Abstract
Melatonin is a neurohormone that has been claimed to be involved in a wide range of physiological functions. Nevertheless, for most of its effects, the mechanism of action is not really known. In mammals, two melatonin receptors, MT1 and MT2, have been cloned. They belong to the G-protein-coupled receptor (GPCR) superfamily. They share some specific short amino-acid sequences, which suggest that they represent a specific subfamily. Another receptor from the same subfamily, the melatonin-related receptor has been cloned in different species including humans. This orphan receptor also named GPR50 does not bind melatonin and its endogenous ligand is still unknown. Nevertheless, this receptor has been shown to behave as an antagonist of the MT1 receptor, which opens new pharmacological perspectives for GPR50 despite the lack of endogenous or synthetic ligands. Moreover, MT1 and MT2 interact together through the formation of heterodimers at least in cells transfected with the cDNA of these two receptors. Lastly, signalling complexes associated with MT1 and MT2 receptors are starting to be deciphered. A third melatonin-binding site has been purified and characterized as the enzyme quinone reductase 2 (QR2). Inhibition of QR2 by melatonin may explain melatonin's protective effect that has been reported in different animal models and that is generally associated with its well-documented antioxidant properties.
Keywords: melatonin, circadian rhythm, circadian clock, GPR50, quinone reductase 2, MRR, MT1, MT2, MT3
Introduction
Melatonin (5-methoxy-N-acetyltryptamine) is a neurohormone synthesized during the night by the pineal gland irrespective of the species considered (Arendt, 1998). In humans and all other diurnal species, plasma levels of melatonin are high during the sleep period, whereas in nocturnal species (that is, the majority of laboratory animals) melatonin concentrations are high during the active period. Its secretion is regulated by circadian and seasonal variations in daylight length. The circadian rhythm of pineal melatonin synthesis and release is driven by the circadian ‘clock' located in the suprachiasmatic nuclei (SCN) of the hypothalamus that project to the pineal gland via a multi-synaptic pathway (Reppert and Weaver, 2002). The clock rhythm is entrained to a 24-h period by environmental light (the photoperiod) that is directly sensed by the retina and conveyed to the SCN via the retino-hypothalamic tract. Melatonin is very lipophilic and is released without storage directly into the blood and the CSF. All the structures that present melatonin-binding sites will receive this information on photoperiod through the melatonin signal. Melatonin also acts directly on melatonin receptors expressed in the SCN to modulate the clock itself.
The discovery of a radioligand allowing binding as well as autoradiography studies was a major breakthrough in the field of melatonin research (Vakkuri et al., 1984). This radioligand, 2-[125I]iodomelatonin (125I-MLT), has a high affinity (in the picomolar range) for melatonin receptors and is still the only available radioligand. This high affinity was the key point for the characterization of the distribution of melatonin-binding sites. Indeed, melatonin-binding sites have the unusual feature of being expressed in very low density even in tissues most sensitive to melatonin. This low density is probably related to the high affinity of melatonin for its endogenous receptors. A few pharmacological studies were also performed with [3H]melatonin but the low specific activity of this radioligand prevented widespread use in tissues with low-density melatonin-binding sites (Kennaway et al., 1994; Browning et al., 2000).
The purpose of this review is to report on the recent developments in the field of melatonin receptor subtypes and binding sites including the recently characterized MT3-binding site, and the orphan GPR50, which modulates melatonin receptor function. All the other topics regarding the physiological role of melatonin, the regulation of its synthesis, the therapeutic perspectives of melatonin ligands and the chemistry of melatonin ligands have been discussed in recent reviews (Masana and Dubocovich, 2001; von Gall et al., 2002b; Simonneaux and Ribelayga, 2003; Witt-Enderby et al., 2003; Arendt, 2005; Boutin et al., 2005; Zlotos, 2005; Maronde and Stehle, 2007).
Melatonin receptors: distribution
In 1994, the first melatonin receptor was cloned from Xenopus laevis immortalized melanophore, but this receptor subtype, Mel1C, was found to be only expressed in non-mammalian species (that is, birds, chicken, fishes) (Ebisawa et al., 1994). Subsequently, two other melatonin receptors, MT1 (Mel1A) and MT2 (Mel1B), were cloned from humans (Reppert et al., 1994, 1995). The official international nomenclature for these receptors is MT1 and MT2 (Dubocovich et al., 2001; Alexander et al., 2007). They both belong to the G-protein-coupled receptor (GPCR) superfamily of membrane receptors and show high homology at the amino-acid level (about 55% overall and 70% within transmembrane domains). Moreover, they share specific short amino-acid sequences, which suggest that they represent a specific subfamily.
In human brain, the MT1 mRNA was detected by RT-PCR or in situ hybridization in SCN, cortex, hippocampus, thalamus and cerebellum, whereas the MT2 mRNA was detected in the retina and in whole brain and the hippocampus, although in lower amounts (Reppert et al., 1994, 1995; Mazzucchelli et al., 1996; Weaver and Reppert, 1996; Al-Ghoul et al., 1998; Uz et al., 2005). The MT2 receptor subtype has not yet been reported in the SCN in humans, most likely due to the limited number of receptors in this small brain structure. Nevertheless, MT2 receptors have been detected in the SCN of other species such as mouse and rat (Liu et al., 1997; Rivera-Bermudez et al., 2004). The localization of the human MT1 receptor protein was recently confirmed in the SCN, hippocampus, nucleus accumbens, amygdala, substantia nigra, hypothalamus and cerebellum by immunohistochemistry or western immunoblotting studies using specific antibodies (Song et al., 1997; Savaskan et al., 2001, 2002a; Dillon et al., 2002; Uz et al., 2005; Wu et al., 2007). Expression of the human MT2 receptor protein was demonstrated in hippocampus and cortex by immunohistochemical studies (Savaskan et al., 2005; Brunner et al., 2006).
So far, no reliable antibody directed against MT1 or MT2 receptors has been reported for species other than humans. Consequently, the receptor expression pattern in other species was only determined at the mRNA level using the reverse transcriptase-PCR (RT-PCR) or in situ hybridization. For instance, the MT1 mRNA was expressed in the majority of the central and peripheral tissues studied in rats (Poirel et al., 2003b).
Expression of the MT1 receptor is also regulated in a circadian manner as demonstrated in the SCN and the pars tuberalis (PT) in rodents. These rhythms are regulated in the SCN by the light–dark cycle even in pinealectomized rats (Masson-Pevet et al., 1996) and by melatonin itself in the PT (Guerrero et al., 2000). The level of MT1 and MT2 receptor expression is altered in ageing and in Alzheimer's disease (Savaskan et al., 2002a, 2005; Wu et al., 2007). Melatonin levels decrease with ageing, which may explain the flattening of circadian rhythms (van Coevorden et al., 1991). This decrease in the robustness of the circadian rhythm can be restored by a chronic treatment of rats with a melatonin agonist (Koster-van Hoffen et al., 1993).
Melatonin receptors: signal transduction
Signal-transduction pathways triggered by MT1 and MT2 receptors were characterized in various primary cell cultures and tissues (Table 1) and in different mammalian cell lines expressing the recombinant receptors (Table 2) (see also Morgan et al., 1994; Masana and Dubocovich, 2001; von Gall et al., 2002b; Witt-Enderby et al., 2003 for more details). These signalling pathways involve the activation of both pertussis toxin-sensitive and -insensitive G proteins. Indeed, both receptors preferentially couple to Gi proteins. MT1, and most likely MT2, couple also to Gq/11 proteins, although to a lesser extent (Brydon et al., 1999a, 1999b; Jarzynka et al., 2006).
Table 1.
Melatonin-regulated signalling responses in cells or tissues expressing endogenous receptors
| Signalling response | Cells/tissues | Functional effect | Receptor subtype predominantly involved | References |
|---|---|---|---|---|
| cAMP formation | Ovine pars tuberalis primary cells | ↓ | MT1 and/or MT2 | Carlson et al. (1989); McNulty et al. (1994) |
| Rabbit cortex explants | ↓ | MT1 and/or MT2 | Stankov et al. (1992) | |
| Rat neonatal pituitary primary cells | ↓ | MT1 and/or MT2 | Vanecek and Vollrath (1989); Slanar et al. (2000) | |
| SCN2.2 cells | ↓ | MT1 and/or MT2 | Rivera-Bermudez et al. (2004) | |
| cGMP formation | Rat neonatal pituitary primary cells | ↓ | MT1 and/or MT2 | Vanecek and Vollrath (1989) |
| pCREB | Mouse SCN slices | ↓ | MT1 | von Gall et al. (2000) |
| Ovine pars tuberalis primary cells | ↓ | MT1 and/or MT2 | McNulty et al. (1994); McNulty et al. (1996) | |
| pJNK, pERK1/2 | MCF-7 cells | ↑ | MT1 | Chan et al. (2002) |
| DAG/PKC | Rat neonatal pituitary primary cells | ↓ | MT1 and/or MT2 | Vanecek and Vollrath (1990) |
| Human monocytes | ↑ | MT1 and/or MT2 | Morrey et al. (1994) | |
| Rat SCN slices | ↑ | MT1 and/or MT2 | Mc Arthur et al. (1997) | |
| Rat SCN slices | ↑ | MT2 | Hunt et al. (2001) | |
| SCN2.2 cells | ↑ | MT2 | Gerdin et al. (2004); Rivera-Bermudez et al. (2004) | |
| c-Fos | Rat neonatal pituitary | ↓ | MT1 and/or MT2 | Sumova and Vanecek (1997) |
| Arachidonic acid | Rat neonatal pituitary primary cells | ↑ | MT1 and/or MT2 | Vanecek and Vollrath (1990) |
| Ca2+-dependent, large conductance K+ channels (BKCa) | Rat cerebral arteries | ↓ | MT1 and/or MT2 | Geary et al. (1997) |
| Rat tail arteries | ↓ | MT1 and/or MT2 | Geary et al. (1997) | |
| K+ conductance | Mouse SCN slices | ↑ | MT1 and/or MT2 | Jiang et al. (1995) |
| Inward-rectifying cation current (Ik) | Mouse SCN slices | ↓ | MT1 and/or MT2 | Jiang et al. (1995) |
| GABAA-mediated current | Rat SCN slices | ↑ | MT1 | Wan et al. (1999) |
| Rat hippocampus slices | ↓ | MT2 | Wan et al. (1999) | |
| Ca2+ influx | Rat neonatal pituitary primary cells | ↓ | MT1 and/or MT2 | Slanar et al. (2000) |
| Intracellular Ca2+ | Rat neonatal pituitary primary cells | ↓ | MT1 and/or MT2 | Vanecek and Klein (1992) |
| ↑ | MT1 | Brydon et al. (1999a) |
↓, decrease, inhibition; ↑, increase, activation.
Table 2.
Melatonin-regulated signalling responses in cell lines expressing the recombinant MT1, or MT2 receptors
| Signalling response | Cell line |
Functional effect |
References | |
|---|---|---|---|---|
| hMT1 | hMT2 | |||
| cAMP formation | NIH3T3 | ↓ | ↓ | Reppert et al. (1995); Godson and Repper (1997) |
| CHO | ↓ | ↓ | Witt-Enderby and Dubocovich (1996); MacKenzie et al. (2002) | |
| HEK293 | ↓ | ↓ | Brydon et al. (1999a, 1999b); Roka et al. (1999) | |
| AtT-20 | ↓ | ND | Nelson et al. (2001) | |
| cGMP formation | HEK293 | = | ↓ | Petit et al. (1999) |
| pCREB | CHO | ↓ | ND | Witt-Enderby et al. (1998) |
| pMEK1/2 | CHO | ↑ | = | Witt-Enderby et al. (2000) |
| pERK1/2 | CHO | ↑ | = | Witt-Enderby et al. (2000) |
| HEK293 | ↑ | ↑ | Daulat et al. (2007) | |
| pJNK | COS-7 | ↑ | ↑ | Chan et al. (2002) |
| PKA activity | CHO | ↓ | nd | Witt-Enderby et al. (1998) |
| K+ currents | AT.-T20 | ↑ | nd | Nelson et al. (2001) |
| Intracellular Ca2+ | HEK293 | ↑ | nd | Brydon et al. (1999a, 1999b) |
| Ca2+ influx | AT.-T20 | ↓ | nd | Nelson et al. (2001) |
| PI turnover | NIH3T3 | ↑ | nd | Godson and Reppert (1997) |
| HEK293 | ↑ | nd | Roka et al. (1999) | |
| CHO | ↑ | ↑ | MacKenzie et al. (2002) | |
Abbreviations: CHO, Chinese hamster ovary; ERK, extracellular signal-regulated kinase; ND, not determined; JNK, c-Jun N-terminal kinase; MEK, mitogen-activated protein kinase kinase; PI, phosphotnositide.
↓, decrease; ↑, increase; =, no effect.
G-protein activation of the MT1 receptor modulates several signal-transduction pathways. Melatonin typically inhibits forskolin-stimulated cAMP formation (Morgan et al., 1989; Brydon et al., 1999a,1999b), protein kinase A activity (Morgan et al., 1994; Witt-Enderby et al., 1998) and phosphorylation of the cAMP-responsive element binding (Witt-Enderby et al., 1998; McNulty et al., 1994). Activation of the MT1 receptor also increases the phosphorylation of the mitogen-activated protein kinase kinases 1 and 2 (MEK1 and MEK2), the extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2) (Witt-Enderby et al., 2000) and c-Jun N-terminal kinase (JNK) via pertussis toxin-sensitive and -insensitive G proteins (Chan et al., 2002). Potentiation of ATP and prostaglandin F2α-induced phosphoinositide turnover through the activation of βγ subunits of pertussis toxin-sensitive G proteins has also been reported (Godson and Reppert, 1997; Roka et al., 1999).
Similarly, activation of the MT2 receptor inhibits forskolin-stimulated cAMP production (Reppert et al., 1995; MacKenzie et al., 2002) and stimulates JNK (Chan et al., 2002) and phosphoinositide turnover (MacKenzie et al., 2002).
Collectively, the repertoire of G-protein-dependent signalling pathways activated by MT1 and MT2 receptors is very similar. Subtype-specific differences have only been reported in some cases. For instance, the MT2 receptor inhibits cGMP formation through the soluble guanylyl cyclase pathway, but MT1 does not in HEK293 cells (Brydon et al., 1999a, 1999b; Petit et al., 1999). Furthermore, activation of PKC in the SCN occurs only through MT2 receptors and not MT1 despite the expression of both subtypes (Hunt et al., 2001).
Proteins interacting with melatonin receptors
Recent evidence indicates that GPCRs not only couple to heterotrimeric G proteins but also physically associate with other intracellular proteins (Bockaert et al., 2004; Pluder et al., 2006). Several approaches have been developed to identify protein complexes associated with the intracellular domains (loops and C-tail) of GPCRs. Indeed, the nature of the interacting proteins that bind to the intracellular parts of GPCRs can determine its targeting to a specific cellular compartment, its association with other signalling or structural proteins and the fine-tuning of its signal transduction such as desensitization and resensitization. Therefore, the identification of the protein complexes associated with GPCRs constitutes an important step towards the development of new drugs that could be used to disrupt or strengthen specific interactions between GPCRs and their associated proteins.
An original proteomic approach was recently described by Daulat et al. (2007) and gave an overview of the protein complexes able to interact with the MT1 and MT2 receptors in HEK293 cells. This approach is based on the tandem affinity purification (TAP) of MT1 and MT2 receptors that are expressed in HEK293 cells as C-terminal TAP-tag fusion proteins. The TAP method is based on two successive affinity chromatography steps to purify the protein of interest and the associated complexes. The protein of interest is expressed as fusion protein of the TAP tag, which is composed of two IgG-binding domains, a TEV (Tobacco Etch Virus) protease cleavage site and a calmodulin-binding domain. The complex is first immobilized on an IgG column, then specifically liberated by the addition of the TEV protease and finally bound to a calmodulin column in the presence of calcium. The final eluate is typically separated by SDS-polyacrylamide gel electrophoresis and the recovered proteins were identified by mass spectrometry. The TAP-tag method presents several advantages compared to other techniques as full-length protein of interest, including membrane proteins, can be expressed in mammalian cells where subcellular localization and post-translational modifications are conserved. Despite the fact that this two-step purification protocol generates only few false positives, control conditions in the absence of the TAP-tagged protein should always be run in parallel to identify these nonspecific proteins. Using this method, we confirmed the coupling of both receptors to Gi proteins and allowed the purification of protein complexes associated with GPCRs under native conditions. Several new and potentially functionally relevant MT1- and MT2-associated proteins were identified; some of them were common to both receptors, and others were specific for each subtype.
Filamin A and insulin receptor substrate 4 (IRS4) were identified as common members of MT1- and MT2-associated complexes. The actin-binding protein filamin A has been already shown to interact with several other members of the GPCR family, including dopamine D2/D3 (Li et al., 2000; Lin et al., 2001) and calcium-sensing receptors (Awata et al., 2001; Hjalm et al., 2001). Interestingly, filamin A was reported to form a signalling complex with D3 receptors and β-arrestins, which can be destabilized by D3 receptor or GRK2/3 activation (Kim et al., 2005). Filamin A interacts also with μ-opioid receptors and regulates receptor trafficking (Onoprishvili et al., 2003). Finally, a role for filamin in endocytic sorting and recycling of internalized calcitonin receptor has been reported (Seck et al., 2003). The role of IRS4 is less well documented. Involvement of IRS4 in fibroblast growth factor receptor signalling (Hinsby et al., 2004) and interaction with the protein phosphatase 4 has been described (Mihindukulasuriya et al., 2004).
The MT1 melatonin receptor was shown to specifically interact with Rac1, Rap-1A, the 2′,3′-cyclic-nucleotide 3′-phosphodiesterase and the protein elongation factor 1-γ (eEF-1Bγ) (Daulat et al., 2007). The small GTPases Rac1 and Rap-1A have been shown to function downstream of 5-HT4 receptors and the cAMP guanine nucleotide exchange factor Epac1 (Maillet et al., 2003). Both Rap and Rac1 have been reported to be activated upon stimulation of several other GPCRs (Pelletier et al., 2003; Weissman et al., 2004). The 2′,3′-cyclic-nucleotide 3′-phosphodiesterase, belonging to the PDE3A family, is involved in the degradation of second messengers such as cAMP and cGMP (Lugnier, 2006). eEF-1Bγ and other elongation factors have been reported to modulate GPCR function by direct interaction with the receptor (McClatchy et al., 2002, 2006; Cho et al., 2003).
Specific interaction partners of the MT2 receptor were also identified by the TAP approach, including catenin δ1 (p120 catenin) and the protein phosphatase 2Cγ (PP2Cγ) (Daulat et al., 2007). Catenin δ1 is a regulator of cadherin stability and an important modulator of Rho GTPase activities (Reynolds, 2007). This protein was shown to interact with mGluR1 receptors and dissociate upon activation of the receptor by L-glutamate (Jones et al., 2002). However, its specific role in GPCR signalling is currently unknown. Several serine/threonine phosphatases participate in the dephosphorylation of activated GPCRs (Shih et al., 1999). Phosphatases of the PP2A and PP2B subfamilies have been reported to target GPCRs, whereas PP2C subfamily members have been shown to dephosphorylate the metabotropic glutamate receptor 3 (Flajolet et al., 2003). Specific dephosphorylation of the MT2 receptor by the PP2Cγ remains to be demonstrated. Taken together, the TAP method defined the protein complexes associated with transfected TAP-tagged MT1 and MT2 receptors in intact HEK293 cells. It will be interesting to expand this study to neuronal and endocrine cells known to express endogenous melatonin receptors, and to study the components of these complexes in native tissues by co-immunoprecipitation experiments.
One interesting feature of the MT1 receptor is the presence of a class III recognition motif for PSD-95/Disc-large/Zona Occludens-1 (PDZ) domains at its C-terminal extremity (D-S-V). PDZ domains are modular protein interaction domains that are specialized for binding to C-terminal peptide motifs of proteins. PDZ domain-based scaffolds typically assemble large molecular complexes at specific subcellular sites, like synapses. Using protein microarrays and quantitative fluorescence polarization, Stiffler et al. (2007) have characterized the binding selectivity of 157 mouse PDZ domains with respect to 217 genome-encoded peptides encompassing the 10 C-terminal residues of mouse proteins, including the MT1 receptor. By this approach, the C-terminal extremity of the MT1 receptor was shown to interact with the channel-interacting PDZ domain protein Cipp, the serine protease HtrA1, and MUPP1. The interaction with MUPP1 has been recently confirmed in the PT and has been shown to promote coupling of MT1 to the cAMP pathway (Guillaume et al., 2008). Another PDZ domain-containing protein has been already described as potential interacting candidate of the MT1 receptor, the neuronal NO synthase (Stricker et al., 1997). Using a C-terminal peptide display strategy, Stricker et al. screened 13 billion distinct C-terminal peptides to select sequences specific to the PDZ domain of neuronal NO synthase and found that the positive peptides had a D-X-V C-terminal consensus sequence. Searching the non-redundant protein database NCBInr revealed 484 matches, including the glutamate receptor 6 and the MT1 receptor. The interaction between nNOS and MT1 has been recently confirmed (Maurice et al., 2008), however the functional consequences on melatonin-mediated cell signalling remain to be demonstrated. The MT2 receptor also contains a class III PDZ domain-binding motif at its C-terminal extremity (-A-D-A-L). However, no information has been reported in the literature regarding this motif, and a functional role of this motif remains to be demonstrated.
Melatonin receptors: MT1/MT2 heterodimerization
Oligomerization (or dimerization) has emerged as a general trademark of GPCRs that is supported by multiple lines of evidence. Recently, the IUPHAR proposed general guidelines for recognition and acceptance of such oligomeric receptors (Pin et al., 2007). Receptor dimers may be composed of the same (homodimers) or different (heterodimers) GPCRs. As GPCR dimerization may have a profound impact on receptor pharmacology, signalling and regulation, the issue is of primary importance for basic science and drug development.
Investigation of GPCR dimerization has been limited for a long time by the use of biochemical techniques that were based on detergent extraction of receptors from their natural environment, the plasma membrane. New non-invasive energy transfer-based approaches were introduced in the field of GPCR dimerization late in the 1990s. BRET (bioluminescence resonance energy transfer) is one of these techniques that allows real-time measurement of protein–protein interactions in intact cells (Pfleger and Eidne, 2006). This approach is based on the energy transfer between two receptor fusion proteins. One receptor is fused to the energy donor Renilla luciferase (Rluc) and the second to the energy acceptor, the yellow variant of the green fluorescence protein (YFP). Significant BRET can be measured if the donor and acceptor are in close proximity (10–100 Å) and if their respective orientation is appropriate. MT1 and MT2 receptors have been among the first GPCRs, whose homo- and heterodimerization have been demonstrated by BRET in transfected HEK293 cells (Ayoub et al., 2002). Interestingly, the propensity of MT2 homodimer formation is 3- to 4-fold lower than that of MT1/MT2 heterodimer and MT1 homodimer formation (Ayoub et al., 2004), suggesting that MT2 may be preferentially engaged into heterodimers in cells co-expressing equimolar quantities of both receptors. It is important to note that melatonin receptors may potentially heterodimerize with other GPCRs. Some other GPCRs have been tested for their ability to heterodimerize with melatonin receptors. The CCR5 chemokine receptor, the β2-adrenoceptor (Ayoub et al., 2002, 2004) and the serotonin 5-HT4 receptor (Berthouze et al., 2005) do not heterodimerize with melatonin receptors, whereas the orphan GPR50 does (Levoye et al., 2006a) as determined by BRET. Further experiments will be necessary to determine the complete heterodimerization profile of melatonin receptors.
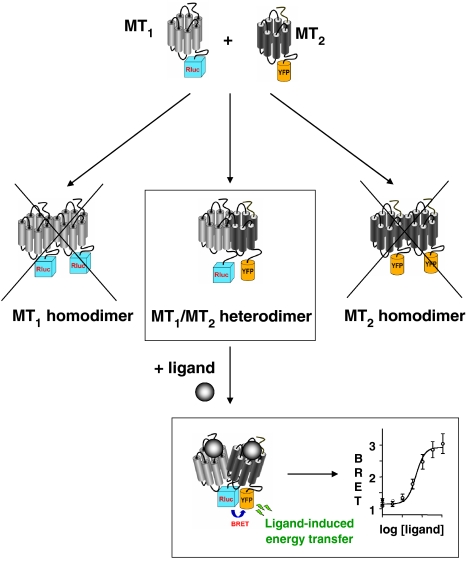
The BRET assay has also been successfully applied to the determination of the pharmacological profile of MT1/MT2 heterodimers. Indeed, a general problem for the determination of the functional properties of heterodimers is the concomitant presence of the respective homodimers. This also applies to MT1 and MT2 receptors, which bind 125I-MLT and most melatonin receptor-specific ligands with similar affinities. The choice of the appropriate energy transfer couple in the BRET assay avoids the ‘contamination' of the heterodimer-specific profile by that of the two corresponding homodimers (Figure 1). For instance, if MT1-Rluc and MT2-YFP fusion proteins are co-expressed, energy transfer can only occur between MT1-Rluc and the MT2-YFP fusion proteins but not between two MT1-Rluc or two MT2-YFP fusion proteins. Accordingly, ligand binding to heterodimers can be monitored in such an assay if the ligand-promoted conformational change within the heterodimer modifies the relative distance and orientation of Rluc and YFP.
Figure 1.
Determination of the pharmacological profile of MT1/MT2 heterodimers by BRET (bioluminescence resonance energy transfer). Co-expression of MT1-Rluc (Renilla luciferase) and MT2-YFP (yellow variant of the green fluorescence protein) fusion proteins results in the formation of at least three different oligomeric receptor species, MT1-Rluc homodimers, MT2-YFP homodimers and MT1-Rluc/MT2-YFP heterodimers. Whereas all three species are detected in classical 125I-MLT-binding assays, BRET occurs only between MT1-Rluc and MT2-YFP. The observed ligand-promoted BRET change of melatonin receptor ligands is therefore exclusively generated by the MT1/MT2 heterodimer and not ‘contaminated' by the other dimeric receptor species.
Using this approach, a specific pharmacological profile has been established for the MT1/MT2 heterodimer and several heterodimer-selective ligands have been identified (Ayoub et al., 2004). Luzindole (N-acetyl-2-benzyltryptamine) and 4P-PDOT (4-phenyl-2-propionamidotetralin) are the two most widely used pharmacological tools to discriminate between MT1 and MT2 in native tissues and animal studies (Dubocovich et al., 1998). Our BRET data indicate that these MT2-selective compounds have similar or even higher affinity for MT1/MT2 heterodimers. This high affinity for MT1/MT2 heterodimers has to be considered for the correct interpretation of data obtained in cellular systems co-expressing MT1 and MT2. Functional consequences of MT1/MT2 heterodimerization, that is, on receptor signalling and trafficking, are currently unknown.
Several candidate tissues for MT1/MT2 heterodimer formation may already be identified. Functional melatonin receptors were first characterized in the retina (Dubocovich, 1983), a tissue that predominantly expresses the MT2 subtype (Dubocovich and Takahashi, 1987). Immunohistochemical experiments in human retina show that both receptors are expressed in virtually every ganglion and photoreceptor cell, strongly suggesting heterodimer formation in these cells (Meyer et al., 2002; Savaskan et al., 2002b, 2007).
Co-expression of MT1 and MT2 in pyramidal neurons of the hippocampus, as shown by RT-PCR (Musshoff et al., 2002), is consistent with the possible existence of MT1/MT2 heterodimers in these cells. An immunohistochemical analysis showed that MT1 and MT2 expression patterns overlap with MT1 predominantly expressed in the CA1 hippocampal subfield and MT2 in the CA3/CA4 subfields (Savaskan et al., 2002a, 2005).
Expression of MT1 and MT2 in SCN neurons is widely accepted (von Gall et al., 2002b): the SCN contains 125I-MLT-binding sites (Reppert et al., 1988), both receptor mRNAs have been detected by in situ hybridization (Reppert et al., 1994; Dubocovich et al., 1998) and MT1 expression in human SCN has been recently confirmed by immunohistochemistry (Wu et al., 2007). Although a detailed analysis has not been performed, the extensive labelling throughout the SCN strongly indicates co-expression of both receptors, at least in sub-populations of SCN neurons. Arteries (Krause et al., 1995) and adipose tissue (Brydon et al., 2001) are two further tissues co-expressing MT1 and MT2.
Modification of the MT1/MT2 ratio is likely to influence the dimerization pattern of these receptors. Such modifications have been observed in the brain and retina of Alzheimer's disease patients (see Levoye et al., 2006c; Wu et al., 2007 for review) and may modify the functional melatonin response. Future efforts have to focus on the identification of MT1/MT2 heterodimers in native tissues and primary cell cultures to firmly establish their physiological relevance. Faster progress in this field is still hampered by the absence of adequate high-affinity melatonin receptor-specific antibodies for co-immunoprecipitation experiments.
Melatonin receptors: in vivo functions
In this review, we will discuss only melatonin receptor functions that have been confirmed in vivo. Consequently, all the results obtained in vitro on tissues or in cell lines either transfected with the receptor subtypes or naturally expressing these receptors will not be discussed here. These data are described in detail in the reviews mentioned above. There are at least two different ways to study the in vivo function of a receptor: the use of receptor-specific pharmacological tools (antagonist and agonist) and knockout mice. Only a few selective ligands have been described for MT1 or MT2 receptors. Most of these ligands are antagonists or partial agonists and most of them have a weak selectivity, which limits their use in vivo (Dubocovich, Delagrange, Olcese, IUPHAR Receptor database, 2007, http://www.iuphar-db.org/GPCR/ChapterMenuForward?chapterID=1291). Today, the 4P-PDOT compound is the only specific molecule available for in vivo studies. 4P-PDOT is an MT2 antagonist with a selectivity of 100–1000 times (Dubocovich et al., 1997; Audinot et al., 2003). Because of this lack of pharmacological tools, only few in vivo physiological functions have been clearly attributed to each melatonin receptor type.
At the level of the SCN, MT1 and MT2 receptors are involved in different functions, which have been mainly studied on tissue slices. Whereas MT1 receptors are necessary for the acute inhibitory action of melatonin or melatonin receptor agonists on neuronal activity in the SCN, MT2 receptors mediate the phase shift of circadian rhythm of neuronal activity in the SCN (Liu et al., 1997; Hunt et al., 2001). These effects have been confirmed for MT1 receptors on SCN slices by both pharmacological experiments and by studies performed on MT1 receptor knockout mice (Liu et al., 1997; Dubocovich et al., 2005). For MT2, these effects have been only confirmed by in vivo treatment with the MT2-selective antagonist 4P-PDOT (Hudson et al., 2005). The interpretation of in vivo results obtained with MT2 knockout mice is more difficult and suggests a functional interaction between these two receptors (Dubocovich et al., 2005). Nevertheless, the resynchronizing effects of melatonin observed for both slices and in vivo treatments, are always reported in the evening corresponding in rats or mice to the light–dark transition (Redman et al., 1983). Phase-response curves obtained in humans confirmed this window of sensitivity for both melatonin and melatonin receptor agonists (Wirz-Justice et al., 2002; Arendt, 2005). This window of sensitivity may be explained by the desensitization of the MT2 receptor at the beginning of the night, which internalizes upon exposure to its endogenous agonist, melatonin, as most GPCRs. In vitro studies have recently validated this hypothesis (Gerdin et al., 2004). This rapid desensitization may turn out to be tissue specific. Indeed, melatonin receptors play an important role in the transmission of the photoperiodic message, which is given by the duration of melatonin synthesis (Bartness and Goldman, 1989). Consequently, to sense this message, melatonin receptors have to stay at the plasma membrane for at least 12 h, as observed in the ovine PT. Prolonged exposure of primary PT cell cultures with melatonin (8–16 h) induced a sensitization of the cells, which increased with the duration of melatonin incubation (Hazlerigg et al., 1993). A decrease of melatonin receptor density was only observed after 24 h of melatonin incubation.
The SCN is the master pacemaker of the organism. The generation of circadian rhythms involves, at the cellular level, clock genes and associated proteins, which are organized in a transcriptional–translational autoregulatory loop that generates molecular oscillations of these clock genes (Ko and Takahashi, 2006). Melatonin receptors are expressed in the SCN and melatonin is able to advance or to resynchronize the clock. However, the molecular mechanisms underlying this chronobiotic effect are poorly understood. Melatonin treatments have no effect on the expression of clock genes in the SCN (Poirel et al., 2003a). The only effect reported recently for melatonin in the SCN is the phase advance in melatonin-treated rats of rev-erbα mRNA expression (Agez et al., 2007). This nuclear orphan receptor is involved in the functional link between the regulatory loops of the molecular clock. In the PT, melatonin regulates clock gene expression. Melatonin infusion in sheep induced cryptochrome1 expression and suppressed the expression of other clock genes (Johnston et al., 2006b). These effects of melatonin probably involve the MT1 receptor. Indeed, the expression of most clock genes is reduced in the PT of MT1 receptor knockout mice but not in MT2 knockout mice (von Gall et al., 2005).
The phenotype of knockout mice either for MT1 or MT2 or both receptor subtypes is not very different from wild-type mice (Jin et al., 2003). Indeed, the changes associated with targeted disruption of either the MT1 or MT2 receptors are subtle and have been reported mainly at the molecular levels, like the regulation of clock genes, transcription factors (CREB) or neuronal activity (Liu et al., 1997; von Gall et al., 2000, 2002a, 2005; Wang et al., 2005). These deletions induce no major behavioural changes under normal conditions according to the limited number of publication related to this field. The lack of phase-shifting effect of melatonin in MT1 knockout mice, as mentioned above, and a deficit in memory, based only on the elevated plus-maze test in MT2 knockout mice were until recently the only behavioural effect reported in vivo (Dubocovich et al., 2005; Larson et al., 2006). Another publication reported that knockout mice for the MT1 receptor exhibited a depressive behaviour in an animal model of depression, the forced swimming test (Weil et al., 2006). These results do not demonstrate that melatonin receptors are directly involved in mood disorders, but suggest that their dysregulation might participate indirectly in the installation of depression. In depressed patients, circadian rhythms are generally also dampened or even disrupted (Turek, 2007). The efficacy of agomelatine as an antidepressant may result in part from its activity as an MT1/MT2 agonist, although melatonin receptor activation alone is not sufficient for effectiveness in treating depression (Dalton et al., 2000; Bertaina-Anglade et al., 2006).
The orphan GPR50: progress on the understanding of its function
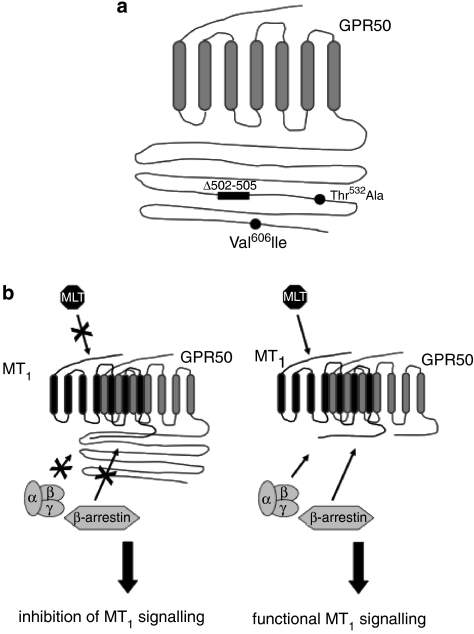
GPR50 is one of the more than 100 orphan GPCRs that resists the de-orphanization efforts of academic and industrial research. GPR50, also called melatonin-related receptor (MRR), was cloned in 1996 and classified as a member of the melatonin receptor subfamily due to its high homology (45%) with MT1 and MT2 at the amino-acid level and due to the presence of characteristic signatures of this subfamily (Reppert et al., 1996). More than 10 years later, the function of GPR50 is still poorly understood. Recent genetic studies provided first hints on potential functions. Three polymorphisms located in exon 2 of the human GPR50 gene have been documented (Figure 2a). All three polymorphisms induce amino-acid changes in the C-tail of the human GPR50 (Δ502–505, Thr532Ala and Val606Ile). The Δ502–505 variant corresponds to the deletion of four amino acids (Thr-Thr-Gly-His) and has been associated in a Scottish population with increased risk of developing either bipolar affective and major depressive disorder in women (Thomson et al., 2005). However, this association was not replicated in a Swedish population (Alaerts et al., 2006). A further genetic study confirmed the existence of these three variants in two independent obese cohorts and described an additional variant upstream of the GPR50 coding region (C-16X2GPR50T) (Bhattacharyya et al., 2006). No association was observed with body mass index, but carriers of all three variants (Δ502–505, Thr532Ala and Val606Ile) had significantly higher fasting circulating triglyceride levels and carriers of the C-16X2GPR50T variant had lower circulating high-density lipoprotein cholesterol levels. The functional differences of these variants and their implication in mental disorders and lipid metabolism remain to be shown. The phenotype of mice with targeted disruption of the GPR50 gene has been described recently (Ivanova et al., 2007). In agreement with the genetic studies, these mice have an altered metabolism (increased metabolic rate). Phenotypic alterations associated with mental disorders have not been studied in these animals.
Figure 2.
GPR50 polymorphisms and functional properties. (a) Localization of the three polymorphisms reported in the literature for GPR50 (Thomson et al., 2005; Alaerts et al., 2006; Bhattacharyya et al., 2006; Feng et al., 2007). 1: Δ502–505 corresponds to the insertion/deletion of the four amino acids Thr-Thr-Gly-His (TTGH) at position 502 (Δ502–505 variant); 2: Thr532Ala corresponds to the substitution of Thr (T) to Ala (A) at position 532; 3: Val606Ile corresponds to the substitution of Val (V) to Ile (I) at position 606. The reader is referred to the publication of Bhattacharyya et al. (2006) for the haplotype frequencies of these three polymorphisms in GPR50. (b) Regulation of MT1 signalling by heterodimerization with GPR50. Engagement of GPR50 (wild type) into heterodimers with MT1 receptors prevents high-affinity agonist binding, heterotrimeric G-protein coupling and β-arrestin binding to the MT1 receptor. When engaged into heterodimers with a C-terminally truncated GPR50 mutant, the MT1 receptor functions properly.
An unexpected function of GPR50 was revealed in co-expression experiments with MT1 and MT2 receptors (Levoye et al., 2006a). GPR50 was shown to constitutively heterodimerize with MT1 and MT2 but not with β2-adrenoceptors and CCR5 chemokine receptors. Whereas engagement of GPR50 into heterodimers with MT1 completely inhibited the high-affinity agonist binding of the latter, engagement of GPR50 with MT2 does not modify the agonist-binding properties of MT2. Binding of GPR50 to MT1 had other profound consequences on MT1 function such as the inhibition of heterotrimeric G-protein coupling and β-arrestin binding (Figure 2b). A more detailed analysis showed that the long C-tail of GPR50 prevents recruitment of intracellular interaction partners such as G proteins and β-arrestins to the MT1 receptor in the heterodimer.
The potential significance of the inhibitory effect of GPR50 on MT1 receptor function was shown in immortalized human endothelial cerebral hCMEC/D3 cells that express both proteins endogenously (Levoye et al., 2006a). Whereas MT1 activity was undetectable in cells expressing GPR50, MT1 receptors became fully functional upon GPR50 silencing, demonstrating that endogenous GPR50 expression levels can indeed regulate MT1 activity. Two major tasks remain to fully understand the physiological significance of MT1/GPR50 heterodimerization: the identification of tissues co-expressing MT1 receptors and GPR50 and the regulation of GPR50 expression. GPR50 mRNA is expressed in the hypothalamus, pituitary, retina, testis, kidney and several other central and peripheral sites known to express MT1 receptors (Reppert et al., 1996; Drew et al., 1998, 2001; Vassilatis et al., 2003). As a first step to study GPR50 expression at the protein level, we recently developed specific antibodies against GPR50 (Ould-Hamouda et al., 2007). Immunohistochemical analysis detected the presence of GPR50 in rat pituitary and in human hippocampus, two structures also expressing MT1 receptors that are thus promising candidate tissues for further analysis.
Formation of MT1/GPR50 heterodimers is constitutive and is expected to depend on the relative expression levels of both proteins. MT1 receptor expression is known to be regulated during development and the circadian cycle (Poirel et al., 2002; Johnston et al., 2003, 2006a). Little is known about the regulation of GPR50 expression. Recently, GPR50 mRNA levels were shown to be downregulated in short-day photoperiods specifically in tanycytes of the ependymal layer of the hypothalamus (Barrett et al., 2006). Hypothalamic expression of GPR50 is also highly responsive to energy status with decreased expression upon fasting and feeding with high-energy diet (Ivanova et al., 2007). The function of MT1 in the heterodimer might also be regulated post-translationally by the proteolysis of the GPR50 C-tail as heterologous expression studies showed that the MT1 receptor is fully functional when engaged in heterodimers with a C-terminally truncated GPR50 mutant (Levoye et al., 2006a) (Figure 2b). This regulatory mechanism may warrant further attention as recent data suggest that GPR50 is indeed sensitive to proteolytic degradation (Ould-Hamouda et al., 2007).
GPR50 may have further functions that remain to be discovered. In particular, the question of an endogenous ligand and the possibility of ligand-dependent signalling is difficult to exclude. GPR50 heterodimerizes with MT2. However, the consequences of GPR50 on the MT2 function are currently unknown but warrant further attention. Heterodimerization between an orphan and non-orphan GPCR as exemplified by the MT1/GPR50 couple, may be a more general strategy of cells to regulate GPCR function and may in addition open new perspectives for drug design (Levoye et al., 2006b; Levoye and Jockers, 2007).
Melatonin-binding site MT3 is QR2: facts, remaining questions and hypotheses
MT3, previously called ML-2, was initially described by Duncan et al. (1988). By 1999, only a dozen articles describing this binding were published. This binding site presented a ‘low' affinity for melatonin (5–50 nM in contrast to the less than 1 nM for MT1 and MT2 receptors, see Molinari et al., 1996 for complete discussion), very fast association and dissociation kinetics and a pharmacological profile slightly different from MT1 and MT2 receptors. A specific ligand for MT3 was identified in 1996, 5-methoxycarbonylamino-N-acetyltryptamine (MCA-NAT), which presents nanomolar affinity for MT3 and only micromolar affinity for MT1 and MT2 (Molinari et al., 1996). Further studies extended the pharmacological characterization of MT3 (Paul et al., 1999). By using a mild extraction procedure and affinity chromatography onto an immobilized analogue of MCA-NAT, a single protein was isolated (Nosjean et al., 2000) that, once sequenced, turned out to be a long forgotten quinone reductase, QR2 (Zhao et al., 1997).
Further studies on this enzyme firmly established that MT3 and QR2 are the same protein. Indeed, transfection of QR2 in Chinese hamster ovary cells revealed an MT3-type binding site (Nosjean et al., 2000). Similarly, tissues from various species showed a correlation between QR2 activity and MT3 binding (Nosjean et al., 2001). Furthermore, and most importantly, QR2−/− mice did not have any measurable MT3-binding sites (Mailliet et al., 2004). Very recently, the co-crystallization of QR2 with melatonin and 2-iodo-melatonin, and the docking of MCA-NAT onto this structure confirmed that QR2 corresponds to MT3 (Calamini et al., 2008).
Nevertheless, different properties of melatonin reported to involve MT3 cannot be explained so far according to what is known today about QR2. For example, MT3 was originally described as a membrane-bound binding site in hamster brains (Duncan et al., 1988), while QR2 is a cytosolic enzyme, which has so far never been reported to be membrane-associated. Specific MT3 binding at the membrane level could be detected in wild-type mice but not in QR2−/− mice. Moreover, MT3-binding sites have a pharmacological profile different from MT1 and MT2 receptors and were reported to be functionally coupled to phosphoinositide hydrolysis in Syrian hamster RPMI 1846 melanoma cells (Pickering and Niles, 1992; Eison and Mullins, 1993). This observation is the key to the nature of MT3. These results may imply that the MT3-binding site present on QR2 induces new functions for this enzyme. Finally, a set of molecules with strong affinity for the MT3 membrane-binding sites does not have the same capacity to inhibit QR2 catalytic activity (Mailliet et al., 2005), suggesting that the characteristics of the MT3-binding site at the membrane level were different from that of the cytosolic site involved in the control of QR2 catalytic activity. However, biophysical experiments did not confirm this hypothesis so far.
To rule out any possible bias, these points should be addressed de novo at the bench level. At least two theories should be explored. These pharmacological differences might be explained by the presence of QR2 monomers at the membrane level. As a reminder, QR2 is a homodimer. The monomer would not have any catalytic activity—in line with the fact that both catalytic sites of the QR2 homodimer are formed of amino acids belonging to each monomer—but would have a large portion of its sequence, of hydrophobic nature and normally implicated in the monomer–monomer interface, available for association with membranes. The simplest explanation, though, would be that a portion of the QR2 homodimers is associated with the plasma membrane. One hypothesis is that QR2 possesses a cryptic myristoylation site. Myristoylation is known to play a role in membrane addressing (Boutin, 1997). This site could become available for N-myristoyltransferase—which catalyses the transfer of myristate moiety onto the N-terminal glycine of target proteins—after cleavage by caspase, as demonstrated for another protein, bid, a member of the bcl2 antiapoptotic protein family (Degli Esposti et al., 2003). These issues should be explored using confocal microscopy, specific antibodies, subcellular fractionations (Antoine et al., 1993) and directed mutagenesis.
In contrast to a common belief (see Testa and Kramer, 2007 for review), QR2 might be an activating enzyme. Indeed, in three different models, the toxicity of menadione is enhanced by QR2: in K562 cells treated with QR2 siRNA (Buryanovskyy et al., 2004), in HT22 cells treated with QR2 shRNA (Chomarat et al., 2007) and in QR2−/− mice (Long et al., 2002). In all these cases, menadione was more toxic for biological systems in the presence of QR2 than in its absence. These are important features of QR2 and its MT3-binding site. Indeed, their role in redox is now clearly stated and strongly suggests that its inhibition is beneficial for living organisms.
Resveratrol is a potent QR2 inhibitor (Buryanovskyy et al., 2004; Calamini et al., 2008). Resveratrol and melatonin have been reported in many studies to have antioxidant activities and protective effects, which might be due to their potencies to inhibit QR2 activity. The fact that inhibition of QR2 expression induces an upregulation of different enzymes with antioxidant properties supports this hypothesis (Buryanovskyy et al., 2004). The ‘antioxidant' effect of melatonin is generally observed at high concentrations (>1 μM) (Reiter et al., 1995) far from the physiological levels. Nevertheless, it should be kept in mind that melatonin can also be ingested with food (Tan et al., 2002; Reiter et al., 2007). Obviously, the local concentration of melatonin in subcellular compartments or in organs has not been reported so far, even after massive melatonin ingestion or treatment.
Although a review should be meant to generalize the observations and to try to make sense out of them, it seems that some other features of QR2 (see also Vella et al., 2005, for discussion) should be listed to complete the overall portrait of MT3/QR2. For instance, Brouillette and Quirion (2007) reported that ageing rats (24 months and beyond) could be separated into two groups: one in which animals can retain a ‘normal' level of learning, and another one with animals that cannot. Microarray experiments with rat brains of both groups showed that QR2 was among the genes that were the most strongly induced in the learning deficiency group. This suggests that QR2 has some deleterious effects in relation to the memory processes.
Finally, it is worth stressing that QR2 does not recognize the classical NAD(P)H co-substrates as a hydride donors, but rather some of their putative breakdown products, such as N-methyl- and N-ribosyl-nicotinamide (Zhao et al., 1997). As described recently by Belenky et al. (2007) in a review on NAD metabolism, these breakdown products are still of unknown origin(s). This is also a quite interesting fact, as without these NAD(P)H breakdown products QR2 is not capable of having any kind of catalytic activity, which strongly suggests an yet new level of QR2/MT3 catalytic activity regulation.
An association between Parkinson's disease or schizophrenia and polymorphisms of QR2 has been reported (Harada et al., 2001, 2003). This polymorphism consists in an insertion of 29-bp nucleotides in the promoter region of the QR2 gene. Human cells expressing a promoter containing this insertion polymorphism demonstrate significantly higher QR2 gene expression (Wang and Jaiswal, 2006) and the catalytic activity thereof. Consequently, some neurological disorders including schizophrenia and Parkinson's disease might be associated with a higher expression of QR2, which would result in a higher susceptibility to external factors.
Conclusions/perspectives
After more than 10 years since the cloning of the melatonin receptor subtypes, their pharmacology is still not very well known. This may be due in part to the reasons mentioned above and to the lack of appropriate pharmacological tools. All the melatonin ligands reported until now are more or less close in chemical structure to that of melatonin or melatonin bio-isosteres. New ligands, mainly full and stable antagonists, not structurally related to melatonin should lead to new pharmacological tools. Nevertheless, the weak acute effect of melatonin in different animal models, with the exception of the clear but subtle effect of melatonin on the circadian rhythm, is also a possible reason. Indeed, melatonin or specific melatonin receptor agonists generally have modulator effects against other molecules such as neurotransmitters and their pharmacology might involve other partners.
The finding that MT3 and QR2 are the same protein, initially taken with much caution by the scientific community, might indeed shed new light on the role of melatonin at high concentrations (that is, >1 μM), in oxidative stress and, somehow, one might now hope to better understand at least some of the antioxidant properties of melatonin through a molecular mechanism of the neurohormone at the QR2 level.
Furthermore, the existence of other not yet identified melatonin receptor types is very plausible. Although two melatonin receptor subtypes have been cloned from mammalian species, the MT2 receptor is not present as a functional receptor in all mammals. For instance, in Siberian hamster the gene encoding the MT2 receptor contains a premature stop codon, which means that the protein is not expressed. Nevertheless, these animals are highly seasonal and melatonin treatments mimic short-day exposure and induce all the characteristic behavioural and physiological modifications induced by short photoperiod (body weight decrease, pelage colour change, gonadal regression, hormonal status, etc.) (Bartness and Goldman, 1988). It is difficult to understand how a single melatonin receptor subtype may be involved in all these changes on top of the circadian effects.
Further complexity may be generated by the formation of heterodimers between MT1 and MT2, at least in cells expressing the two melatonin receptor subtypes. The orphan receptor GPR50 does not bind melatonin and its endogenous ligand is still unknown. Nevertheless, this receptor has been shown to behave as an antagonist of the MT1 receptor, which opens new pharmacological perspectives for GPR50 despite the lack of identified endogenous or synthetic ligands and emphasize the complexity of the melatonin receptor field.
Acknowledgments
This study was supported by grants from the Institut de Recherches SERVIER, the Fondation pour la Recherche Médicale (‘Equipe FRM'), the Fédération pour la Recherche sur le Cerveau/FRC Neurodon and the Association pour la Recherche sur le Cancer (ARC). We are grateful to P Chen for comments on the paper and to Dr MA Ayoub for providing the template for Figure 1.
Conflict of interest
JA Boutin and P Delagrange are employees of the Institut de Recherches Servier.
References
- Agez L, Laurent V, Pevet P, Masson-Pevet M, Gauer F. Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neuroscience. 2007;144:522–530. doi: 10.1016/j.neuroscience.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Al-Ghoul WM, Herman MD, Dubocovich ML. Melatonin receptor subtype expression in human cerebellum. NeuroReport. 1998;9:4063–4068. doi: 10.1097/00001756-199812210-00011. [DOI] [PubMed] [Google Scholar]
- Alaerts M, Venken T, Lenaerts AS, De Zutter S, Norrback KF, Adolfsson R, et al. Lack of association of an insertion/deletion polymorphism in the G protein-coupled receptor 50 with bipolar disorder in a Northern Swedish population. Psychiatr Genet. 2006;16:235–236. doi: 10.1097/01.ypg.0000242193.28526.b3. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels, 2nd edition (2007 Revision) Br J Pharmacol. 2007;150 Suppl 1:S1. doi: 10.1038/sj.bjp.0707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine B, Boutin JA, Siest G. Heterogeneity of hepatic UDP-glucuronosyltransferase activities: investigations of isoenzymes involved in p-nitrophenol glucuronidation. Comp Biochem Physiol C Toxicol Pharmacol. 1993;106:241–248. doi: 10.1016/0742-8413(93)90278-s. [DOI] [PubMed] [Google Scholar]
- Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod. 1998;3:13–22. doi: 10.1530/ror.0.0030013. [DOI] [PubMed] [Google Scholar]
- Arendt J. Melatonin: characteristics, concerns, and prospects. J Biol Rhythms. 2005;20:291–303. doi: 10.1177/0748730405277492. [DOI] [PubMed] [Google Scholar]
- Audinot V, Mailliet F, Lahaye-Brasseur C, Bonnaud A, Le Gall A, Amosse C, et al. New selective ligands of human cloned melatonin MT1 and MT2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:553–561. doi: 10.1007/s00210-003-0751-2. [DOI] [PubMed] [Google Scholar]
- Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein. J Biol Chem. 2001;276:34871–34879. doi: 10.1074/jbc.M100775200. [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, et al. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol Pharmacol. 2004;66:312–321. doi: 10.1124/mol.104.000398. [DOI] [PubMed] [Google Scholar]
- Barrett P, Ivanova E, Graham ES, Ross AW, Wilson D, Ple H, et al. Photoperiodic regulation of cellular retinoic acid-binding protein 1, GPR50 and nestin in tanycytes of the third ventricle ependymal layer of the Siberian hamster. J Endocrinol. 2006;191:687–698. doi: 10.1677/joe.1.06929. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD. Effects of melatonin on long-day responses in short-day housed adult Siberian hamsters. Am J Physiol. 1988;255:R823–R830. doi: 10.1152/ajpregu.1988.255.5.R823. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD. Mammalian pineal melatonin: a clock for all seasons. Experientia. 1989;45:939–945. doi: 10.1007/BF01953051. [DOI] [PubMed] [Google Scholar]
- Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Bertaina-Anglade V, la Rochelle CD, Boyer PA, Mocaer E. Antidepressant-like effects of agomelatine (S 20098) in the learned helplessness model. Behav Pharmacol. 2006;17:703–713. doi: 10.1097/FBP.0b013e3280116e5c. [DOI] [PubMed] [Google Scholar]
- Berthouze M, Ayoub M, Russo O, Rivail L, Sicsic S, Fischmeister R, et al. Constitutive dimerization of human serotonin 5-HT4 receptors in living cells. FEBS Lett. 2005;579:2973–2980. doi: 10.1016/j.febslet.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Luan J, Challis B, Keogh J, Montague C, Brennand J, et al. Sequence variants in the melatonin-related receptor gene (GPR50) associate with circulating triglyceride and HDL levels. J Lipid Res. 2006;47:761–766. doi: 10.1194/jlr.M500338-JLR200. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Roussignol G, Becamel C, Gavarini S, Joubert L, Dumuis A, et al. GPCR-interacting proteins (GIPs): nature and functions. Biochem Soc Trans. 2004;32:851–855. doi: 10.1042/BST0320851. [DOI] [PubMed] [Google Scholar]
- Boutin JA. Myristoylation. Cell Signal. 1997;9:15–35. doi: 10.1016/s0898-6568(96)00100-3. [DOI] [PubMed] [Google Scholar]
- Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–419. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Brouillette J, Quirion R.Transthyretin: a key gene involved in the maintenance of memory capacities during aging Neurobiol Aging 2007(in press) [DOI] [PubMed]
- Browning C, Beresford I, Fraser N, Giles H. Pharmacological characterization of human recombinant melatonin mt(1) and MT2 receptors. Br J Pharmacol. 2000;129:877–886. doi: 10.1038/sj.bjp.0703130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner P, Sozer-Topcular N, Jockers R, Ravid R, Angeloni D, Fraschini F, et al. Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer's disease. Eur J Histochem. 2006;50:311–316. [PubMed] [Google Scholar]
- Brydon L, Petit L, de Coppet P, Barrett P, Morgan PJ, Strosberg AD, et al. Polymorphism and signalling of melatonin receptors. Reprod Nutr Dev. 1999b;39:315–324. doi: 10.1051/rnd:19990304. [DOI] [PubMed] [Google Scholar]
- Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R. Functional expression of mt2 (mel1b) melatonin receptors in human paz6 adipocytes. Endocrinology. 2001;142:4264–4271. doi: 10.1210/endo.142.10.8423. [DOI] [PubMed] [Google Scholar]
- Brydon L, Roka F, Petit L, de Coppet P, Tissot M, Barrett P, et al. Dual signaling of human Mel1a melatonin receptors via G(I2), G(I3), and G(Q/11) proteins. Mol Endocrinol. 1999a;13:2025–2038. doi: 10.1210/mend.13.12.0390. [DOI] [PubMed] [Google Scholar]
- Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh TC, Wu JM, et al. Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry. 2004;43:11417–11426. doi: 10.1021/bi049162o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamini B, Santarsiero BD, Boutin JA, Mesacar AD.Kinetic, thermodynamic and X-ray structural insights on the interaction of melatonin and analogs with quinone reductase 2 Biochem J 2008(in press) [DOI] [PMC free article] [PubMed]
- Carlson LL, Weaver DR, Reppert SM. Melatonin signal transduction in hamster brain: inhibition of adenylyl cyclase by a pertussis toxin-sensitive G protein. Endocrinology. 1989;125:2670–2676. doi: 10.1210/endo-125-5-2670. [DOI] [PubMed] [Google Scholar]
- Chan AS, Lai FP, Lo RK, Voyno-Yasenetskaya TA, Stanbridge EJ, Wong YH. Melatonin mt1 and MT2 receptors stimulate c-Jun N-terminal kinase via pertussis toxin-sensitive and -insensitive G proteins. Cell Signal. 2002;14:249–257. doi: 10.1016/s0898-6568(01)00240-6. [DOI] [PubMed] [Google Scholar]
- Cho DI, Oak MH, Yang HJ, Choi HK, Janssen GM, Kim KM. Direct and biochemical interaction between dopamine D3 receptor and elongation factor-1Bbetagamma. Life Sci. 2003;73:2991–3004. doi: 10.1016/s0024-3205(03)00707-0. [DOI] [PubMed] [Google Scholar]
- Chomarat P, Coge F, Guenin SP, Mailliet F, Vella F, Mallet C, et al. Cellular knock-down of quinone reductase 2: a laborious road to successful inhibition by RNA interference. Biochimie. 2007;89:1264–1275. doi: 10.1016/j.biochi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Dalton EJ, Rotondi D, Levitan RD, Kennedy SH, Brown GM. Use of slow-release melatonin in treatment-resistant depression. J Psychiatry Neurosci. 2000;25:48–52. [PMC free article] [PubMed] [Google Scholar]
- Daulat AM, Maurice P, Froment C, Guillaume JL, Broussard C, Monsarrat B, et al. Purification and identification of G protein-coupled receptor protein complexes under native conditions. Mol Cell Proteomics. 2007;6:835–844. doi: 10.1074/mcp.M600298-MCP200. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M, Ferry G, Masdehors P, Boutin JA, Hickman JA, Dive C. Post-translational modification of Bid has differential effects on its susceptibility to cleavage by caspase 8 or caspase 3. J Biol Chem. 2003;278:15749–15757. doi: 10.1074/jbc.M209208200. [DOI] [PubMed] [Google Scholar]
- Dillon DC, Easley SE, Asch BB, Cheney RT, Brydon L, Jockers R, et al. Differential expression of high-affinity melatonin receptors (MT1) in normal and malignant human breast tissue. Am J Clin Pathol. 2002;118:451–458. doi: 10.1309/1T4V-CT1G-UBJP-3EHP. [DOI] [PubMed] [Google Scholar]
- Drew JE, Barrett P, Mercer JG, Moar KM, Canet E, Delagrange P, et al. Localization of the melatonin-related receptor in the rodent brain and peripheral tissues. J Neuroendocrinol. 2001;13:453–458. doi: 10.1046/j.1365-2826.2001.00651.x. [DOI] [PubMed] [Google Scholar]
- Drew JE, Barrett P, Williams LM, Conway S, Morgan PJ. The ovine melatonin-related receptor: cloning and preliminary distribution and binding studies. J Neuroendocrinol. 1998;10:651–661. doi: 10.1046/j.1365-2826.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Cardinali DP, Delagrange P, Krause DN, Strosberg D, Sugden D, et al. Melatonin Receptors. The IUPHAR Compendium of Receptor Characterization and Classification. IUPHAR Media: London; 2001. pp. 270–277. [Google Scholar]
- Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, Manna E. Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res. 2005;39:113–120. doi: 10.1111/j.1600-079X.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Masana MI, Iacob S, Sauri DM. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:365–375. doi: 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Takahashi JS. Use of 2-[125I]iodomelatonin to characterize melatonin binding sites in chicken retina. Proc Natl Acad Sci USA. 1987;84:3916–3920. doi: 10.1073/pnas.84.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML, Yun K, AlGhoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–1220. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Takahashi JS, Dubocovich ML. 2-[125I]iodomelatonin binding sites in hamster brain membranes: pharmacological characteristics and regional distribution. Endocrinology. 1988;122:1825–1833. doi: 10.1210/endo-122-5-1825. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Karne S, Lerner MR, Reppert SM. Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc Natl Acad Sci USA. 1994;91:6133–6137. doi: 10.1073/pnas.91.13.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eison AS, Mullins UL. Melatonin binding sites are functionally coupled to phosphoinositide hydrolysis in Syrian hamster RPMI 1846 melanoma cells. Life Sci. 1993;53:PL393–PL398. doi: 10.1016/0024-3205(93)90494-n. [DOI] [PubMed] [Google Scholar]
- Feng Y, Wigg K, King N, Vetro A, Kiss E, Kapornai K, et al. GPR50 is not associated with childhood-onset mood disorders in a large sample of Hungarian families. Psychiatr Genet. 2007;17:347–350. doi: 10.1097/YPG.0b013e3281ac232f. [DOI] [PubMed] [Google Scholar]
- Flajolet M, Rakhilin S, Wang H, Starkova N, Nuangchamnong N, Nairn AC, et al. Protein phosphatase 2C binds selectively to and dephosphorylates metabotropic glutamate receptor 3. Proc Natl Acad Sci USA. 2003;100:16006–16011. doi: 10.1073/pnas.2136600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Melatonin directly constricts rat cerebral arteries through modulation of potassium channels. Am J Physiol. 1997;273 3 Part 2:H1530–H1536. doi: 10.1152/ajpheart.1997.273.3.H1530. [DOI] [PubMed] [Google Scholar]
- Gerdin MJ, Masana MI, Rivera-Bermudez MA, Hudson RL, Earnest DJ, Gillette MU. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB J. 2004;18:1646–1656. doi: 10.1096/fj.03-1339com. [DOI] [PubMed] [Google Scholar]
- Godson C, Reppert SM. The Mel(1a) melatonin receptor is coupled to parallel signal transduction pathways. Endocrinology. 1997;138:397–404. doi: 10.1210/endo.138.1.4824. [DOI] [PubMed] [Google Scholar]
- Guerrero HY, Gauer F, Schuster C, Pevet P, MassonPevet M. Melatonin regulates the mRNA expression of the mt(1) melatonin receptor in the rat pars tuberalis. Neuroendocrinology. 2000;71:163–169. doi: 10.1159/000054533. [DOI] [PubMed] [Google Scholar]
- Guillaume JL, Daulat AM, Maurice P, Levoye A, Migaud M, Brydon L, et al. The PDZ protein MUPP1 promotes Gi coupling and signalling of the MT1 melatonin receptor J Biol Chem 2008. in press [DOI] [PubMed]
- Harada S, Fujii C, Hayashi A, Ohkoshi N. An association between idiopathic Parkinson's disease and polymorphisms of phase II detoxification enzymes: glutathione S-transferase M1 and quinone oxidoreductase 1 and 2. Biochem Biophys Res Commun. 2001;288:887–892. doi: 10.1006/bbrc.2001.5868. [DOI] [PubMed] [Google Scholar]
- Harada S, Tachikawa H, Kawanishi Y. A possible association between an insertion/deletion polymorphism of the NQO2 gene and schizophrenia. Psychiatr Genet. 2003;13:205–209. doi: 10.1097/00041444-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Hazlerigg DG, Gonzalez BA, Lawson W, Hastings MH, Morgan PJ. Prolonged exposure to melatonin leads to time-dependent sensitization of adenylate cyclase and down-regulates melatonin receptors in pars tuberalis cells from ovine pituitary. Endocrinology. 1993;132:285–292. doi: 10.1210/endo.132.1.7678217. [DOI] [PubMed] [Google Scholar]
- Hinsby AM, Olsen JV, Mann M. Tyrosine phosphoproteomics of fibroblast growth factor signaling: a role for insulin receptor substrate-4. J Biol Chem. 2004;279:46438–46447. doi: 10.1074/jbc.M404537200. [DOI] [PubMed] [Google Scholar]
- Hjalm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J Biol Chem. 2001;276:34880–34887. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- Hudson RL, Ginter PS, Stepien I, Dubocovich ML.Distinct melatonin receptors are involved in melatonin (MLT)-mediated phase shifts of circadian rhythms of wheel-running activity in vivo and of neuronal firing in the suprachiasmatic nucleus (SCN) brain slice 2005Society for Neuroscience: Washington, DC; Program no. 766.21 AbstractViewer/Itinerary Planner [Google Scholar]
- Hunt AE, AlGhoul WM, Gillette MU, Dubocovich ML. Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol. 2001;280:C110–C118. doi: 10.1152/ajpcell.2001.280.1.C110. [DOI] [PubMed] [Google Scholar]
- Ivanova EA, Bechtold D, Dupre S, Brennand J, Barrett P, Luckman S, et al. Altered metabolism in the melatonin-related receptor (GPR50) knock out mouse. Am J Physiol Endocrinol Metab. 2007;294:E176–E182. doi: 10.1152/ajpendo.00199.2007. [DOI] [PubMed] [Google Scholar]
- Jarzynka MJ, Passey DK, Ignatius PF, Melan MA, Radio NM, Jockers R, et al. Modulation of melatonin receptors and G-protein function by microtubules. J Pineal Res. 2006;41:324–336. doi: 10.1111/j.1600-079X.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Nelson C, Allen C. Melatonin activates an outward current and inhibits lh in rat suprachiasmatic nucleus neurons. Brain Res. 1995;687:125–132. doi: 10.1016/0006-8993(95)00478-9. [DOI] [PubMed] [Google Scholar]
- Jin XW, von GC, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003;23:1054–1060. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JD, Klosen P, Barrett P, Hazlerigg DG. Regulation of MT melatonin receptor expression in the foetal rat pituitary. J Neuroendocrinol. 2006a;18:50–56. doi: 10.1111/j.1365-2826.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- Johnston JD, Messager S, Ebling FJ, Williams LM, Barrett P, Hazlerigg DG. Gonadotrophin-releasing hormone drives melatonin receptor down-regulation in the developing pituitary gland. Proc Natl Acad Sci USA. 2003;100:2831–2835. doi: 10.1073/pnas.0436184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JD, Tournier BB, Andersson H, Masson-Pevet M, Lincoln GA, Hazlerigg DG. Multiple effects of melatonin on rhythmic clock gene expression in the mammalian pars tuberalis. Endocrinology. 2006b;147:959–965. doi: 10.1210/en.2005-1100. [DOI] [PubMed] [Google Scholar]
- Jones SB, Lanford GW, Chen YH, Morabito M, Kim K, Lu Q. Glutamate-induced delta-catenin redistribution and dissociation from postsynaptic receptor complexes. Neuroscience. 2002;115:1009–1021. doi: 10.1016/s0306-4522(02)00532-8. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Hugel HM, Rowe SA. Characterization of the chicken brain melatonin-binding protein using iodinated and tritiated ligands. J Pineal Res. 1994;17:137–148. doi: 10.1111/j.1600-079x.1994.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Kim KM, Gainetdinov RR, Laporte SA, Caron MG, Barak LS. G protein-coupled receptor kinase regulates dopamine D3 receptor signaling by modulating the stability of a receptor-filamin-beta-arrestin complex. A case of autoreceptor regulation. J Biol Chem. 2005;280:12774–12780. doi: 10.1074/jbc.M408901200. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15 Spec no 2:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Koster-van Hoffen GC, Mirmiran M, Bos NP, Witting W, Delagrange P, Guardiola-Lemaitre B. Effects of a novel melatonin analog on circadian rhythms of body temperature and activity in young, middle-aged, and old rats. Neurobiol Aging. 1993;14:565–569. doi: 10.1016/0197-4580(93)90040-i. [DOI] [PubMed] [Google Scholar]
- Krause DN, Barrios VE, Duckles SP. Melatonin receptors mediate potentiation of contractile responses to adrenergic nerve stimulation in rat caudal artery. Eur J Pharmacol. 1995;276:207–213. doi: 10.1016/0014-2999(95)00028-j. [DOI] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Uz T, Arslan AD, Kurtuncu M, Imbesi M, et al. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci Lett. 2006;393:23–26. doi: 10.1016/j.neulet.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, et al. The orphan GPR50 receptor specifically inhibits MT(1) melatonin receptor function through heterodimerization. EMBO J. 2006a;25:3012–3023. doi: 10.1038/sj.emboj.7601193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Jockers R. Do orphan G-protein-coupled receptors have ligand-independent functions? New insights from receptor heterodimers. EMBO Rep. 2006b;7:1094–1098. doi: 10.1038/sj.embor.7400838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoye A, Jockers R. Alternative drug discovery approaches for orphan GPCRs. Drug Discov Today. 2007;13:52–58. doi: 10.1016/j.drudis.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Levoye A, Jockers R, Ayoub MA, Delagrange P, Savaskan E, Guillaume JL. Are G protein-coupled receptor heterodimers of physiological relevance? Focus on melatonin receptors. Chronobiol Int. 2006c;23:419–426. doi: 10.1080/07420520500521863. [DOI] [PubMed] [Google Scholar]
- Li M, Bermak JC, Wang ZW, Zhou QY. Modulation of dopamine D(2) receptor signaling by actin-binding protein (ABP-280) Mol Pharmacol. 2000;57:446–452. doi: 10.1124/mol.57.3.446. [DOI] [PubMed] [Google Scholar]
- Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci USA. 2001;98:5258–5263. doi: 10.1073/pnas.011538198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- Long DJ, II, Iskander K, Gaikwad A, Arin M, Roop DR, Knox R, et al. Disruption of dihydronicotinamide riboside:quinone oxidoreductase 2 (NQO2) leads to myeloid hyperplasia of bone marrow and decreased sensitivity to menadione toxicity. J Biol Chem. 2002;277:46131–46139. doi: 10.1074/jbc.M208675200. [DOI] [PubMed] [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- MacKenzie RS, Melan MA, Passey DK, Witt-Enderby PA. Dual coupling of MT1 and MT2 melatonin receptors to cyclic AMP and phosphoinositide signal transduction cascades and their regulation following melatonin exposure. Biochem Pharmacol. 2002;63:587–595. doi: 10.1016/s0006-2952(01)00881-4. [DOI] [PubMed] [Google Scholar]
- Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, et al. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat Cell Biol. 2003;5:633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- Mailliet F, Ferry G, Vella F, Berger S, Coge F, Chomarat P, et al. Characterization of the melatoninergic MT3 binding site on the NRH:quinone oxidoreductase 2 enzyme. Biochem Pharmacol. 2005;71:74–88. doi: 10.1016/j.bcp.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Mailliet F, Ferry G, Vella F, Thiam K, Delagrange P, Boutin JA. Organs from mice deleted for NRH:quinone oxidoreductase 2 are deprived of the melatonin binding site MT3. FEBS Lett. 2004;578:116–120. doi: 10.1016/j.febslet.2004.10.083. [DOI] [PubMed] [Google Scholar]
- Maronde E, Stehle JH. The mammalian pineal gland: known facts, unknown facets. Trends Endocrinol Metab. 2007;18:142–149. doi: 10.1016/j.tem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Masana MI, Dubocovich ML. Melatonin receptor signaling: finding the path through the dark. Sci STKE. 2001;2001:PE39. doi: 10.1126/stke.2001.107.pe39. [DOI] [PubMed] [Google Scholar]
- Masson-Pevet M, Bianchi L, Pevet P. Circadian photic regulation of melatonin receptor density in rat suprachiasmatic nuclei: comparison with light induction of fos-related protein. J Neurosci Res. 1996;43:632–637. doi: 10.1002/(SICI)1097-4547(19960301)43:5<632::AID-JNR13>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Maurice P, Daulat AM, Broussard C, Mozo J, Clary G, HotellierA generic approach for the purification of signaling complexes that specifically interact with the carboxy-terminal domain of G protein-coupled receptors Mol Cell Proteomics 2008(in press) [DOI] [PMC free article] [PubMed]
- Mazzucchelli C, Pannacci M, Nonno R, Lucini V, Fraschini F, Stankov BM. The melatonin receptor in the human brain: cloning experiments and distribution studies. Brain Res Mol Brain Res. 1996;39:117–126. doi: 10.1016/0169-328x(96)00017-4. [DOI] [PubMed] [Google Scholar]
- Mc Arthur A, Hunt A, Gillette M. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinology. 1997;138:627–634. doi: 10.1210/endo.138.2.4925. [DOI] [PubMed] [Google Scholar]
- McClatchy DB, Fang G, Levey AI. Elongation factor 1A family regulates the recycling of the M4 muscarinic acetylcholine receptor. Neurochem Res. 2006;31:975–988. doi: 10.1007/s11064-006-9103-1. [DOI] [PubMed] [Google Scholar]
- McClatchy DB, Knudsen CR, Clark BF, Kahn RA, Hall RA, Levey AI. Novel interaction between the M4 muscarinic acetylcholine receptor and elongation factor 1A2. J Biol Chem. 2002;277:29268–29274. doi: 10.1074/jbc.M203081200. [DOI] [PubMed] [Google Scholar]
- McNulty S, Ross AW, Barrett P, Hastings MH, Morgan PJ. Melatonin regulates the phosphorylation of CREB in ovine pars tuberalis. J Neuroendocrinol. 1994;6:523–532. doi: 10.1111/j.1365-2826.1994.tb00615.x. [DOI] [PubMed] [Google Scholar]
- McNulty S, Ross AW, Shiu KY, Morgan PJ, Hastings MH. Phosphorylation of CREB in ovine pars tuberalis is regulated both by cyclic AMP-dependent and cyclic AMP-independent mechanisms. J Neuroendocrinol. 1996;8:635–645. [PubMed] [Google Scholar]
- Meyer P, Pache M, Loeffler KU, Brydon L, Jockers R, Flammer J, et al. Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002;86:1053–1057. doi: 10.1136/bjo.86.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihindukulasuriya KA, Zhou G, Qin J, Tan TH. Protein phosphatase 4 interacts with and down-regulates insulin receptor substrate 4 following tumor necrosis factor-alpha stimulation. J Biol Chem. 2004;279:46588–46594. doi: 10.1074/jbc.M408067200. [DOI] [PubMed] [Google Scholar]
- Molinari EJ, North PC, Dubocovich ML. 2-[I-125]iodo-5-methoxycarbonylamino-N-acetyltryptamine: a selective radioligand for the characterization of melatonin ML(2) binding sites. Eur J Pharmacol. 1996;301:159–168. doi: 10.1016/0014-2999(95)00870-5. [DOI] [PubMed] [Google Scholar]
- Morgan P, Barret P, Howell H, Helliwel R. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int. 1994;24:101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Morgan P, Lawson W, Davidson G, Howell H. Melatonin inhibits cyclic AMP in cultured ovine pars tuberalis cells. J Mol Endocrinol. 1989;3:R5–R8. [Google Scholar]
- Morrey KM, McLachlan JA, Serkin CD, Bakouche O. Activation of human monocytes by the pineal hormone melatonin. J Immunol. 1994;153:2671–2680. [PubMed] [Google Scholar]
- Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ. Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus. 2002;12:165–173. doi: 10.1002/hipo.1105. [DOI] [PubMed] [Google Scholar]
- Nelson CS, Ikeda M, Gompf HS, Robinson ML, Fuchs NK, Yoshioka T, et al. Regulation of melatonin 1a receptor signaling and trafficking by asparagine-124. Mol Endocrinol. 2001;15:1306–1317. doi: 10.1210/mend.15.8.0681. [DOI] [PubMed] [Google Scholar]
- Nosjean O, Ferro M, Cogé F, Beauverger P, Henlin JM, Lefoulon F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- Nosjean O, Nicolas JP, Klupsch F, Delagrange P, Canet E, Boutin JA. Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. Biochem Pharmacol. 2001;61:1369–1379. doi: 10.1016/s0006-2952(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Onoprishvili I, Andria ML, Kramer HK, Ancevska-Taneva N, Hiller JM, Simon EJ. Interaction between the mu opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol Pharmacol. 2003;64:1092–1100. doi: 10.1124/mol.64.5.1092. [DOI] [PubMed] [Google Scholar]
- Ould-Hamouda H, Chen P, Levoye A, Sozer-Topcular N, Daulat AM, Guillaume JL, et al. Detection of the human GPR50 orphan seven transmembrane protein by polyclonal antibodies mapping different epitopes. J Pineal Res. 2007;43:10–15. doi: 10.1111/j.1600-079X.2007.00437.x. [DOI] [PubMed] [Google Scholar]
- Paul P, Lahaye C, Delagrange P, Nicolas JP, Canet E, Boutin JA. Characterization of 2-[I-125]iodomelatonin binding sites in Syrian hamster peripheral organs. J Pharmacol Exp Ther. 1999;290:334–340. [PubMed] [Google Scholar]
- Pelletier S, Duhamel F, Coulombe P, Popoff MR, Meloche S. Rho family GTPases are required for activation of Jak/STAT signaling by G protein-coupled receptors. Mol Cell Biol. 2003;23:1316–1333. doi: 10.1128/MCB.23.4.1316-1333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Lacroix I, deCoppet P, Strosberg AD, Jockers R. Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3′-5′-monophosphate pathway. Biochem Pharmacol. 1999;58:633–639. doi: 10.1016/s0006-2952(99)00134-3. [DOI] [PubMed] [Google Scholar]
- Pfleger KD, Eidne KA. Illuminating insights into protein–protein interactions using bioluminescence resonance energy transfer (BRET) Nat Methods. 2006;3:165–174. doi: 10.1038/nmeth841. [DOI] [PubMed] [Google Scholar]
- Pickering DS, Niles LP. Expression of nanomolar-affinity binding sites for melatonin in Syrian hamster RPMI 1846 melanoma cells. Cell Signal. 1992;4:201–207. doi: 10.1016/0898-6568(92)90083-k. [DOI] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, et al. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- Pluder F, Morl K, Beck-Sickinger AG. Proteome analysis to study signal transduction of G protein-coupled receptors. Pharmacol Ther. 2006;112:1–11. doi: 10.1016/j.pharmthera.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Poirel VJ, Boggio V, Dardente H, Pevet P, Masson-Pevet M, Gauer F. Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience. 2003a;120:745–755. doi: 10.1016/s0306-4522(03)00344-0. [DOI] [PubMed] [Google Scholar]
- Poirel VJ, Cailotto C, Streicher D, Pevet P, Masson-Pevet M, Gauer F. MT1 melatonin receptor mRNA tissular localization by PCR amplification. Neuroendocrinol Lett. 2003b;24:33–38. [PubMed] [Google Scholar]
- Poirel VJ, Masson-Pevet M, Pevet P, Gauer F. MT1 melatonin receptor mRNA expression exhibits a circadian variation in the rat suprachiasmatic nuclei. Brain Res. 2002;946:64–71. doi: 10.1016/s0006-8993(02)02824-x. [DOI] [PubMed] [Google Scholar]
- Redman J, Armstrong S, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–1091. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow-Walden L, Chuang J, et al. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995;18:1–11. doi: 10.1111/j.1600-079x.1995.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, Simopoulos AP, Maldonado MD, Flores LJ, et al. Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed functions. World Rev Nutr Diet. 2007;97:211–230. doi: 10.1159/000097917. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel(1b) melatonin receptor. Proc Natl Acad Sci USA. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T, Mahle CD, Kolakowski LJ. Cloning of a melatonin-related receptor from human pituitary. FEBS Lett. 1996;386:219–224. doi: 10.1016/0014-5793(96)00437-1. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Science. 1988;242:78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- Reynolds AB. p120-catenin: past and present. Biochim Biophys Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Bermudez MA, Masana MI, Brown GM, Earnest DJ, Dubocovich ML. Immortalized cells from the rat suprachiasmatic nucleus express functional melatonin receptors. Brain Res. 2004;1002:21–27. doi: 10.1016/j.brainres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Roka F, Brydon L, Waldhoer M, Strosberg AD, Freissmuth M, Jockers R, et al. Tight association of the human Mel(1a)-melatonin receptor and G(i): precoupling and constitutive activity. Mol Pharmacol. 1999;56:1014–1024. doi: 10.1124/mol.56.5.1014. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F, et al. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer's disease. J Pineal Res. 2005;38:10–16. doi: 10.1111/j.1600-079X.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Jockers R, Ayoub M, Angeloni D, Fraschini F, Flammer J, et al. The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer's disease. Curr Alzheimer Res. 2007;4:47–51. doi: 10.2174/156720507779939823. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Olivieri G, Brydon L, Jockers R, Krauchi K, Wirz JA, et al. Cerebrovascular melatonin MT1-receptor alterations in patients with Alzheimer's disease. Neurosci Lett. 2001;308:9–12. doi: 10.1016/s0304-3940(01)01967-x. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Olivieri G, Meier F, Brydon L, Jockers R, Ravid R, et al. Increased melatonin 1a-receptor immunoreactivity in the hippocampus of Alzheimer's disease patients. J Pineal Res. 2002a;32:59–62. doi: 10.1034/j.1600-079x.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Wirz-Justice A, Olivieri G, Pache M, Krauchi K, Brydon L, et al. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem. 2002b;50:519–526. doi: 10.1177/002215540205000408. [DOI] [PubMed] [Google Scholar]
- Seck T, Baron R, Horne WC. Binding of filamin to the C-terminal tail of the calcitonin receptor controls recycling. J Biol Chem. 2003;278:10408–10416. doi: 10.1074/jbc.M209655200. [DOI] [PubMed] [Google Scholar]
- Shih ML, Lin FB, Scott JD, Wang HY, Malbon CC. Dynamic complexes of beta(2)-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J Biol Chem. 1999;274:1588–1595. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- Slanar O, Pelisek V, Vanecek J. Melatonin inhibits pituitary adenylyl cyclase-activating polypeptide-induced increase of cyclic AMP accumulation and [Ca2+](I) in cultured cells of neonatal rat pituitary. Neurochem Int. 2000;36:213–219. doi: 10.1016/s0197-0186(99)00120-5. [DOI] [PubMed] [Google Scholar]
- Song Y, Chan CW, Brown GM, Pang SF, Silverman M. Studies of the renal action of melatonin: evidence that the effects are mediated by 37 kDa receptors of the Mel1a subtype localized primarily to the basolateral membrane of the proximal tubule. FASEB J. 1997;11:93–100. doi: 10.1096/fasebj.11.1.9034171. [DOI] [PubMed] [Google Scholar]
- Stankov B, Biella G, Panara C, Lucini V, Capsoni S, Fauteck J, et al. Melatonin signal transduction and mechanism of action in the central nervous system: using the rabbit cortex as a model. Endocrinology. 1992;130:2152–2159. doi: 10.1210/endo.130.4.1312448. [DOI] [PubMed] [Google Scholar]
- Stiffler MA, Chen JR, Grantcharova VP, Lei Y, Fuchs D, Allen JE, et al. PDZ domain binding selectivity is optimized across the mouse proteome. Science. 2007;317:364–369. doi: 10.1126/science.1144592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker NL, Christopherson KS, Yi BA, Schatz PJ, Raab RW, Dawes G, et al. PDZ domain of neuronal nitric oxide synthase recognizes novel C-terminal peptide sequence. Nat Biotechnol. 1997;15:336–342. doi: 10.1038/nbt0497-336. [DOI] [PubMed] [Google Scholar]
- Sumova A, Vanecek J. Melatonin inhibits GnRH-induced increase of cFOS immunoreactivity in neonatal rat pituitary. J Neuroendocrinol. 1997;9:135–139. doi: 10.1046/j.1365-2826.1997.d01-1076.x. [DOI] [PubMed] [Google Scholar]
- Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- Testa B, Kramer SD. The biochemistry of drug metabolism—an introduction: Part 2. Redox reactions and their enzymes. Chem Biodivers. 2007;4:257–405. doi: 10.1002/cbdv.200790032. [DOI] [PubMed] [Google Scholar]
- Thomson PA, Wray NR, Thomson AM, Dunbar DR, Grassie MA, Condie A, et al. Sex-specific association between bipolar affective disorder in women and GPR50, an X-linked orphan G protein-coupled receptor. Mol Psychiatry. 2005;10:470–478. doi: 10.1038/sj.mp.4001593. [DOI] [PubMed] [Google Scholar]
- Turek FW. From circadian rhythms to clock genes in depression. Int Clin Psychopharmacol. 2007;22 Suppl 2:S1–S8. doi: 10.1097/01.yic.0000277956.93777.6a. [DOI] [PubMed] [Google Scholar]
- Uz T, Arslan AD, Kurtuncu M, Imbesi M, Akhisaroglu M, Dwivedi Y, et al. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res. 2005;136:45–53. doi: 10.1016/j.molbrainres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Vakkuri O, Leppaluoto J, Vuolteenaho O. Development and validation of a melatonin radioimmunoassay using radioiodinated melatonin as a tracer. Acta Endocrinol. 1984;106:152–157. doi: 10.1530/acta.0.1060152. [DOI] [PubMed] [Google Scholar]
- van Coevorden A, Mockel J, Laurent E, Kerkhofs M, L'Hermite-Baleriaux M, Decoster C, et al. Neuroendocrine rhythms and sleep in aging men. Am J Physiol. 1991;260:E651–E661. doi: 10.1152/ajpendo.1991.260.4.E651. [DOI] [PubMed] [Google Scholar]
- Vanecek J, Klein DC. Melatonin inhibits gonadotropin-releasing hormone-induced elevation of intracellular Ca2+ in neonatal rat pituitary cells. Endocrinology. 1992;130:701–707. doi: 10.1210/endo.130.2.1733718. [DOI] [PubMed] [Google Scholar]
- Vanecek J, Vollrath L. Melatonin inhibits cyclic AMP and cyclic GMP accumulation in the rat pituitary. Brain Res. 1989;505:157–159. doi: 10.1016/0006-8993(89)90129-7. [DOI] [PubMed] [Google Scholar]
- Vanecek J, Vollrath L. Melatonin modulates diacylglycerol and arachidonic acid metabolism in the anterior pituitary of immature rats. Neurosci Lett. 1990;110:199–203. doi: 10.1016/0304-3940(90)90811-m. [DOI] [PubMed] [Google Scholar]
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci USA. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella F, Ferry G, Delagrange P, Boutin JA. NRH:quinone reductase 2: an enzyme of surprises and mysteries. Biochem Pharmacol. 2005;71:1–12. doi: 10.1016/j.bcp.2005.09.019. [DOI] [PubMed] [Google Scholar]
- von Gall C, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm DP, et al. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci. 2002a;5:234–238. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002b;309:151–162. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- von Gall C, Weaver DR, Kock M, Korf HW, Stehle JH. Melatonin limits transcriptional impact of phosphoCREB in the mouse SCN via the Mel1a receptor. NeuroReport. 2000;11:1803–1807. doi: 10.1097/00001756-200006260-00002. [DOI] [PubMed] [Google Scholar]
- von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann NY Acad Sci. 2005;1040:508–511. doi: 10.1196/annals.1327.105. [DOI] [PubMed] [Google Scholar]
- Wan Q, Man HY, Liu F, Braunton J, Niznik HB, Pang SF, et al. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat Neurosci. 1999;2:401–403. doi: 10.1038/8062. [DOI] [PubMed] [Google Scholar]
- Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22:2231–2237. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Jaiswal AK. Nuclear factor Nrf2 and antioxidant response element regulate NRH:quinone oxidoreductase 2 (NQO2) gene expression and antioxidant induction. Free Radic Biol Med. 2006;40:1119–1130. doi: 10.1016/j.freeradbiomed.2005.10.063. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Reppert SM. The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. NeuroReport. 1996;8:109–112. doi: 10.1097/00001756-199612200-00022. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ. Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull. 2006;68:425–429. doi: 10.1016/j.brainresbull.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Weissman JT, Ma JN, Essex A, Gao Y, Burstein ES. G-protein-coupled receptor-mediated activation of rap GTPases: characterization of a novel Galphai regulated pathway. Oncogene. 2004;23:241–249. doi: 10.1038/sj.onc.1207014. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Werth E, Renz C, Muller S, Krauchi K. No evidence for a phase delay in human circadian rhythms after a single morning melatonin administration. J Pineal Res. 2002;32:1–5. doi: 10.1034/j.1600-079x.2002.10808.x. [DOI] [PubMed] [Google Scholar]
- Witt-Enderby PA, Bennett J, Jarzynka MJ, Firestine S, Melan MA. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci. 2003;72:2183–2198. doi: 10.1016/s0024-3205(03)00098-5. [DOI] [PubMed] [Google Scholar]
- Witt-Enderby PA, Dubocovich ML. Characterization and regulation of the human ML1A melatonin receptor stably expressed in Chinese hamster ovary cells. Mol Pharmacol. 1996;50:166–174. [PubMed] [Google Scholar]
- Witt-Enderby PA, MacKenzie RS, McKeon RM, Carroll EA, Bordt SL, Melan MA. Melatonin induction of filamentous structures in non-neuronal cells that is dependent on expression of the human mt1 melatonin receptor. Cell Motil Cytoskeleton. 2000;46:28–42. doi: 10.1002/(SICI)1097-0169(200005)46:1<28::AID-CM4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Witt-Enderby PA, Masana MI, Dubocovich ML. Physiological exposure to melatonin supersensitizes the cyclic adenosine 3′,5′-monophosphate-dependent signal transduction cascade in Chinese hamster ovary cells expressing the human mt1 melatonin receptor. Endocrinology. 1998;139:3064–3071. doi: 10.1210/endo.139.7.6102. [DOI] [PubMed] [Google Scholar]
- Wu YH, Zhou JN, Van Heerikhuize J, Jockers R, Swaab DF. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer's disease. Neurobiol Aging. 2007;28:1239–1247. doi: 10.1016/j.neurobiolaging.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Yang XL, Holtzclaw WD, Talalay P. Unexpected genetic and structural relationships of a long-forgotten flavoenzyme to NAD(P)H:quinone reductase (DT-diaphorase) Proc Natl Acad Sci USA. 1997;94:1669–1674. doi: 10.1073/pnas.94.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotos DP. Recent advances in melatonin receptor ligands. Arch Pharm (Weinheim) 2005;338:229–247. doi: 10.1002/ardp.200400996. [DOI] [PubMed] [Google Scholar]