Abstract
Background and purpose:
Kinins are implicated in many pathophysiological conditions, and recent evidence has suggested their involvement in colitis. This study assessed the role of the kinin B1 receptors in a mouse model of colitis.
Experimental approach:
Colitis was induced in mice by 2,4,6-trinitrobenzene sulphonic acid (TNBS), and tissue damage and myeloperoxidase activity were assessed. B1 receptor induction was analysed by organ bath studies, binding assay and reverse transcription PCR.
Key results:
TNBS-induced colitis was associated with tissue damage, neutrophil infiltration and time-dependent increase of colon B1 receptor-mediated contraction, with the maximal response observed at 72 h. The upregulation of the B1 receptor at this time point was also confirmed by means of binding studies. B1 receptor mRNA levels were elevated as early as 6 h after colitis induction and remained high for up to 48 h. TNBS-evoked tissue damage and neutrophil influx were reduced by the selective B1 receptor antagonist SSR240612, and in B1 receptor knockout mice. In vivo treatment with inhibitors of protein synthesis, nuclear factor-κB activation, inducible nitric oxide synthase (iNOS) or tumour necrosis factor α (TNFα) significantly reduced B1 receptor agonist-induced contraction. Similar results were observed in iNOS and TNF receptor 1-knockout mice.
Conclusions and implications:
These results provide convincing evidence on the role of B1 receptors in the pathogenesis of colitis. Therefore, the blockade of kinin B1 receptors might represent a new therapeutic option for treating inflammatory bowel diseases.
Keywords: inflammation, kinin B1 receptor, colitis, pro-inflammatory peptides, inflammatory bowel disease treatment
Introduction
Bradykinin (BK) and its related peptides are effectors of the kallikrein–kinin system. They are formed from high and low molecular weight kininogens, by the action of serine protease kallikreins, in both plasma and peripheral tissues (Moreau et al., 2005). There is compelling evidence indicating that kinins are rapidly generated after tissue injury. They seem to modulate several events observed during the inflammatory process, including vasodilatation, increase of vascular permeability, plasma extravasation, cell migration, pain, hyperalgesia and synthesis of inflammatory mediators such as eicosanoids, cytokines and nitric oxide (Calixto et al., 2000, 2004). Most kinin effects are mediated by the activation of the two G-protein-coupled receptors named B1 and B2 (Moreau et al., 2005). B2 receptors are distributed in a constitutive manner throughout the central and peripheral tissues and present high affinity for BK and Lys-BK peptides. Nevertheless, B1 receptors display high affinity for the metabolites des-Arg9-BK and Lys-des-Arg9-BK. Interestingly, B1 receptors, with rare exceptions, are normally absent, but they can be extensively and rapidly upregulated either in vivo or in vitro (Calixto et al., 2000).
Inflammatory bowel diseases (IBDs) are chronic inflammatory conditions of the gastrointestinal tract, clinically present as two disorders: Crohn's disease and ulcerative colitis. The inflammation of the intestinal mucosa in IBDs is characterized by an influx of neutrophils and macrophages, accompanied by the production of cytokines, eicosanoids, proteolytic enzymes and free radicals that lead to inflammation and ulceration (Bamias and Cominelli, 2007; Van Limbergen et al., 2007). Of note, several studies have demonstrated that the kallikrein–kinin system plays relevant pathophysiological roles in the gastrointestinal tract (Cuthbert and Margolius, 1982). Animal models of colitis and human IBD suggest that kinins contribute greatly to the development and maintenance of gastrointestinal diseases, such as IBD. In 1973, Zeitlin and Smith demonstrated that kallikrein contents are increased in patients with ulcerative colitis. Later, a specific plasma kallikrein inhibitor, Bz-Pro-Phe-boroArg (P8720), was shown to attenuate intestinal inflammation (for review, see Stadnicki, 2005). Recently, we have demonstrated that in a model of colitis induced by 2,4,6-trinitrobenzene sulphonic acid (TNBS) there was a marked increase of BK-elicited contraction in vitro, associated with an enhancement of specific B2 receptor-binding sites in the mouse colon (Hara et al., 2007). Moreover, treatment with selective B2 receptor antagonists was able to ameliorate dextran sulphate sodium-induced colitis in both mice and rats (Arai et al., 1999; Kamata et al., 2002). Finally, an alteration in both the distribution and levels of B1 receptors was observed in the intestinal tissue of patients with IBD, suggesting a role for this receptor in intestinal inflammation (Stadnicki et al., 2005).
The present study employed pharmacological and molecular approaches to investigate the role of B1 receptors in the TNBS mouse model of colitis. We also attempted to investigate some of the mechanisms involved in B1 receptor upregulation in this paradigm. Herein, we report for the first time, that either pharmacological blockade or genetic disruption of B1 receptors markedly improves TNBS-induced colitis. Our study also demonstrates that functional upregulation of B1 receptors in TNBS-induced colitis is dependent on de novo protein synthesis, nuclear factor (NF)-κB activation, tumour necrosis factor α (TNFα) release and inducible nitric oxide synthase (iNOS) induction. The characterization of kinin B1 receptor upregulation in the inflamed colon points to this receptor as a potential therapeutic target in IBD.
Materials and methods
Animals
All experimental procedures followed the ‘Principles of Laboratory Animal Care' from the National Institutes of Health (NIH) publication number 85-23, and were approved by the Animal Ethics Committee of the Universidade Federal de Santa Catarina.
Experiments were conducted using male Swiss (30–40 g), C57Bl6, B1R knockout, TNF receptor 1 knockout and iNOS knockout mice (25–30 g) fed on a standard commercial diet. They were kept in controlled room temperature (22±2 °C) and humidity (60–80%) under a 12 h light–dark cycle. B1 receptor, TNF receptor-1 and iNOS knockout mice are on the C57Bl6 background, constructed as described previously (Rothe et al., 1993; MacMicking et al., 1995; Pesquero et al., 2000).
Induction and evaluation of experimental colitis
Colitis was induced according to the methodology described by Wallace et al. (1989) and adapted by Hara et al. (2007). After a fasting period of 18–24 h with free access to 5% glucose solution, mice were randomly divided into control and colitis groups. The animals were anaesthetized with 2,2,2-tribromoethanol (0.125 g kg−1, i.p.) and then a catheter was carefully inserted into the colon (4 cm proximal to the anus). To induce colitis, 0.1 mL of the hapten-forming reagent TNBS (in 50% ethanol) was slowly administered. To assure the distribution of TNBS within the entire colon, mice were carefully maintained at a 45° angle (head down position) for 2 min, and then returned to their cages. Control mice received the same volume of saline solution. At 72 h following TNBS administration, the animals were killed and the colon was removed, dissected and opened lengthwise. The severity of colon damage was macroscopically assessed using the criteria previously established for TNBS-induced colitis. A score ranging from 0 to 10 was employed, as follows: 0, no damage; 1, hyperaemia without ulcers; 2, hyperaemia and wall thickening without ulcers; 3, one ulceration site without wall thickening; 4, two or more ulceration sites; 5, 0.5 cm extension of inflammation or major damage; 6–10, 1 cm extension of inflammation or severe damage and the score was increased by 1 for every 0.5 cm of damage up to a maximal score of 10; 0 or 1, absence or presence of diarrhoea; 0 or 1, absence or presence of stricture; 0, 1 or 2, absence or presence of mild or severe adhesion.
Organ bath studies
After different intervals of time following TNBS administration (6–96 h), the animals (n=6 per group) were killed by cervical dislocation and the entire colons were removed, isolated (4 cm) and carefully cleaned and separated from adherent tissues (but not opened) (Hara et al., 2007). Saline-treated animals were used as controls. Briefly, full thickness longitudinal colon segments (1.5 cm) were set up vertically in individual 5-mL organ baths containing Krebs–Henseleit solution (mM: NaCl, 113; KCl, 4.7; CaCl2, 2.5; NaHCO3, 25; MgSO4, 1.1; KH2PO4, 0.9 and D-glucose, 11; pH 7.4), maintained at 37 °C and continuously gassed with 95% of O2 and 5% of CO2. Preparations were set up in a force-displacement transducer (Narco Biosystems, Houston, TX, USA) under a resting tension of 1 g, and were allowed to equilibrate for at least 40 min before the incubation of any drug, during which the bath solution was renewed every 15 min. Isometric contractions were recorded on a polygraph. The contractile responses in all experiments are expressed as the percentage of the contraction induced by carbachol (CCh) (100 μM). Complete cumulative concentration–response curves for the selective kinin B1 receptor agonist des-Arg9-BK (0.1–10 000 nM) were obtained as previously described by Van Rossum (1963). The contact time of each concentration of the kinin B1 receptor agonist, des-Arg9-BK, with colon strips was at least 1 min and the time to complete the full cumulative concentration–response curve was about 12 min. No more than one complete curve was recorded for each colon. In some experiments, a single concentration of des-Arg9-BK (1 μM) was used. All experiments were performed in the presence of captopril (3 μM) to prevent the degradation of peptides.
In a separate group of experiments, the colon segments were set up 72 h following TNBS treatment, and concentration–response curves for des-Arg9-BK (0.1–10 000 nM) were performed in the absence or presence of the selective B1 receptor antagonist R-715 (1–10 nM) or the selective B2 receptor antagonist FR173657 (100 nM) (El Sayah et al., 2006). The antagonists were added to the preparation 10 min before the construction of the concentration–response curves.
Myeloperoxidase activity assay
The polymorphonuclear infiltration was indirectly measured by means of myeloperoxidase (MPO) activity according to the methodology described by Souza et al. (2000). Briefly, the entire colons obtained from mice treated with saline or TNBS (72 h earlier) were homogenized at 5% (w v−1) in EDTA, NaCl/phosphate buffer (pH 4.7) and centrifuged at 12 500 g for 15 min, at 4 °C. The pellet was resuspended in hexadecyltrimethyl ammonium bromide 0.5% in Na phosphate buffer (pH 5.4) and the samples were frozen in liquid nitrogen and thawed three times. Upon thawing, the samples were re-centrifuged (12 500 g, 15 min, 4 °C) and 25 μL of the supernatant was used for the MPO assay. The enzymatic reaction was assessed with 1.6 mM tetramethylbenzidine, 80 mM NaPO4 and 0.3 mM hydrogen peroxide. The absorbance was measured at 690 nm and the results are expressed in optical density per milligram of tissue.
In vivo treatment protocols
To determine the involvement of kinin B1 receptors in the experimental colitis model, two treatment protocols were employed using the selective non-peptide B1 receptor antagonist SSR240612. The animals received SSR240612 twice a day (10 mg kg−1, p.o., every 12 h) from the beginning of the fasting period (preventive treatment) or starting at 24 h after colitis induction (therapeutic treatment). Similar treatment protocols were carried out using dexamethasone (1 mg kg−1, s.c., every 12 h) as a positive control. After 72 h, the animals were killed and the severity of colitis was evaluated by means of macroscopic score determination and MPO activity. In some experiments, the colitis was induced in wild-type (WT; C57Bl6) or B1R knockout mice and evaluated as described above.
To assess the possible mechanisms involved in kinin B1 receptor upregulation in TNBS-induced colitis, the animals were systemically treated from the fasting period to death with several groups of drugs. The colitis was induced as previously described and following 72 h, the animals were killed and the functional upregulation of B1 receptors was evaluated in colonic tissue by means of organ bath studies. Control animals received saline solution (NaCl 0.9%, 10 mL kg−1) at the same intervals of time. The contribution of de novo protein synthesis to B1R upregulation in experimental colitis was assessed in animals treated with the glucocorticoid dexamethasone (1 mg kg−1, every 12 h, s.c.) or the protein synthesis inhibitor cycloheximide (2.5 mg kg−1, every 12 h, i.p.). The role of transcription factor NF-κB in the functional upregulation of B1R was evaluated in a separate group of animals, treated with the NF-κB inhibitor pyrrolidinedithiocarbamate (PDTC) (30 mg kg−1, every 24 h, i.p.).
To investigate the participation of the cytokine TNFα in TNBS-induced kinin B1 receptor upregulation, separate groups of animals received the chimaeric anti-TNFα monoclonal antibody infliximab (2 mg kg−1, every 24 h, s.c.) or the TNFα synthesis inhibitor thalidomide (50 mg kg−1, every 24 h, p.o.). To estimate the contribution of the enzyme iNOS, other animals were treated with the selective iNOS inhibitor 1400W (10 mg kg−1, every 12 h, s.c.). In some experimental groups, colitis was induced in WT (C57Bl6), TNF receptor-1 or iNOS knockout mice.
The choice of the doses of each drug was based on previous data described in the literature (Kankuri et al., 2001; Rejdak et al., 2001; Cuzzocrea et al., 2002; Steed et al., 2003; Gougat et al., 2004; Shi et al., 2006; Rodrigues et al., 2007; Fries et al., 2008; Genovese et al., 2008). At the schedules adopted for in vivo treatments in our study, we have not observed any clear toxic effects, such as piloerection, hypoactivity or abdominal constrictions.
[3H]des-Arg10-kallidin-binding studies
The density of B1 receptors in control and inflamed mouse colons was assessed by means of binding studies. At 72 h after TNBS treatment, the entire colons were removed and their macroscopic damage was graded as previously described. For the membrane preparation, six inflamed (scored from 4 to 10) and six control colons were used. Membrane preparation was carried out according to the method described by Scherrer et al. (1999); Hara et al. (2007), with some modifications. The colons were homogenized in a cold sucrose solution (0.25 M) and centrifuged at 1000 g for 10 min at 4 °C. The first supernatant was stored and the resulting pellet was re-suspended in cold sucrose solution (0.25 M), homogenized and centrifuged at 1000 g for 10 min at 4 °C. The second supernatant was mixed with the first, and then diluted in 10 mL of buffer (25 mM (N-trishydroxymethyl)methyl-2-aminoethane sulphonic acid (TES), 1 mM phenanthroline; pH 6.8) and centrifuged at 35 000 g for 30 min at 4 °C. The remaining pellet was diluted in 2 mL of binding buffer (25 mM TES, 1 mM phenanthroline, 1 mM dithiothreitol, 140 μg mL−1 bacitracin, 10 μM captopril, 0.1% bovine serum albumin; pH 6.8). Protein levels were determined according to the method of Bradford (1976) and used at 0.8 mg mL−1.
For the binding assay, the membranes (125 μL) were incubated in binding buffer at 25 °C in the presence of increasing concentrations of [3H]des-Arg10-kallidin (specific activity 82.00 Ci mmol−1) (0.03–10 nM). As nonspecific binding, unlabelled des-Arg9-Leu8-BK was included at a final concentration of 1 μM. After 90 min of incubation, the membranes were transferred to filters presoaked in 0.1% aqueous polyethyleneimine and then washed immediately under vacuum eight times with 1 mL of cold Tris-HCl (50 mM, pH 7.4) buffer solution. The radioactivity was counted by liquid scintillation techniques. Specific binding was defined as the difference in the amount of radioactivity bound in the absence and presence of unlabelled des-Arg9-Leu8-BK, which enabled the determination of the maximal specific binding on saturation curves (Bmax) and the dissociation constant (KD).
Reverse-transcription PCR
Semiquantitative reverse transcription -PCR was performed as described previously by Leite et al. (2007) with some adaptations. Total RNA was extracted from entire colons obtained from mice treated with saline or TNBS (6–96 h) by using 1.5 mL of TRIzol reagent, according to the manufacturer's recommendations. The reverse transcription was performed with the reverse transcriptase M-MLV (200 U). The reaction was carried out using 5 μg of RNA, 1 μL of oligo dT (0.5 μg mL−1), 1 μL of dNTP mix (10 mM), 40 U of RNAse OUT, 2 μL of dithiothreitol (0.1 mM) and 4 μL of M-MLV buffer reaction (250 mM Tris-HCl (pH 8.3), 375 mM KCl and 15 mM MgCl2). The cDNA was obtained as described in reverse transcriptase M-MLV protocol in a total of 20 μL and stored at 4 °C until use. The PCR reaction was performed using 0.5 μg of cDNA, 0.2 μL of dNTP mix (10 mM), 0.3 μL of MgCl2 (50 mM), 0.5 μL of the antisense primer (10 μM) for B1 receptors (5′-GACATAAATCAGTGGGTTC-3), or β-actin (CGTCTCCGGAGTCCATCACA), 0.5 μL of the sense primer (10 μM) for B1 receptors (5′-AACCGTTTCAACTGGCCC-3′), or β-actin (TCCTTCGTTGCCGGTCCACA), 1 μL of Taq polymerase buffer and 0.4 μL of Taq polymerase, in a total of 10 μL. The PCR was performed in a DNA thermal cycler (Eppendorf Mastercycler Personal, Hamburg, Germany) and the conditions for PCR were 5 min at 95 °C followed by 31 cycles of 15 s at 95 °C, 30 s at 52 °C and 1.5 min at 72 °C. The PCR products were size fractionated in 1.8% agarose gels stained with 1 μL of GoldView. The DNA band sizes were confirmed by the 50 bp DNA Ladder.
Statistical analysis
All values are expressed as means±s.e.mean, except the KD values (the molar concentrations of agonist required to produce 50% of the maximal specific binding) that are given as the geometric means accompanied by their 95% confidence limits. Statistical analysis was performed by means of unpaired Student's t-test or by ANOVA followed by Student–Newman–Keuls test when appropriate. P-values less than 0.05 were considered as indicative of significance.
Drugs and chemical reagents
The nomenclatures of drugs and molecular targets adopted in this study are according to Alexander et al. (2008). The following drugs and reagents were used: ((E)-3-(6-acetamindo-3-pyridyl)-N[N-2-4-dichloro-3-[(2-methyl-8-quinolinyl)oxy-methyl]phenyl]-N-methylamino-carbonyl-ethyl]acrylamide) (FR 173657) (kindly donated by Fujisawa Pharmaceutical Co., Osaka, Japan); DNA Ladder, reverse transcriptase M-MLV, RNAse OUT, TRIzol reagent (Invitrogen, Scotland, UK); Taq polymerase (Luwig, Porto Alegre, Brazil) [3H]des-Arg10-kallidin (specific activity 82.00 Ci mmol−1) (Perkin Elmer Life and Analytical Science, Paris, France); dNTP mix (Promega, Madison, WI, USA); [(2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-{[(6-methoxy-2-naphthyl)sulphonyl]amino}propanol)amino]-3-(4-{[2R,6S)-2,6-dimethylpiperidinyl]methyl}phenyl)-N-isopropyl-N-ethylpropanamide hydrochloride] (SSR240612) (kindly donated by Sanofi Recherche, Montpellier, France); bacitracin, bovine serum albumin protease free, captopril, CCh, cycloheximide, des-Arg9-BK, des-Arg9-[Leu8]-BK, dexamethasone, dithiothreitol, hexadecyltrimethyl ammonium bromide, phenanthroline, polyethyleneimine, PDTC, (TES, tetramethylbenzidine, 2,2,2-tribromoethanol, TNBS, Trizma base (minimum 99.9% titration), (all from Sigma Chemical Co., St Louis, MO, USA); N-[[3-(aminomethyl)phenyl]methyl]-ethanimidamide dihydrochloride (1400W), thalidomide (Tocris Bioscience, Ellis Ville, MO, USA). The R-715 was kindly donated by Dr Férnand Gobeil (Department of Pharmacology, University of Sherbrooke, Sherbrooke, QC, Canada). Stock solutions were stored at 1–10 mM (−20 °C) and diluted to the desired concentration just before use in phosphate-buffered saline. In some cases, the drugs were dissolved in absolute ethanol or dimethyl sulphoxide. The concentration of solvents in the organ bath never exceeded 0.02%, which had no effect per se on the tone of the preparations or on B1 receptor agonist-induced contraction.
Results
Kinin B1 receptor upregulation in TNBS-induced colitis
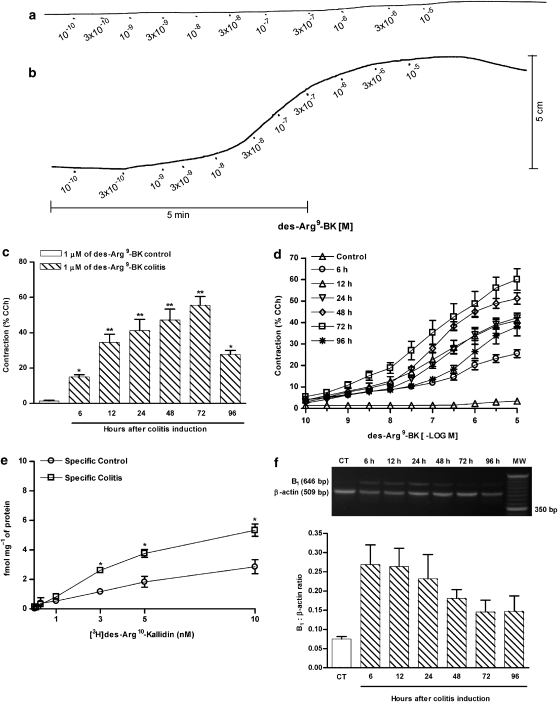
We initially assessed the contractile response induced by the selective B1 receptor agonist des-Arg9-BK in the isolated colons under normal (Figure 1a) and inflammatory conditions (Figure 1b) as demonstrated in typical records. At different time points following colitis induction, the entire colon was removed and prepared for organ bath studies. As shown in Figure 1c, a single concentration of des-Arg9-BK (1 μM) caused a marked and time-dependent increase of contraction in colon preparations obtained from TNBS-treated animals. The increase in the contractile response was significant as early as 6 h after colitis induction, reaching the maximal response at 72 h, and decreasing thereafter. Conversely, des-Arg9-BK displays only a slight contractile response in preparations obtained from control mice (Figures 1c and d). The induction of colitis by TNBS caused a striking change in the concentration–response curves for des-Arg9-BK, with an increase of about 15-fold in the maximal response (Rmax), when assessed 72 h following TNBS treatment, in comparison to control animals (Figure 1d). The preincubation of the preparations with the selective B1 receptor antagonist R-715 (1–30 nM), but not the selective B2 receptor antagonist FR173657 (100 nM), caused a concentration-dependent shift to the right of the concentration–response curves for des-Arg9-BK accompanied by a reduction of the Rmax, indicating mixed competitive and non-competitive mechanisms (data not shown).
Figure 1.
Upregulation of kinin B1 receptors in 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis. Colitis was induced by intracolonic administration of TNBS (2.5 mg per mouse, in 50% ethanol), and the colons were collected at different time points following TNBS treatment (6–96 h) for experimental analysis. (a) Typical record showing cumulative concentration–response curve for des-Arg9-BK in a colon preparation obtained from control mice. (b) Typical record showing cumulative concentration–response curve for des-Arg9-BK in a colon preparation obtained 72 h after colitis induction. (c) Contractile responses to des-Arg9-BK (1 μM) in colon preparations obtained at different time intervals following colitis induction (6–96 h). (d) Mean cumulative concentration–response curves for des-Arg9-BK in colon preparations obtained from control or TNBS-treated mice (6–96 h). Results are expressed as percentages of the contraction induced by carbachol (CCh 100 μM). (e) [3H]des-Arg10-kallidin saturation binding assay performed on colon preparations obtained 72 h following saline or TNBS administration. (f) Representative reverse transcription-PCR analysis showing B1 receptor mRNA expression and quantification of the ratio between B1 receptor and β-actin mRNA expression obtained at different time intervals following colitis induction (6–96 h). Each point or column represents the means±s.e.mean, of 5–7 mice per group. *P<0.05 and **P<0.01 versus control group.
The upregulation of kinin B1 receptors in TNBS-induced colitis was further confirmed by binding studies. The data indicate a marked increase in B1 receptor densities (without a significant alteration of affinity), according to assessment 72 h following colitis induction (Figure 1e). In control mice, the Bmax obtained was 2.9±0.5 fmol mg−1 of protein, with a KD value of 4.1 nM (95% confidence interval of 2.9–6.0 nM). In TNBS-treated mice, an increase of about 1.9-fold in the Bmax value (5.3±0.4 fmol mg−1 of protein) was observed, whereas the KD value was not significantly modified (KD of 3.7 nM; 95% confidence interval of 3.5–3.8 nM). The increase in specific binding sites for B1 receptors was preceded by a 3.6-fold rise in B1 receptor mRNA expression, which was evident as early as 6 h after colitis induction, and remained upregulated for up to 48 h (Figure 1f).
Kinin B1 receptor blockade ameliorates TNBS-induced colitis
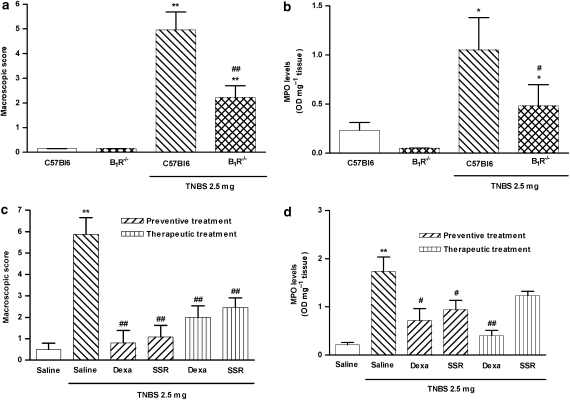
As kinin B1 receptors were found to be upregulated in the colonic tissue obtained from TNBS-treated mice, we next evaluated whether or not the B1 receptor might be implicated in the pathophysiology of colitis in mice. For this purpose, we induced colonic inflammation in WT (C57Bl6) and B1 receptor−/− mice as previously described. As shown in Figure 2a, genetic disruption of B1 receptors resulted in a significant reduction (55±3.6%) of the macroscopic score observed for colonic tissue, in comparison to WT mice. In addition, the MPO activity in the colon tissue was significantly reduced in B1 receptor−/− mice by 54±7.1% (Figure 2b). Corroborating these observations, Swiss mice subjected to the preventive treatment with SSR240612 (selective B1 receptor antagonist) exhibited a striking decrease in both macroscopic score and MPO activity, with reduction percentages of 82±9.1 and 46±10.9%, respectively (Figures 2c and d). Similar results were observed for the preventive treatment with dexamethasone (reduction of 86±9.9 and 59±14.2%, respectively). To analyse the therapeutic potential of the B1 receptor blockade in our model, the animals were treated with SSR240612 after colitis establishment. Therapeutic treatment with the B1 receptor antagonist, that is, starting 24 h after induction of colitis, resulted in a significant reduction of the macroscopic score (58±7.7%; P<0.01) and a nonsignificant reduction in MPO activity (29±5.3%; P=0.1201). The positive control dexamethasone, given similarly, diminished macroscopic score and the MPO activity by 66±8.8 and 77±6.2%, respectively (Figures 2c and d).
Figure 2.
Pharmacological blockade or genetic disruption of B1 receptor diminished 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis. Colitis was induced by intracolonic administration of TNBS (2.5 mg per mouse, in 50% ethanol) in wild-type (WT) (C57Bl6) and B1 receptor−/− mice, or in Swiss mice treated with vehicle or B1 receptor antagonist SSR240612 (10 mg kg−1, p.o. every 12 h) from the beginning of the fasting period in the colitis induction protocol (preventive treatment) or 24 h after colitis induction (therapeutic treatment). At 72 h following TNBS administration, the severity of the colitis was assessed by macroscopic score and myeloperoxidase (MPO) activity determination. (a) Macroscopic score and (b) MPO activity in colonic tissues from WT or B1R−/− mice. (c) Macroscopic score of colons from vehicle- or SSR240612-treated Swiss mice. (d) MPO activity in colonic tissue from Swiss mice treated with vehicle or SSR240612. Each column represents the means ±s.e.mean, of 5–7 mice per group. *P<0.05 and **P<0.01 versus control group; #P<0.05 and ##P<0.01 versus TNBS-treated group.
Mechanisms underlying functional B1 receptor upregulation after TNBS-induced colitis
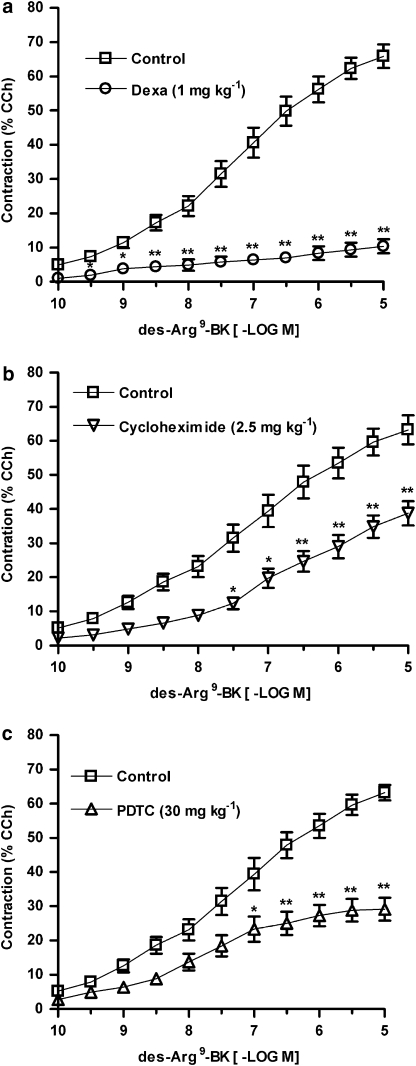
We subsequently evaluated some of the possible mechanisms underlying B1 receptor upregulation in TNBS-induced colitis. Initially, to assess the requirement of de novo protein synthesis, mice were systemically treated with the glucocorticoid dexamethasone (1 mg kg−1, s.c., every 12 h) or with the inhibitor of protein synthesis cycloheximide (2.5 mg kg−1, i.p., every 12 h). The results depicted in Figures 3a and b demonstrate that both treatments resulted in a significant decrease of B1 receptor-mediated contractile response in the mouse colon (reduction in Rmax of 83±1.8 and 39±5.7%, respectively).
Figure 3.
The B1 receptor functional upregulation in 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis is dependent on de novo protein synthesis and nuclear factor (NF)-κB activation. Mice were treated with the glucocorticoid dexamethasone (Dexa) (1 mg kg−1, s.c., every 12 h), the protein synthesis inhibitor cycloheximide (2.5 mg kg−1, i.p., every 12 h), or the NF-κB inhibitor pyrrolidinedithiocarbamate (PDTC) (30 mg kg−1, i.p., every 24 h). At 72 h following colitis induction by TNBS (2.5 mg per mouse, in 50% ethanol), mice were killed, the colons were isolated and cumulative concentration–response curves for des-Arg9-BK were obtained. Mean cumulative concentration–response curves for des-Arg9-BK in preparations obtained from mice treated with vehicle or (a) dexamethasone, (b) cycloheximide or (c) PDTC. Results are expressed as percentages of the contraction induced by carbachol (CCh 100 μM). Each point represents the means±s.e.mean, of at least six preparations. *P<0.05 and **P<0.01 versus vehicle-treated group.
We next attempted to determine the involvement of the transcriptional factor NF-κB in the functional upregulation of B1 receptors after colitis induction. The administration of the NF-κB inhibitor PDTC (30 mg kg−1 i.p., every 24 h) caused a marked decrease in des-Arg9-BK-induced contractile response (reduction in Rmax of 54±5.2%; Figure 3c).
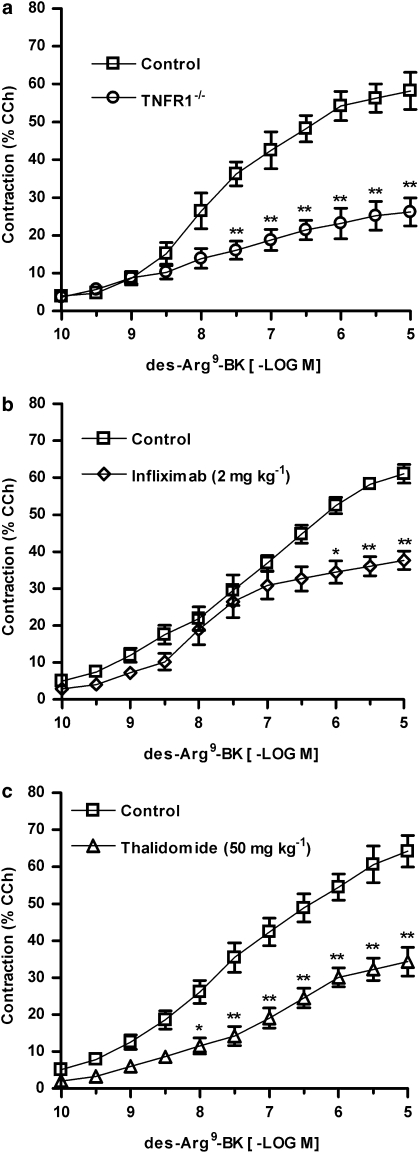
NF-κB regulates the expression of many inflammatory mediators such as the cytokine TNFα, which displays a pivotal role in ulcerative colitis (Ben-Neriah and Schmidt-Supprian, 2007). As TNFα has been widely associated with B1 receptor upregulation in several inflammation models (Calixto et al., 2004), we subsequently evaluated the relevance of TNFα for the functional B1 receptor upregulation in experimental colitis. To test this hypothesis, the colitis was induced as described beforehand in WT or TNF receptor-1−/− mice, and after 72 h the contractile responses elicited by des-Arg9-BK were assessed as described before. Data in Figure 4a demonstrate that B1 receptor-mediated contraction was significantly reduced in TNF receptor-1−/− mice (reduction in Rmax of 55±6.8%) when compared to WT mice. Moreover, the treatment of Swiss mice with the anti-TNFα antibody infliximab (2 mg kg−1, s.c., every 24 h) or thalidomide (50 mg kg−1 p.o., every 24 h) also caused a significant decrease of the maximal contractile response induced by des-Arg9-BK, in colon preparations obtained 72 h after TNBS treatment (38±4.9 and 47±6.0%, respectively) (Figures 4b and c).
Figure 4.
Tumour necrosis factor α (TNFα) plays an important role in kinin B1 receptor functional upregulation in 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis. Colitis was induced by intracolonic administration of TNBS (2.5 mg per mouse, in 50% ethanol) in wild-type (WT) (C57Bl6) or TNF receptor-1−/− mice, or in Swiss mice treated with vehicle, the anti-TNFα antibody infliximab (2.0 mg kg−1, s.c.), or the TNFα synthesis inhibitor thalidomide (50 mg kg−1, p.o.), every 24 h. At 72 h following colitis induction the mice were killed, the colons were isolated and cumulative concentration–response curves for des-Arg9-BK were obtained. Mean cumulative concentration–response curves for des-Arg9-BK in control colon preparations or in colon preparations from (a) TNF receptor-1−/− mice, (b) infliximab- or (c) thalidomide-treated mice. Results are expressed as percentages of the contraction induced by carbachol (CCh 100 μM). Each point represents the means±s.e.mean, of at least six preparations. *P<0.05 and **P<0.01 versus respective control group.
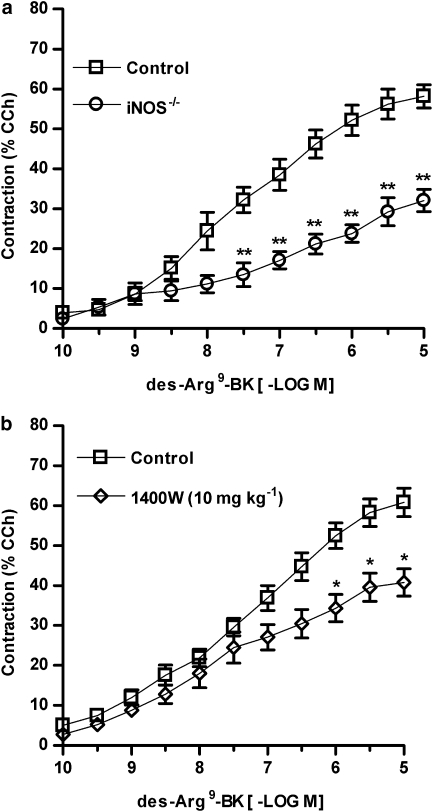
Besides the involvement of TNFα, the induction of iNOS enzyme also appears to contribute to functional B1 receptor upregulation in this model, as the contraction induced by des-Arg9-BK following TNBS administration was markedly reduced (45±4.8%) in colon preparations obtained from iNOS−/− mice in comparison to WT mice (Figure 5a). Similar results were observed in Swiss mice treated with the selective inhibitor of iNOS activity 1400W (10 mg kg−1, s.c., every 12 h) (Figure 5b). In 1400W-treated mice, we observed a reduction of 33±4.2% in Rmax in comparison to control animals.
Figure 5.
The induction of inducible nitric oxide synthase (iNOS) is implicated in kinin B1 receptor functional upregulation in 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis. Colitis was induced by intracolonic administration of TNBS (2.5 mg per mouse, in 50% ethanol) in wild-type (WT) (C57Bl6) or iNOS−/− mice, or in Swiss mice treated with vehicle or the selective iNOS inhibitor 1400W (10 mg kg−1, s.c., every 12 h). After 72 h, the mice were killed, the colons were isolated and cumulative concentration–response curves for des-Arg9-BK were obtained. Mean cumulative concentration–response curves for des-Arg9-BK in control colon preparations or in colon preparations from (a) iNOS−/− mice or (b) 1400W-treated mice. Results are expressed as percentages of the contraction induced by carbachol (CCh 100 μM). Each point represents the means±s.e.mean, of at least six preparations. *P<0.05 and **P<0.01 versus respective control group.
Discussion
It has been postulated that kinins could contribute to the inflammatory response and symptoms of IBD (Stadnicki, 2005). The present report extends this notion and demonstrates the importance of the induction of kinin B1 receptors for the initiation and maintenance of TNBS-induced colitis in mice.
The relevance of kinins has been well demonstrated in several biological systems (Calixto et al., 2000, 2004; Moreau et al., 2005). Regarding the gastrointestinal tract, only a few studies have so far assessed the physiological roles played by kinins. It has been shown previously that kallikrein–kinin system is implicated in enzymatic processing of mucoproteins, ion transport across the epithelium and regulation of local blood flow (Cuthbert and Margolius, 1982; Cuthbert and MacVinish, 1986). Activation of plasma kallikrein has been documented in patients with the active phase of ulcerative colitis (Stadnicki et al., 1997). Furthermore, a selective plasma kallikrein inhibitor has been shown to attenuate acute and chronic enterocolitis in the Lewis rat (Stadnicki et al., 1998). Devani et al. (2005) demonstrated that levels of tissue inflammation observed in Crohn's disease and ulcerative colitis samples correlate well with the kallikrein depletion from goblet cells and increased interstitial kallikrein reactivity.
Our group has recently demonstrated that BK produces a concentration-related contraction of colon preparations obtained from control mice by activating B2 receptors, whereas the selective B1 receptor agonist des-Arg9-BK causes only a slight contractile response in this preparation (Hara et al., 2007). These results suggest the presence of constitutive kinin B2 receptors (but not B1 receptors) in the mouse colon, a pattern of expression described for most tissues (Calixto et al., 2004). Nevertheless, in epithelial cells of human intestinal tissue, both kinin receptors have been detected under normal conditions (Stadnicki et al., 2005). In this study, kinin B1 receptors have been found to be expressed in the basal area of enterocytes in the normal intestine, and in the apical portion of enterocytes in the inflamed tissue (Stadnicki et al., 2005). This immunolocalization might give an alternative explanation for the modest contraction induced by des-Arg9-BK in preparations obtained from control mice.
Under inflammatory conditions, a shift of kinin receptor subtypes from B2 to B1 has been reported in both in vitro and in vivo models (Phagoo et al., 1999; Rashid et al., 2004). Conversely, we have previously demonstrated a marked increase of B2 receptor-mediated contractile responses and specific binding sites in colon preparations obtained from TNBS-treated mice (Hara et al., 2007). In the present work, B1 receptors were also found to be greatly upregulated in experimental colitis, as indicated by functional, biochemical and molecular experimental approaches. First, B1 receptor mRNA expression was noticeably increased between 6 and 48 h following colitis induction in mice. Furthermore, binding assay experiments revealed a significant increase of specific kinin B1 receptor-binding sites, according to assessment 72 h after colitis induction, at the same time point in which des-Arg9-BK-induced contraction was maximal. We have also shown that des-Arg9-BK-induced contraction was markedly inhibited by the selective B1 receptor antagonist R-715, whereas the selective B2 receptor antagonist FR173657 had no effect. Our data corroborate and extend those from Stadnicki et al. (2005) showing that B1 receptor protein expression is significantly increased in the intestines of patients with IBD. On the basis of present results, it is not possible to precisely indicate the exact site(s) or cell type(s) involved in B1 receptor upregulation. Increased expression of B1 receptors might well occur in enteric nerves, in mucosa or muscle cells. We have observed that B1 receptor-mediated contraction in mouse colon is a tetrodotoxin-insensitive event (data not shown). Therefore, one might suggest that modulation of B1 receptors takes place in the muscle cells, although this remains to be further investigated.
It has been previously shown that treatment with the non-peptide B1 receptor antagonist SSR240612 prevents both the intestinal tissue damage and the neutrophil accumulation following splanchnic artery occlusion-reperfusion injury (Gougat et al., 2004). The present study undoubtedly demonstrates an important role for kinin B1 receptors in TNBS-induced colitis in mice. Our results revealed that B1 receptor knockout mice were more resistant to TNBS-induced colitis and presented a reduced cellular influx. Similarly, the preventive oral treatment with the selective B1 receptor antagonist SSR240612 diminished the tissue damage and MPO activity observed in TNBS-treated mice, suggesting that the B1 receptor is involved in the onset of colonic inflammation. In an interesting manner, the B1 receptor antagonist SSR240612 was also able to reduce the tissue damage even after inflammation had been established. These data highlight the potential of selective non-peptide B1 receptor antagonists as new alternatives for treating IBD.
Many reports showed that glucocorticoids, or protein synthesis inhibitors, are able to reduce the upregulation of B1 receptors, indicating its dependence on de novo protein synthesis (Calixto et al., 2004). The present results show that in vivo treatment with either dexamethasone or cycloheximide was able to reduce des-Arg9-BK-induced contraction, suggesting an interference with B1 receptor upregulation. Studies conducted by Ni et al. (1998) revealed the existence of a binding site for NF-κB in the promoter region of the human B1 receptor gene, which seems to be essential for the control of receptor transcription following exposure to certain inflammatory agents such as TNFα. In this context, it has been demonstrated that specific NF-κB blockers are able to prevent B1 receptor upregulation both in vitro and in vivo (Calixto et al., 2004). Our current results support the published data indicating the participation of NF-κB in the kinin B1 receptor upregulation following the induction of colitis.
An imbalance in the production of pro- and anti-inflammatory mediators has been suggested during IBD. More specifically, the correlation between increased TNFα production and IBD development has been exemplified in several animal models by the use of specific neutralizing antibodies, antisense oligonucleotides or cytokine gene receptor knockouts. The central role of TNFα in the pathophysiology of human Crohn's disease has been confirmed by the effect of a chimaeric monoclonal antibody against TNFα (infliximab) in patients with a refractory clinical state (Järnerot et al., 2005). However, the role of this cytokine in chronic inflammation of the colon has not yet been completely elucidated. The present results provide convincing evidence on the role of TNFα in the upregulation of B1 receptors following TNBS colitis induction in mice. First, B1 receptor-mediated contraction was markedly reduced in TNF receptor-1−/− mice, when compared to WT mice. Subsequently, in vivo treatment with either infliximab or thalidomide also significantly decreased the contractile response induced by des-Arg9-BK, in colon preparations obtained 72 h after TNBS administration. On the basis of the present data, it is possible to infer that TNFα might regulate TNBS-induced colitis, at least in part, by controlling the upregulation of kinin B1 receptors. In fact, a role for TNFα in B1 receptor upregulation has already been demonstrated (Rocha et al., 2005).
Increased NO production has been described in human IBD, as well as in animal models of intestinal inflammation. However, there are some controversial results on the precise role of NO in modulating intestinal inflammation. It has been shown that iNOS−/− mice are less susceptible to dextran sulphate sodium-induced colitis, whereas iNOS deficiency does not alter the severity of TNBS-induced colitis (Beck et al., 2004). Additionally, treatment with the selective iNOS inhibitor 1400W dramatically reduces the severity of TNBS-induced colitis in rats (Kankuri et al., 2001). We found that either inhibition of iNOS activity or deletion of the iNOS gene reduced des-Arg9-BK-induced contraction in TNBS-treated mice. To our knowledge, this is the first report showing the control of kinin B1 receptor expression by NO production. The resistance to dextran sulphate sodium or TNBS-induced colitis reported in the iNOS−/− mice appears to involve reduced leukocyte recruitment, alterations in the cell subsets of the inflammatory cell infiltrate, reduced cytokine expression and decreased TNFα production. The present study reveals an additional mechanism, by which NO might alter TNBS-induced colitis via kinin B1 receptor upregulation.
Although progress in research has increased our comprehension about the mechanisms involved in IBD, the limitations of current medical approaches continue to drive the search for better therapeutic agents. The present work has clearly revealed that B1 receptors are highly upregulated following TNBS colitis induction in mice, via mechanisms involving de novo protein synthesis, NF-κB and iNOS activation, and TNFα generation. We have also demonstrated that both B1 receptor gene deletion and pharmacological blocking of B1 receptors are able to markedly prevent the inflammatory changes observed during TNBS-induced colitis. Concerning the pathological significance of our experimental model, B1 receptors appear to play an important role in the inflammatory alterations observed in TNBS-induced colitis. By some means, changes in these receptors produces a marked alteration of mouse colon contractility. Whether or not these changes are of clinical relevance remain to be evaluated. In our opinion, it is tempting to correlate the clinical signs observed during colitis with the upregulation of B1 receptor-mediated contractile responses.
The evidence provided in the present study opens up new and important approaches towards the possible use of selective B1 receptor antagonists for the treatment of human IBD. In fact, the pharmaceutical industry is spending much effort to identify new orally active and selective kinin B1 receptor antagonists (Campos et al., 2006). Despite the lack of knowledge regarding the potential side effects resulting from B1 receptor antagonism, this strategy might well represent an attractive alternative for the management of IBD and other inflammatory diseases that remain without an adequate therapy.
Acknowledgments
This study was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), Programa de Apoio aos Núcleos de Excelência (PRONEX) and Fundação de Apoio a Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC). GFP is a PhD student in Pharmacology and received a grant from CNPq. AOG is a PhD student in the Molecular Biology Program and received a grant from CAPES. DBH, DFPL and ESF hold postdoctoral fellowships from CNPq.
Abbreviations
- BK
bradykinin
- CCh
carbachol
- IBD
inflammatory bowel disease
- iNOS
inducible nitric oxide synthase
- KD
dissociation constant
- MPO
myeloperoxidase
- NF-κB
nuclear factor-κB
- Rmax
maximal response
- TNBS
2,4,6-trinitrobenzene sulphonic acid
- TNFα
tumour necrosis factor α
- WT
wild type
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y, Takanashi H, Kitagawa H, Wirth KJ, Okayasu I. Effect of icatibant, a bradykinin B2 receptor antagonist, on the development of experimental ulcerative colitis in mice. Dig Dis Sci. 1999;44:845–851. doi: 10.1023/a:1026694732602. [DOI] [PubMed] [Google Scholar]
- Bamias G, Cominelli F. Immunopathogenesis of inflammatory bowel disease: current concepts. Curr Opin Gastroenterol. 2007;23:365–369. doi: 10.1097/MOG.0b013e3281c55eb2. [DOI] [PubMed] [Google Scholar]
- Beck PL, Xavier R, Wong J, Ezedi I, Mashimo H, Mizoguchi A, et al. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am J Physiol Gastrointest Liver Physiol. 2004;286:G137–G147. doi: 10.1152/ajpgi.00309.2003. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y, Schmidt-Supprian M. Epithelial NF-κB maintains host gut microflora homeostasis. Nat Immunol. 2007;8:479–481. doi: 10.1038/ni0507-479. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;74:3805–3809. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Cabrini DA, Ferreira J, Campos MM. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: key G-protein-coupled receptors and their role in inflammatory and painful processes. Br J Pharmacol. 2004;143:803–818. doi: 10.1038/sj.bjp.0706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos MM, Leal PC, Yunes RA, Calixto JB. Non-peptide antagonists for kinin B1 receptors: new insights into their therapeutic potential for the management of inflammation and pain. TIPS. 2006;27:646–651. doi: 10.1016/j.tips.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, MacVinish LL. Transporting epithelia as targets for kinin effects. Adv Exp Med Biol. 1986;198A:203–210. doi: 10.1007/978-1-4684-5143-6_28. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, Margolius HS. Kinins stimulate net chloride secretion by the rat colon. Br J Pharmacol. 1982;75:587–598. doi: 10.1111/j.1476-5381.1982.tb09178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, Serraino I, Britti D, et al. Pyrrolidine dithicarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496–510. doi: 10.1038/sj.bjp.0704463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Devani M, Vecchi M, Ferrero S, Avesani EC, Arizzi C, Chao L, et al. Kallikrein–kinin system in inflammatory bowel disease: intestinal involvement and correlation with the degree of tissue inflammation. Dig Liver Dis. 2005;37:665–673. doi: 10.1016/j.dld.2005.01.021. [DOI] [PubMed] [Google Scholar]
- El Sayah M, Medeiros R, Fernandes ES, Campos MM, Calixto JB. Mechanisms underlying up-regulation in the pig iris sphincter in vitro. Mol Pharmacol. 2006;69:1701–1708. doi: 10.1124/mol.105.021097. [DOI] [PubMed] [Google Scholar]
- Fries W, Muja C, Crisafulli C, Cuzzocrea S, Mazzon E. Dynamics of enterocyte tight junctions—effect of experimental colitis and two different anti-TNF strategies. Am J Physiol Gastrointest Liver Physiol. 2008;294:G938–G947. doi: 10.1152/ajpgi.00469.2007. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muià C, Esposito E, et al. TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock. 2008;29:32–41. doi: 10.1097/shk.0b013e318059053a. [DOI] [PubMed] [Google Scholar]
- Gougat J, Bernard F, Sarran L, Planchenault C, Poncelet M, Maruani J. SSR240612 [(2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-{[(6-methoxy-2-naphthyl)sulfonyl]amino}propanoyl)amino]-3-(4-{[2R,6S)-2,6-dimethylpiperidinyl] methylphenyl)-N-isopropyl-N-methylpropanamide hydrochloride], a new nonpeptide antagonist of the bradykinin b1 receptor: biochemical and pharmacological characterization. J Pharmacol Exp Ther. 2004;309:661–669. doi: 10.1124/jpet.103.059527. [DOI] [PubMed] [Google Scholar]
- Hara DB, Fernandes ES, Campos MM, Calixto JB. Pharmacological and biochemical characterization of bradykinin B2 receptors in the mouse colon: influence of the TNBS-induced colitis. Regul Pept. 2007;141:25–34. doi: 10.1016/j.regpep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, Grännö C, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805–1811. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Kamata K, Hayashi I, Mizuguchi Y, Arai K, Saeki T, Ohno T, et al. Suppression of dextran sulfate sodium-induced colitis in kininogen-deficient rats and non-peptide B2 receptor antagonist-treated rats. Jpn J Pharmacol. 2002;90:59–66. doi: 10.1254/jjp.90.59. [DOI] [PubMed] [Google Scholar]
- Kankuri E, Vaali K, Knowles RG, Lähde M, Korpela R, Vapaatalo H, et al. Suppression of acute experimental colitis by a highly selective inducible nitric-oxide synthase inhibitor, N-[3-(aminomethyl)benzyl]acetamidine. J Pharmacol Exp Ther. 2001;298:1128–1132. [PubMed] [Google Scholar]
- Leite DF, Echevarria-Lima J, Ferreira SC, Calixto JB, Rumjanek VM. ABCC transporter inhibition reduces zymosan-induced peritonitis. J Leukoc Biol. 2007;82:630–637. doi: 10.1189/jlb.0107042. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The Kallikrein–kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- Ni A, Chao L, Chao J. Transcription factor nuclear factor-κB regulates the inducible expression of the human B1 receptor gene in inflammation. J Biol Chem. 1998;273:2784–2791. doi: 10.1074/jbc.273.5.2784. [DOI] [PubMed] [Google Scholar]
- Pesquero JB, Araujo RC, Heppentall PA, Stucky CL, Silva JA, Walther T, et al. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci USA. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phagoo SB, Poole S, Leeb-Lundberg LMF. Autoregulation of bradykinin receptors: agonists in the presence of interleukin-1β shift the repertoire of receptor subtypes from B2 to B1 in human lung fibroblast. Mol Pharmacol. 1999;56:325–333. doi: 10.1124/mol.56.2.325. [DOI] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Matsumoto M, Ueda H. Switching of bradykinin-mediated nociception following partial sciatic nerve injury in mice. J Pharmacol Exp Ther. 2004;308:1158–1164. doi: 10.1124/jpet.103.060335. [DOI] [PubMed] [Google Scholar]
- Rejdak K, Rejdak R, Sieklucka-Dziuba M, Stelmasiak Z, Grieb P. 2-Deoxyglucose enhances epileptic tolerance evoked by transient incomplete brain ischemia in mice. Epilepsy Res. 2001;43:271–278. doi: 10.1016/s0920-1211(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Rocha AC, Fernandes ES, Passos GF, Calixto JB, Campos MM. Assessment of TNF alpha contribution to the functional up-regulation of kinin B1 receptors in the mouse paw after treatment with LPS. Int Immunopharmacol. 2005;11:1593–1600. doi: 10.1016/j.intimp.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Rodrigues GB, Passos GF, Di Giunta G, Figueiredo CP, Rodrigues EB, Grumman A, et al. Preventive and therapeutic anti-inflammatory effects of systemic and topical thalidomide on endotoxin-induced uveitis in rats. Exp Eye Res. 2007;84:553–560. doi: 10.1016/j.exer.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Rothe H, Ongören C, Rösen P, Kolb H. Genetic analysis of aberrant tumor necrosis factor alpha production in prediabetic BB-rats. Transplant Proc. 1993;25:2833–2834. [PubMed] [Google Scholar]
- Scherrer D, Schmidlin F, Haddad E, Kassel O, Landry Y, Gies J. Glucocorticoids increase bradykinin B2 receptor gene transcription in cultured guinea-pig tracheal smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:153–159. doi: 10.1007/pl00005337. [DOI] [PubMed] [Google Scholar]
- Shi X, Semkova I, Müther PS, Dell S, Kociok N, Joussen AM. Inhibition of TNF-alpha reduces laser-induced choroidal neovascularization. Exp Eye Res. 2006;83:1325–1334. doi: 10.1016/j.exer.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Souza DG, Coutinho SF, Silveira MR, Cara DC, Teixeira MM. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur J Pharmacol. 2000;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- Stadnicki A. Tissue and plasma kallikrein in inflammatory bowel disease. Dig Liver Dis. 2005;37:665–673. doi: 10.1016/j.dld.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Stadnicki A, Gonciarz M, Niewiarowski TJ, Hartleb J, Rudnicki M, Merrell NB, et al. Activation of plasma contact and coagulation systems and neutrophils in the active phase of ulcerative colitis. Dig Dis Sci. 1997;42:2355–2366. doi: 10.1023/a:1018891323205. [DOI] [PubMed] [Google Scholar]
- Stadnicki A, Pastucha E, Nowaczyk G, Mazurek U, Plewka D, Machnik G, et al. Immunolocalization and expression of kinin B1R and B2R receptors in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2005;289:361–366. doi: 10.1152/ajpgi.00369.2004. [DOI] [PubMed] [Google Scholar]
- Stadnicki A, Sartor RB, Janardham R, Stadnicka I, Adam A, Blais C, et al. Kallikrein–kininogen system activation and bradykinin (B2) receptors in indomethacin induced enterocolitis in genetically susceptible Lewis rat. Gut. 1998;43:365–374. doi: 10.1136/gut.43.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed PM, Tansey MG, Zalevsky J, Zhukovsky EA, Desjarlais JR, Szymkowski DE, et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301:1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- Van Limbergen J, Russell RK, Nimmo ER, Ho GT, Arnott ID, Wilson DC, et al. Genetics of the innate immune response in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:338–355. doi: 10.1002/ibd.20096. [DOI] [PubMed] [Google Scholar]
- Van Rossum JM. Cumulative dose–response curves. Arch Int Pharmacodyn. 1963;143:299–330. [PubMed] [Google Scholar]
- Wallace JM, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]