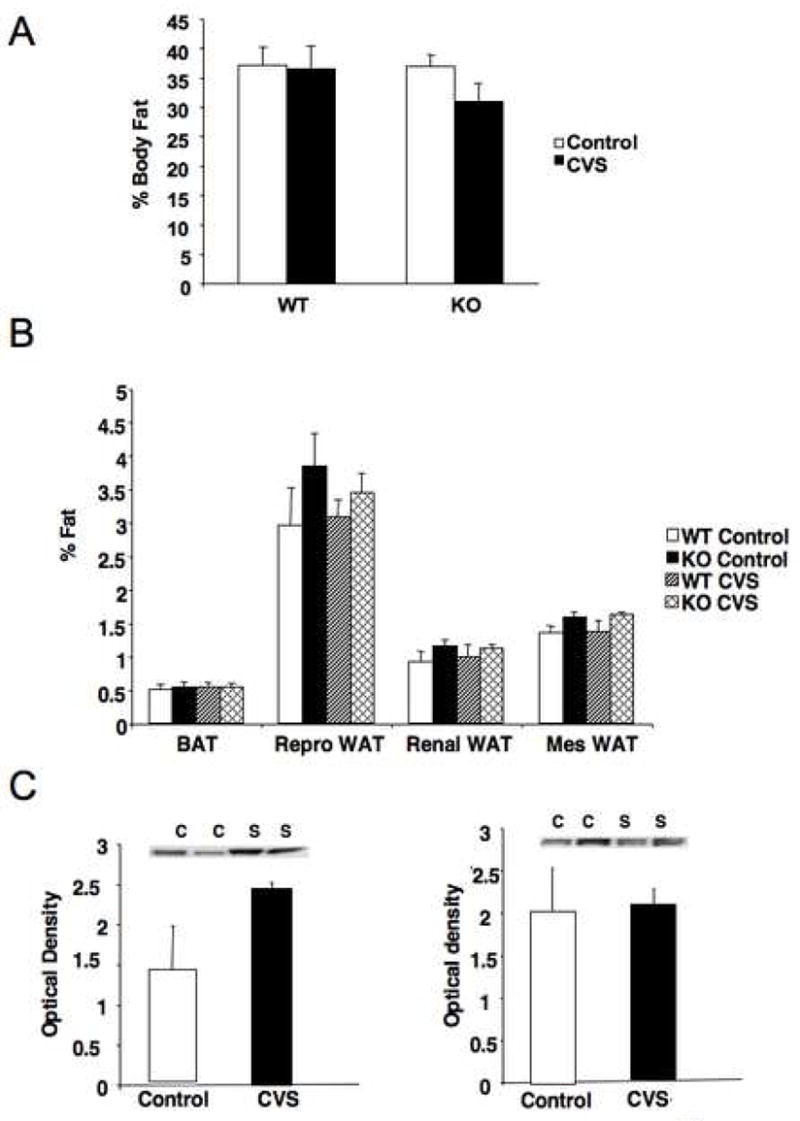
Stress-sensitive mice have increased mesenteric fat, but stress does not lead to changes in overall adiposity. (A) No significant differences were observed in the total percentage of body fat between wild type (WT) and CRFR2-deficient (KO) mice, or between stress groups, although the KO mice did show a trend for reduced body fat following chronic variable stress (CVS). (B) When individual fat pads were weighed and normalized to body weight, no significant differences were observed for brown adipose tissue (BAT), reproductive white adipose tissue (Repro WAT) or renal white adipose tissue (Renal WAT). However, there was a main effect of genotype on the percentage of mesenteric white adipose tissue (Mes WAT, P < 0.05), with KO mice having more mesenteric adipose tissue. (C) No significant differences between control (C) and CVS (S) groups in UCP1 expression in BAT were observed in either WT (left panel) or KO mice (right panel).
In order to examine possible changes in non-shivering thermogenesis, we determined levels of UCP1 in brown adipose tissue. No stress-induced changes were observed (Fig. 4C). We have previously shown that KO mice have basally elevated levels of UCP1 compared to WT littermates (21).
Effects of stress on HF diet intake during limited access
As CVS led to both genotypes consuming ~ 95% of their total calories in the form of high fat diet, possible genotypic differences in consumption may have been masked by a ceiling effect. Thus, we examined the effects of CVS on HF intake during limited access where HF diet was available for only one hour per day, with house chow available ad lib. There was a significant interaction of genotype and stress treatment (F = 5.6, P < 0.05), with KO mice increasing their intake of HF diet during the limited access time in response to CVS, while WT mice actually showed reduced HF intake in response to CVS when the diet was available for only one hour each day (Fig. 5A). There was also a main effect of time (F = 9.5, P < 0.0001), with consumption increasing over the course of the experiment, and a significant interaction between time and stress (F = 1.9, P < 0.05). Chow intake was decreased over time (F = 13.2, P < 0.0001), with a significant interaction between time and stress treatment (F = 4.6, P < 0.0001; Fig. 5B). Analysis of total daily caloric intake revealed a significant interaction between genotype and stress (F = 5.8, P < 0.05), with KO mice increasing overall consumption during stress and WT mice decreasing consumption (Fig. 5C). There was also a main effect of time (F = 7.6, P < 0.0001) and interactions between time and stress (F = 4.4, P < 0.0001) and time, stress, and genotype (F = 1.9, P < 0.05). Overall caloric intake decreased over time, with KO mice exposed to CVS showing the least change in total caloric intake. Overall diet intake was affected by an interaction between genotype and stress treatment (F = 4.6, P < 0.05; Fig. 5D). Analysis of total chow intake revealed a trend for KO mice to consume less chow (F = 3.2, P < 0.10), particularly under basal conditions. This effect was shown to be significant with post hoc testing (P < 0.05; Fig. 5E). There was a significant interaction between genotype and stress on over all caloric intake (F = 5.8, P < 0.05; Fig. 5F), with stress decreasing overall intake in WT mice and slightly increasing intake in KO mice. Post hoc testing also revealed an effect genotype (P < 0.05) on overall caloric intake, with KO mice consuming fewer total calories.
Fig. 5.
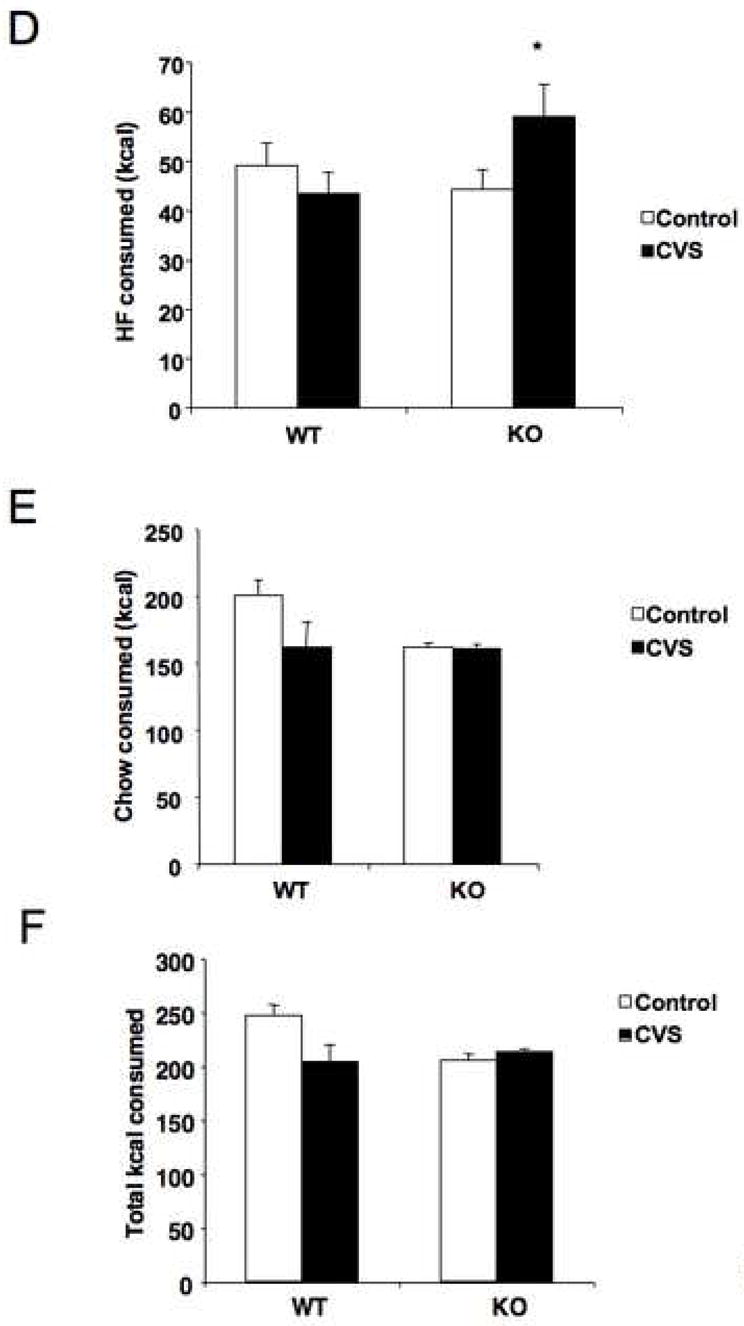
Stress-sensitive mice increased consumption of a high fat diet (HF) during chronic variable stress (CVS) when access was restricted. (A) Analysis by repeated measures ANOVA revealed an effect of time, with HF consumption increasing over the course of the study (F = 9.5, P < 0.0001). There was also a significant interaction between genotype and CVS, with CRFR2-deficient (KO) mice increasing HF intake during CVS and wild type (WT) mice decreasing HF intake (F = 5.6, P < 0.05). Post hoc testing at individual time points revealed a significant interaction between stress and genotype on days 3, 6, and 13 (P < 0.05). *P < 0.05 post hoc effect, CVS compared to control, #P < 0.05 post hoc effect, KO compared to WT. (B) There were no main effects of either genotype or CVS treatment revealed by repeated measures ANOVA on chow intake, but there was a decrease over time (F = 13.2, P < 0.0001). Post hoc testing at individual time points revealed a significant interaction between genotype and CVS treatment on days 5 and 8 (P < 0.05). *P < 0.05 post hoc effect, CVS compared to control. (C) Daily total caloric intake decreased over time by repeated measures ANOVA (F = 7.6, P < 0.0001), and there was a significant interaction between genotype and CVS treatment (F = 5.8, P < 0.05), with CVS decreasing overall caloric intake in WT mice and increasing it in KO mice. Post hoc testing at individual time points revealed a ignificant interaction between genotype and CVS treatment on days 5 and 6 (P < 0.05). *P < 0.05 post hoc effect, CVS compared to control. (D) Analysis of overall intake of HF revealed a significant interaction between genotype and CVS (*P < 0.05). CVS increased HF consumption in KO mice and decreased HF consumption in WT mice relative to control groups. (E) Analysis of total house chow intake revealed a main effect of genotype (P < 0.05), with KO mice consuming less chow, particularly under basal conditions. (F) There was a significant interaction between genotype and CVS treatment on overall caloric intake, with WT mice decreasing total intake during CVS and KO mice increasing total intake (P < 0.05).
Discussion
As stress is often linked to an increased consumption of calorically-dense foods and an elevated risk of obesity, we have utilized a genetic mouse model of elevated stress sensitivity to examine the contribution of this phenotype to dietary preferences, binge-eating, and energy balance. Our results show that mice increased the percent of calories obtained from a high fat diet, as well as their overall caloric intake during chronic variable stress (CVS). Supportive of our hypothesis, CVS exposure and macronutrient-enriched preferred diet availability resulted in genotype-specific effects on weight gain, caloric efficiency, and stress-induced corticosterone levels. When access to the high fat diet was restricted, CVS also led to a genotype-specific increase in fat consumption in stress-sensitive mice.
To examine the influence of stress on macronutrient-enriched diet preference, we analyzed intake in terms of the percent of total calories. Under basal conditions, both genotypes consumed approximately 80% of their calories in the form of high fat diet. During CVS, mice consumed a significantly greater proportion of their total calories (nearing 100%) from this diet, indicating a selective preference for fat during CVS. The absence of a genotypic difference during unrestricted access is likely related to a threshold effect of maximal high fat diet intake reached by both groups. Therefore, in order to investigate stress-induced genotypic disparities, further examined the outcome during a limited high fat access model with CVS.
In concert with the increase in high fat intake observed during unlimited access, CVS also led to a decrease in high protein intake by both genotypes. This decrease was especially apparent in the stress-sensitive KO mice that appeared to show a basally higher intake of the high protein diet. This difference may be related to metabolic alterations in these mice. We have previously shown that CRFR2-deficient mice have an increased lean body mass and increased respiratory exchange ratio when exposed to a chronic high fat diet as well as increased basal thermogenesis (20, 21). Further studies will be required to determine the role of this metabolic phenotype in macronutrient preferences, especially as it relates to protein intake. To date, studies of stress and macronutrient intake have largely focused on fat and carbohydrates, and thus relatively little is known about the preference for dietary protein.
Relative intake of the high carbohydrate diet was also decreased in both genotypes during CVS. Interestingly, KO mice showed a trend for a reduced basal preference for the high carbohydrate diet compared to WT mice. While not significant, there may be an increase in intake of this diet by the end of the two weeks of stress exposure, although relative consumption remained low. Studies have shown an increased consumption of snacks high in both fat and sugar following an acute laboratory stressor in humans (2, 4, 23). However these studies often have not distinguished between the effects on individual macronutrients and overall caloric content. Further stress-induced increases in the high carbohydrate intake in our study may have been masked by the much more substantial consumption of the high fat diet. As all of the macronutrient-enriched diets used are nutritionally complete, the combination of macronutrients available in each diet may also contribute to the changes in preference observed during CVS.
In our studies, CVS exposure led to an overall increase in total caloric intake in both genotypes. Previous reports of stress and food consumption in rodents have shown that stress decreased caloric intake (24, 25), although work in the Syrian hamster has shown increases in food intake following chronic stress (26, 27). However, the majority of such studies have examined only consumption of standard chow and utilized more severe stress paradigms. There is a growing body of evidence in both rodents and humans showing that intake of palatable foods is increased during periods of stress, even if overall caloric intake is reduced (4, 7, 13). We have previously shown that the high fat diet used in these studies is significantly preferred to our house chow or to a high carbohydrate diet, and that withdrawal from such a diet precipitates an increased stress state that elevates a motivation for diet reinstatement (22). Our results presented here support the hypothesis that stress exposure results in increased consumption of preferred diets, especially those high in fat.
In addition to changes in macronutrient preferences, our results revealed increased energy utilization as evidenced by a decreased rate of weight gain and decreased caloric efficiency during CVS. Although there was a main effect of stress on the rate of weight gain, WT mice showed a minimal effect of CVS. However, the rate of gain was substantially reduced in KO mice during CVS exposure. The stress-induced reduction in average caloric efficiency was also most dramatic in the stress-sensitive KO mice in which CVS produced a two-fold greater change from control levels. While caloric efficiency remained relatively stable in the control groups of both genotypes, it fluctuated greatly during CVS exposure indicating a likely influence of the individual stressors utilized in our CVS model. These results support our previous studies showing that stress-sensitive KO mice exposed to repeated stress respond with a greater impact on weight loss and food intake (21).
To assess a continued responsivity of the hypothalamic-pituitary-adrenal (HPA) stress axis for both genotypes throughout the course of CVS exposure, corticosterone levels were measured following the 15 min restraint stress each week. Our results found that the WT mice continued to respond to this stressor, as evidenced by a maintenance of plasma corticosterone levels in response to a physical restraint over the course of 17 days of CVS. However, the stress-sensitive KO mice actually showed a diminution of the corticosterone response over the study time, in contrast to their previously described elevated corticosterone responsivity (19). In the present study, we hypothesize that the stress-sensitive mice may be displaying an enhanced sensitivity to this effect of preferred diet such that with increased time on the diet (one week versus two or three weeks), the stress response was diminished. Work by Mary Dallman and colleagues has demonstrated that access to a choice of a diet high in fat can significantly dampen the HPA response to stress (7, 13, 14). Notably, the “choice” of a high fat diet, rather than the exposure to a chronic high fat diet alone, may be important for the attenuation of the stress response observed in these studies, possibly explaining why mice continue to consume the other diets despite the heightened preference for high fat (14). Length of diet is likely also important and may explain why the HPA response of the KO mice changes over time (28). In our study, WT mice do not show a reduced response over time, indicating that inherent stress sensitivity or dysregulation may play a role in dietary modulation of the stress axis. It is possible that WT mice would show a similar dampening of the HPA axis with more prolonged diet exposure or access to a diet with a higher fat content. The data do suggest, however, that KO mice may have a reduced threshold for the stress dampening effects of a high fat diet. Links between stress and reward pathways may underlie this increased sensitivity to the preferred diet, as consumption of rewarding substances has been shown to reduce central CRF expression (29–32).
As an index of energy storage and availability, fed serum glucose levels were examined prior to sacrifice. Glucose levels were significantly different between genotypes and treatment groups following 17 days of macronutrient choice preference. Under non-stressed conditions, the stress-sensitive KO mice showed significantly reduced serum glucose compared to WT mice. Interestingly, following CVS, WT mice showed a reduction in glucose to levels similar to that of KO mice. Such a reduction may be related to the decreased weight gain and caloric efficiency that occurred during stress exposure despite increased caloric intake. Our previous studies have shown that during chronic high fat diet exposure, the stress-sensitive KO mice do not show the predicted rise in glucose, and remain insulin sensitive (21). Therefore, the genotypic difference observed under the non-stressed conditions may be related to a diet-induced elevation in WT glucose levels to which the KO mice are resistant. We have also previously reported that KO mice remain resistant to diet-induced increases in overall body fat, which may contribute to the lower glucose levels in our current study (21).
To investigate the genotypic effects of CVS and preferred dietary intake on body composition and energy storage, overall adiposity was analyzed in these mice. Surprisingly, there were no main effects of either genotype or stress treatment on overall adiposity. However, there appeared to be a decrease in body fat specific to the stress-sensitive KO mice during CVS despite increases in dietary fat intake. We have previously reported that these mice show decreased body fat content following either a repeated stress or chronic high fat diet exposure, supporting a role of heightened stress sensitivity in overall energy utilization (20, 21). To further examine a possible differential distribution of specific adipose stores, we dissected select fat pads, including brown adipose tissue (BAT), renal, mesenteric, and reproductive adipose tissue. No differences were observed between groups in the relative amounts of BAT, reproductive, or renal fat. However, KO mice had a significantly elevated proportion of mesenteric fat despite no differences in overall adiposity. This finding is interesting in light of data in both humans and animal models suggesting that increased stress and glucocorticoid levels are associated specifically with increased visceral fat deposits (5, 33, 34). The trend toward reduced overall adiposity observed in KO mice was likely due to changes in other adipose stores, particularly subcutaneous fat. Taken together, these results support the use of the CRFR2-deficient mice as a model of increased stress-sensitivity, particularly in relation to feeding behavior and metabolism.
To further examine potential mechanisms for stress-mediated reductions in caloric efficiency, we examined levels of UCP1 in BAT from these mice. We found no stress-induced differences in UCP1 expression within either genotype. Previously, we have reported that KO mice have basally elevated UCP1 levels, indicative of increased basal non-shivering thermogenesis (20, 21). However, the lack of stress-induced changes observed in the current study make it unlikely that changes in caloric efficiency are related to changes in thermogenesis.
Our hypothesis predicted a genotypic difference in the effect of stress on high fat intake, where KO mice would show a greater increase in high fat consumption during CVS. However, as both genotypes consumed greater than 95% of their calories from this diet, determination of possible genotypic effects were likely masked by a ceiling effect. Therefore, we utilized a limited access paradigm to assess possible genotypic differences in high fat intake during CVS. Our results were supportive of our hypothesis, as mice provided with only one hour of daily access to the preferred high fat diet during CVS showed a significant interaction between genotype and stress treatment such that stress-sensitive KO mice increased their intake of this diet relative to controls, while WT mice actually showed a decrease in high fat consumption during this limited access exposure. This distinction from results during unrestricted access may suggest that a stress-sensitive phenotype is more prone to binge-eating behavior during stress. Stress is often cited as a necessary trigger for binge-eating, in conjunction with prior food restriction (17, 18, 35). Our data indicated that stress-sensitive mice might provide a model of stress-induced bingeing without the requirement of previous restriction. Stress-sensitive mice also tended to reduce chow intake during limited access to high fat when compared to WT mice under control conditions, with WT mice taking longer to show a decrease in chow consumption in response to high fat diet consumption. There was also an interaction between genotype and stress on overall caloric intake, consistent with studies comparing rats resistant or prone to binge eating (36). KO mice showed an overall increase in caloric intake in response to CVS, similar to that observed during unrestricted access due to the increased high fat consumption. WT mice, however, decreased their caloric intake in response to CVS, highlighting the importance of overall diet composition and timing of access to preferred diets on the relationship between stress and dietary intake (37).
The results from these studies support the hypothesis that stress promotes an increased preference for a diet high in fat. During macronutrient choice preference, mice increased their intake of high fat diet during CVS while decreasing their intake of the high protein and high carbohydrate diets as part of an overall increase in calories. These results support previous studies examining an involvement of stress pathways in dietary preferences (38, 39). We were able to further discern specific stress effects during limited access to high fat where stress-sensitive KO mice increased consumption during CVS when access to the diet was restricted, while WT mice did not. As a genetic model of stress sensitivity, KO mice also responded to CVS with reduced caloric efficiency and weight gain. These results support human studies showing that certain individuals increase their overall caloric intake, and intake of preferred foods in response to stress and may suggest a genetic predisposition toward this behavior related to stress pathway regulation (2–4), as well as providing another link between stress sensitivity and binge eating (5, 6).
Our data also suggest that prolonged consumption of preferred diets as modeled in macronutrient-enriched choices appears to dampen the physiological response to stress in the stress-sensitive KO mice. Although the mechanisms underlying stress influences on feeding are still being actively investigated, a number of studies point to an important role for CRF in both the paraventricular nucleus and central amygdala, areas directly involved in the stress response that also communicate with centers governing energy balance and reward, including the lateral hypothalamus, nucleus accumbens, and ventral tegmental area affecting both the homeostatic and hedonic aspects of palatable feeding (7, 32, 33). Utilization of the CRFR2-deficient stress-sensitive genetic mouse model provides further insight into the whole animal influence of stress on both central and peripheral mechanisms related to changes in dietary preferences and caloric utilization.
Acknowledgments
This work was supported by The University of Pennsylvania Diabetes Center DK019525. S. L. T. was supported in part by the Systems and Integrative Biology training grant. We thank K. Carlin and Y. Xiong for technical support and Dr. K. Semsar for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89(6):2522–5. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 2.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26(1):37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 3.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav. 1999;66(3):511–5. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- 4.Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. Food selection changes under stress. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Gluck ME, Geliebter A, Lorence M. Cortisol stress response is positively correlated with central obesity in obese women with binge eating disorder (BED) before and after cognitive-behavioral treatment. Ann N Y Acad Sci. 2004;1032:202–7. doi: 10.1196/annals.1314.021. [DOI] [PubMed] [Google Scholar]
- 6.Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med. 2004;66(6):876–81. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- 7.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100(20):11696–701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bligh ME, Douglass LW, Castonguay TW. Corticosterone modulation of dietary selection patterns. Physiol Behav. 1993;53(5):975–82. doi: 10.1016/0031-9384(93)90277-m. [DOI] [PubMed] [Google Scholar]
- 9.Kumar BA, Leibowitz SF. Impact of acute corticosterone administration on feeding and macronutrient self-selection patterns. Am J Physiol. 1988;254(2 Pt 2):R222–8. doi: 10.1152/ajpregu.1988.254.2.R222. [DOI] [PubMed] [Google Scholar]
- 10.Tannenbaum BM, Tannenbaum GS, Anisman H. Impact of life-long macronutrient choice on neuroendocrine and cognitive functioning in aged mice: differential effects in stressor-reactive and stressor-resilient mouse strains. Brain Res. 2003;985(2):187–97. doi: 10.1016/s0006-8993(03)03196-2. [DOI] [PubMed] [Google Scholar]
- 11.Prasad C, delaHoussaye AJ, Prasad A, Mizuma H. Augmentation of dietary fat preference by chronic, but not acute, hypercorticosteronemia. Life Sci. 1995;56(16):1361–71. doi: 10.1016/0024-3205(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 12.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145(5):2174–85. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- 13.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–62. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 14.la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146(5):2193–9. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- 15.Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996;60(3):1039–42. doi: 10.1016/0031-9384(96)00135-7. [DOI] [PubMed] [Google Scholar]
- 16.Buwalda B, Blom WA, Koolhaas JM, van Dijk G. Behavioral and physiological responses to stress are affected by high-fat feeding in male rats. Physiol Behav. 2001;73(3):371–7. doi: 10.1016/s0031-9384(01)00493-0. [DOI] [PubMed] [Google Scholar]
- 17.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77(1):45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 18.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34(2):183–97. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- 19.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24(4):410–4. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 20.Carlin KM, Vale WW, Bale TL. Vital functions of corticotropin-releasing factor (CRF) pathways in maintenance and regulation of energy homeostasis. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0511320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144(6):2580–7. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- 22.Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61(9):1021–9. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 23.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62(6):853–65. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu N, Oomura Y, Kai Y. Stress-induced anorexia in rats mediated by serotonergic mechanisms in the hypothalamus. Physiol Behav. 1989;46(5):835–41. doi: 10.1016/0031-9384(89)90045-0. [DOI] [PubMed] [Google Scholar]
- 25.Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 1999;92(1):327–41. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- 26.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1284–93. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- 27.Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R283–90. doi: 10.1152/ajpregu.00330.2006. [DOI] [PubMed] [Google Scholar]
- 28.Legendre A, Harris RB. Exaggerated response to mild stress in rats fed high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1288–1294. doi: 10.1152/ajpregu.00234.2006. [DOI] [PubMed] [Google Scholar]
- 29.Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24(47):10594–602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res. 2007;176(2):251–8. doi: 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laugero KD, Bell ME, Bhatnagar S, Soriano L, Dallman MF. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis? Endocrinology. 2001;142(7):2796–804. doi: 10.1210/endo.142.7.8250. [DOI] [PubMed] [Google Scholar]
- 33.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19(4):275–80. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Rebuffe-Scrive M, Walsh UA, McEwen B, Rodin J. Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Physiol Behav. 1992;52(3):583–90. doi: 10.1016/0031-9384(92)90351-2. [DOI] [PubMed] [Google Scholar]
- 35.Artiga AI, Viana JB, Maldonado CR, Chandler-Laney PC, Oswald KD, Boggiano MM. Body composition and endocrine status of long-term stress-induced binge-eating rats. Physiol Behav. 2007;91(4):424–31. doi: 10.1016/j.physbeh.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. Int J Obes (Lond) 2007;31(9):1357–67. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 37.Harris RB, Zhou J, Mitchell T, Hebert S, Ryan DH. Rats fed only during the light period are resistant to stress-induced weight loss. Physiol Behav. 2002;76(4–5):543–50. doi: 10.1016/s0031-9384(02)00754-0. [DOI] [PubMed] [Google Scholar]
- 38.Heinrichs SC, Koob GF. Corticotropin-releasing factor modulates dietary preference in nutritionally and physically stressed rats. Psychopharmacology (Berl) 1992;109(1–2):177–84. doi: 10.1007/BF02245497. [DOI] [PubMed] [Google Scholar]
- 39.Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J Pharmacol Exp Ther. 2004;310(3):1027–34. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]


