The plant cell wall is a highly complex and dynamic structure made up of cellulose, hemicelluloses, pectins, lignin, and numerous proteins and inorganic molecules (Carpita and McCann, 2000). The hemicellulose xyloglucan (XyG) is found in the cell walls of all vascular plants and is the major hemicellulose in the primary cell wall of eudicots and nongraminaceous monocots, where it is believed to play an important role in cell wall structure and function. Models of the plant cell wall postulate that XyG functions to cross-link adjacent cellulose microfibrils to form a cellulose-XyG network that constitutes the major load-bearing structure of the primary cell wall (Keegstra et al., 1973; Obel et al., 2007; O'Neill and York, 2003). It is also thought to act as a spacer preventing cellulose microfibrils from aggregating (Thompson, 2005).
In this issue of The Plant Cell, Cavalier et al. (pages 1519–1537) show that XyG provides some degree of mechanical strength to the primary cell wall, but the consequences of a lack of any detectable XyG in Arabidopsis seedlings is not catastrophic, as would be expected if it were a critical component of the load-bearing capacity of the expanding primary cell wall. The authors employ state-of-the art methodology to assess XyG content in XyG xylotransferase mutants, showing that the XyG xylotransferases XXT1 and XXT2 are required for XyG biosynthesis and opening new questions about the role of XyG in primary cell walls that challenge current thinking in this important area of fundamental plant biology.
XyG consists of a β-(1→4)-glucan backbone substituted with α-(1→6)-xylosyl residues in a regular pattern, as well as occasional galactosyl or fucosyl residues. A number of enzymes participate in XyG biosynthesis, including β-(1→4)-glucan synthase, α-fucosyltransferases, β-galactosyltransferases, and α-xylosyltransferases. Arabidopsis contains seven genes that encode putative XyG xylosyltransferases (XXTs), at least two of which (XXT1 and XXT2) exhibit activities capable of forming XyG from appropriate substrates in vitro (Faik et al., 2002; Cavalier and Keegstra, 2006; Fauré et al., 2007). Recent work by Zabotina et al. (2008) showed that XXT5 in Arabidopsis is involved in XyG biosynthesis in vivo, since mutant plants have altered XyG composition. In their work, Cavalier et al. set out to examine the putative role of XXT1 and XXT2 in XyG biosynthesis in more detail through the isolation and characterization of single and double T-DNA insertional mutants of these two genes.
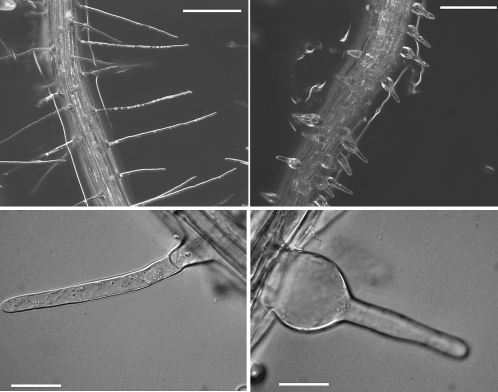
First, the authors obtained the xxt1 and xxt2 single mutant T-DNA insertion lines, which were crossed to obtain the xxt1 xxt2 double mutant. RT-PCR analysis confirmed the absence of the corresponding transcripts from the single and double mutant plants. The gross phenotype of the single mutant lines was indistinguishable from that of wild-type plants, whereas the double mutant plants grew more slowly, were smaller, and showed severely shortened and abnormal root hairs (see figure), which could be complemented by expression of either 35Spro:XXT1 or 35Spro:XXT2. Biochemical analyses of the mutant plants suggested that XXT1 and XXT2 encode active XXTs that participate in XyG biosynthesis in Arabidopsis.
Figure 1.
Root Hair Phenotype of the xxt1 xxt2 Double Mutant.
Wild-type root hairs (left) compared with the xxt1 xxt2 double mutant (right). Bars = 200 μm in top panels, 50 μm in bottom left panels, and 25 μm in the bottom right.
Surprisingly, despite a moderate abnormal phenotype, the double mutant plants were found to contain no trace of detectable XyG. Since this result was unexpected, the authors went to great lengths to assess XyG content of the mutant lines and confirm its absence in the double mutant. Four different types of analysis were conducted, all of which suggested that the double mutant lacks any detectable XyG: (1) oligosaccharide mass profiling using MALDI-TOF mass spectrometry, (2) immunohistochemistry using XyG-directed antibodies, (3) glycosyl linkage analysis, and (4) analysis of cell wall polysaccharides digested with the enzyme preparation Driselase, which provides a diagnostic indicator of XyG. The lack of XyG in the double mutant, despite expression of XXT5 (previously shown to participate in XyG biosynthesis), suggested that XXT1 and XXT2 are epistatic to XXT5 (i.e., the activity of one or the other is required for XXT5 to function).
The most notable phenotypes associated with the lack of XyG in the xxt1 xxt2 double mutant were the abnormal root hairs and significant changes in the mechanical properties of the hypocotyls of etiolated seedlings. The authors concluded that the root hair phenotype showed that XyG biosynthesis is important in these specialized cells that are undergoing rapid tip growth. Although both the stiffness and ultimate stress parameters suggested a moderate decrease in the integrity and strength of the cell wall, the absence of catastrophic changes despite the total lack of XyG suggests a radical rethinking of our models of the primary cell wall. The authors discuss the possibilities that (1) the double mutant contains an aberrant form of XyG that could not be detected by the methods employed, or (2) the lack of XyG is being compensated for, either by an altered β-glucan backbone capable of functioning as a cellulose cross-linking glucan in a similar fashion to XyG, or by modification of other components of the cell wall (such as pectin cross-linking).
The work of Cavalier et al. represents a major contribution to the study of plant cell walls. The results show that XXT1 and XXT2 play key roles in XyG biosynthesis and call for a reexamination of the role of XyG in the primary cell wall.
References
- Cavalier, D.M., and Keegstra, K. (2006). Two xyloglucan xylosyltransferases catalyze the addition of multiple xylosyl residues to cellohexaose. J. Biol. Chem. 281 34197–34207. [DOI] [PubMed] [Google Scholar]
- Cavalier, D.M., Lerouxel, O., Neumetzler, L., Yamauchi, K., Reinecke, A., Freshour, G., Zabotina, O.A., Hahn, M.G., Burgert, I., Pauly, M., Raikhel, N.V., and Keegstra, K. (2008). Disruption of two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20 1519–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita, N., and McCann, M. (2000). The cell wall. In Biochemistry and Molecular Biology of Plants, B.B. Buchanan, G. Wilhelm, and R.L. Jones, eds (Rockville, IL: American Society of Plant Physiologists), pp. 52–108.
- Faik, A., Price, N.J., Raikhel, N.V., and Keegstra, K. (2002). An Arabidopsis gene encoding an a-xylosyltransferase involved in xyloglucan biosynthesis. Proc. Natl. Acad. Sci. USA 99 7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauré, R., Cavalier, D.M., Keegstra, K., Cottaz, S., and Driguez, H. (2007). Glycosynthase-assisted synthesis of xylo-gluco-oligosaccharide probes for a-xylosyltransferases. Eur. J. Org. Chem. 2007 4313–4319. [Google Scholar]
- Keegstra, K., Talmadge, K.W., Bauer, W.D., and Albersheim, P. (1973). The structure of plant cell walls. III. A model of the walls of suspension-cultured sycamore cells based on the interconnections of the macromolecular components. Plant Physiol. 51 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel, N., Neumetzler, L., and Pauly, M. (2007). Hemicelluloses and cell expansion. In The Expanding Cell, J.-P. Verbelen and K. Vissenberg, eds (Berlin: Springer-Verlag), pp. 57–88.
- O'Neill, M.A., and York, W.S. (2003). The composition and structure of plant primary cell walls. In The Plant Cell Wall, J.K.C. Rose, ed (Boca Raton, FL: CRC Press), pp. 1–54.
- Thompson, D.S. (2005). How do cell walls regulate plant growth? J. Exp. Bot. 56 2275–2285. [DOI] [PubMed] [Google Scholar]
- Zabotina, O., van de Ven, M., Freshour, G., Drakakaki, G., Cavalier, D.M., Mouille, G., Hahn, M.G., Keegstra, K., and Raikhel, N.V. (2008). The Arabidopsis XXT5 protein encodes a putative α-1,6-xylosyltransferase that is involved in xylogulan biosynthesis. Plant J., in press. [DOI] [PubMed]