Abstract
Background and purpose:
Substance P (SP) and chemokines play critical roles in acute pancreatitis. SP elevates cytosolic calcium in pancreatic acinar cells and elevated cytosolic calcium is thought to be an early event in the pathogenesis of acute pancreatitis. SP induces production of chemokines MCP-1, MIP-1α and MIP-2 in pancreatic acinar cells, however the exact mechanism by which SP stimulates the production of these pro-inflammatory mediators remain undetermined. The aim of the present study is to investigate the role of calcium in SP-induced chemokine production in pancreatic acinar cells and to establish the signal transduction mechanisms involved.
Experimental approach:
An in vitro model of isolated mouse pancreatic acinar cells was used. Western blotting analysis, ELISA and calcium measurement were performed.
Key results:
SP increased chemokine secretion through the activation of PKCα/βII, MAPKinases (ERK and JNK), NFκB and AP-1 in pancreatic acinar cells. These effects were blocked by pretreatment of the cells with the specific calcium chelator BAPTA-AM. Moreover, SP-induced activation of PKCα/βII, ERK, JNK, NF-κB, AP-1 and chemokine production was inhibited by the specific phospholipase C inhibitor U73122.
Conclusions and implications:
SP-induced chemokine production in pancreatic acinar cells resulted from PLC-induced elevated intracellular calcium and PKCα/βII activation, subsequently leading to the activation of MAPKinases (ERK and JNK) and transcription factors NF-κB and AP-1. The present study demonstrates the critical role of calcium in SP-induced chemokine production in pancreatic acinar cells. Drugs targeting the SP-calcium mediated signaling pathways could prove beneficial in improving the treatment of acute pancreatitis.
Keywords: SP, calcium, PLC, PKCα/βII, MAPKinases, chemokines
Introduction
Substance P (SP) is a 11 amino acid neuropeptide that is released from nerve endings in many tissues. It has been shown to play an important role in asthma, inflammatory bowel disease, arthritis and other inflammatory processes (Bowden et al., 1994; Thurston et al., 1996). Subsequent to its release from nerve endings, SP binds to a G protein-coupled receptor (neurokinin 1 (NK1) receptor) on effector cells, increases microvascular permeability and promotes plasma extravasation from the intravascular to the extravascular space. Pancreatic acinar cells are known to express NK1 receptors, and SP has been detected within the pancreas (Patto et al., 1992; Sjodin and Gylfe, 1992). Studies have found that pancreatic levels of SP and the expression of NK1R on pancreatic acinar cells are increased during experimental acute pancreatitis (Bhatia et al., 1998). The ability of acinar cells to produce SP may play critical roles in pancreatic diseases, especially acute pancreatitis and may provide the first signals required for recruiting inflammatory cells into the pancreas during the initiation of pancreatitis. Genetic deletion of NK1 receptors reduces the severity of pancreatitis and pancreatitis-associated lung injury (Bhatia et al., 2003). Furthermore, knockout mice deficient in the preprotachykinin-A gene, which encodes for SP, are protected against acute pancreatitis and associated lung injury (Bhatia et al., 2003). These results suggest an important pro-inflammatory role for SP and NK1 receptors in acute pancreatitis and associated lung injury.
Chemokines are a family of small (8–10 kDa) inducible cytokines with activating and chemotactic effects on leukocyte subsets. They can be broadly subdivided on a structural basis into the CC subfamily, in which the first two cysteine residues are adjacent, and the CXC subfamily, in which the first two of the four conserved cysteine residues are separated by another amino acid. CC chemokines, such as monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1 α (MIP-1α), are believed to principally affect monocytes, whereas CXC chemokines such as macrophage inflammatory protein-2 (MIP-2), are believed to act on neutrophils. Recently, our group has shown that blockade of SP receptor with its potent selective antagonist, CP96345, significantly protects mice against acute pancreatitis and also attenuates the increase in CC chemokines MCP-1, MIP-1α and CXC chemokine MIP-2 production in both pancreas and lungs (Lau et al., 2005; Sun and Bhatia, 2007). Moreover, in vitro, SP stimulates the production of inflammatory mediators MCP-1, MIP-1α and MIP-2 in pancreatic acinar cells via activation of nuclear factor kappa B (NF-κB) (Ramnath and Bhatia, 2006). Similarly, in human astrocytoma cells, SP induces the activation of NF-κB and gene expression of the chemokine interleukin-8 (IL-8) (Lieb et al., 1997). We have previously demonstrated that the mitogen-activated protein kinases (MAPKs) ERK and JNK are involved in SP-induced chemokine production in pancreatic acinar cells (Ramnath et al., 2007).
Studies have shown that SP induces elevation of cytosolic calcium ([Ca2+]i), in Chinese hamster ovary cells expressing the SP receptor (Mochizuki-Oda et al., 1993), in dorsal horn neurons (Ansel et al., 1996) and in rat pancreatic acinar cell line (Gallacher et al., 1990). The elevation of [Ca2+]i induced by SP was blocked by the NK1 receptor antagonist CP96345 (Mochizuki-Oda et al., 1994). Another NK1 receptor antagonist, GR 82334, completely inhibited the increase in [Ca2+]i that was recorded in polymorphonuclear leukocytes when exposed to SP (Tanabe et al., 1996). There is evidence suggesting that elevated Ca2+ in pancreatic acinar cells is involved in the development of acute pancreatitis (Frick et al., 1997).
SP, chemokines and Ca2+ play critical roles in the pathogenesis of acute pancreatitis. To our knowledge, no study has explored the interplay between SP and Ca2+ in inducing the production of pro-inflammatory mediators in pancreatic acinar cells. Therefore, the aim of the present study was to investigate the role of Ca2+ in SP-induced chemokine production in an in vitro model of isolated pancreatic acinar cells and also, to study the underlying signalling mechanisms involved.
Materials and methods
Animals
All animal experiments were approved by the Animal Ethics Committee of National University of Singapore and carried out in accordance with established International Guiding Principles for Animal Research. Male Swiss albino mouse (25–30 g) were maintained in the Animal Housing Unit of this University in an environment with controlled temperature (21–24 °C) and lighting (12:12 h light–darkness cycle). Standard laboratory chow and drinking water were provided ad libitum. A period of 2 days was allowed for animals to acclimatize before any experimental manipulations were undertaken.
Test system used
Pancreatic acinar cells were obtained from mouse pancreas by collagenase treatment as described previously (Bhatia et al., 2002). Briefly, pancreas from three Swiss mice (20–25 g) were removed, infused with buffer A (in mM: 140 NaCl, 4.7 KCl, 1.13 MgCl2, 1 CaCl2, 10 glucose, 10 HEPES, pH 7.2) containing 200 IU mL−1 collagenase (Worthington, Lakewood, NJ, USA) and 0.5 mg mL−1 soybean trypsin inhibitor (Sigma-Aldrich, St Louis, MO, USA) and incubated in a shaking water bath for 10 min at 37 °C. The digested tissue was centrifuged through 50 mg mL−1 bovine serum albumin and washed two times with buffer A for further experiments. A cell suspension (in buffer A) consisting of only small clumps, around 3–5 acinar cells, was used to carry out the following experiments.
Viability of mouse pancreatic acinar cells
Viability of the pancreatic acinar cells was determined by Trypan blue dye exclusion assay. One drop of 0.4% Trypan blue dye was added to one drop of the isolated acinar cells. The viability was determined under the light microscope (Carl Zeiss, Oberkochen, Germany). The number of unstained cells/clumps was expressed as a percentage of the total number of cells/clumps. This process was repeated for different fields and the average was then calculated. In all experiments, cell viability was greater than 95%.
Experimental design
Pancreatic acinar cells (500 μL of cell suspension) were treated with SP (Sigma-Aldrich) at a concentration of 1 μM for 0, 3, 5, 10, 15, 30, 45 and 60 min at 37 °C. We have previously shown that SP at a concentration of 1 μM significantly upregulated chemokine production and NF-κB activation when compared to the placebo-treated control (Ramnath and Bhatia, 2006). Following treatment with SP, the cells were lysed to detect for PKCα/βII activation by western blot analysis. In some experiments, cells were either pretreated with the specific Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis-acetoxymethyl ester (BAPTA-AM), Sigma-Aldrich, at 1, 5 and 10 μM, or the selective phospholipase C (PLC) inhibitor, 1-[6-((17b-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione (U73122), Calbiochem (Darmstadt, Germany)/Merck (Darmstadt, Germany), at 1, 2.5 μM or the PKC inhibitor (chelerythrine chloride), Calbiochem/Merck, at 10 μM for 30 min and then stimulated with 1 μM SP or vehicle (dimethyl sulphoxide, DMSO, Sigma-Aldrich) for 10 or 45 min at 37 °C. Subsequently the supernatant was used for chemokine detection and the pellet was used for either nuclear extract, to detect NF-κB (p65) and AP-1(c-Jun) activation, or cell lysis for western blot analysis to detect PKCα/βII, ERK and JNK.
Preparation of total cell lysates for western blot analysis
After treatment, pancreatic acinar cells were homogenized on ice in radioimmunoprecipitation assay buffer supplemented with 1 mM phenylmethylsulphonyl fluoride and the protease inhibitor cocktail (Sigma-Aldrich) containing pepstatin, leupeptin, chymostatin, antipain and aprotinin (5 μg mL−1 of each), and centrifuged at 4 °C for 15 min at 14 000 × g. The supernatants were collected and stored at −80 °C until use. Protein concentrations were determined by using Bio-Rad protein assay. In all 5 μL of sample was added to 250 μL of Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA) and read at 595 nm after a short incubation (5 min) at room temperature.
Western blot analysis
Cell lysates (50 μg) were separated on 12% SDS-polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). Non-specific binding was blocked by 1 h incubation of the membranes, at room temperature, in 5% nonfat dry milk in phosphate-buffered saline Tween 20 (PBST) (0.05%Tween 20 in phosphate-buffered saline). The blots were then incubated overnight at 4 °C with the primary antibodies (at a 1:1000 dilutions in the buffer containing 2.5% nonfat dry milk in PBST) phospho-PKCα/βII, phospho-ERK1/2, total ERK1/2, phospho-SAPK/JNK, total SAPK/JNK (Cell Signaling Technology, Danvers, MA, USA) and hypoxanthine-guanine phosphoribosyl transferase (HPRT), purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA. HPRT was used as the housekeeping protein. PKCα/βII antibody detects PKCα only when phosphorylated at threonine 638 and PKC βII only when phosphorylated at threonine 641. It reacts weakly with phosphorylated PKC βI and γ. After which the membranes were washed four times with PBST, and finally incubated for 1 h at room temperature with goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology) at 1:2000 dilutions in the buffer containing 2.5% nonfat dry milk in PBST. The blots were developed for visualization using enhanced chemiluminescence detection kit (Pierce, Rockford, IL, USA). The densities of the bands were quantified using a UVP GelDoc-It Imaging Systems.
Nuclear cell extract preparation
Nuclear cell extracts were prepared by employing a kit from Active Motif (SciMed, Singapore, Asia). In brief, cells were washed, collected in ice-cold PBS in the presence of phosphatase inhibitors, to limit further protein modifications and then centrifuged (4 °C) at 24 × g for 5 min. The pellets were resuspended in a hypotonic buffer, treated with detergent and centrifuged (4 °C) at 14 000 × g for 30 s. After collection of the cytoplasmic fraction, the nuclei were lysed and nuclear proteins solubilized in lysis buffer containing proteasome inhibitors. Protein concentrations were determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA).
NF-κB DNA-binding activity
The binding of NF-κB to DNA was measured in nuclear extracts with an ELISA-based TransAM NF-κB p65 assay kit (Active Motif, SciMed, Asia). This assay uses multi-well plates coated with an unlabelled oligonucleotide containing the consensus binding site for NF-κB (5′ -GGGACTTTCC-3′) (Parry and Mackman, 1994). Nuclear proteins (5 μg) were added to each well and incubated for 1 h at room temperature to allow NF-κB DNA binding. Subsequently, by using an antibody that is directed against NF-κB p65 subunit, the NF-κB complex bound to the oligonucleotide is detected. Addition of the secondary antibody conjugated to HRP provides sensitive colorimetric readout that is easily quantified by spectrophotometry. Absorbance was read at 450 nm within 5 min.
AP-1 DNA-binding activity
TransAM AP-1 kits are designed specifically to detect and quantify AP-1 activation. Similar to TransAM NF-κB p65, TransAM AP-1 kits contain a 96-well plate on which has been immobilized an oligonucleotide that contains a TPA- responsive element TRE (5′ -TGAGTCA-3′). AP-1 dimers contained in nuclear extract (5 μg) specifically binds to this oligonucleotide. The primary antibodies used recognize accessible epitopes on c-Jun proteins upon DNA binding. Secondary antibody conjugated to HRP gives the colorimetric reaction. Absorbance was read at 450 nm within 5 min.
Chemokine detection
Pancreatic acinar cell supernatants were assayed for MCP-1, MIP-1α and MIP-2 using a sandwich ELISA, according to the manufacturer's instructions (Duoset kit; R&D Systems, Minneapolis, MN, USA). For MCP-1 assay, anti-MCP-1 primary antibody was aliquoted onto ELISA plates and incubated at room temperature overnight. Samples (100 μL of cell supernatant) and standards were incubated at room temperature for 2 h, the plates were washed and a biotinylated anti-MCP-1 antibody was added and left at room temperature for 2 h. Plates were washed again, and streptavidin bound to horseradish peroxidase was added for 20 min, at room temperature. After a further wash, tetramethylbenzidine was added for colour development, and the reaction was terminated with 2 M H2SO4. Absorbance was measured at 450 nm. The same procedure was followed for the detection of the remaining chemokines MIP-1α and MIP-2.
Cytosolic calcium ([Ca2+]i) measurement
Cytosolic calcium was measured as described previously (Melendez and Ibrahim, 2004; Zhi et al., 2006). Briefly, cells were loaded with 1 mg mL−1 Fura2-AM (Invitrogen, Carlsbad, CA, USA) in calcium assay buffer (in mM: 145 NaCl, 5 KCl, 1 MgSO4, 1 CaCl2, 10 HEPES, pH 7.4) containing 1.8 mg mL−1 glucose and 2 mg mL−1 BSA. After removal of excess reagents by dilution and centrifugation at 4 °C, the cells were re-suspended in calcium assay buffer and warmed to 37 °C in the cuvette; after a baseline measurement was obtained, the cells were stimulated by the addition of 1 μM SP. Fluorescence was measured at 340 and 380 nm.
Statistical analysis
Results are presented as means+s.e. with six replicates for each condition. Three independent experiments (n=3) were carried out. The significance of changes was evaluated by using ANOVA and Tukey's method was used as a post hoc test for the difference between groups. A P-value ⩽0.05 was taken as the level of significance.
Results
SP induces phosphorylation of PKCα/βII in a time-dependent manner
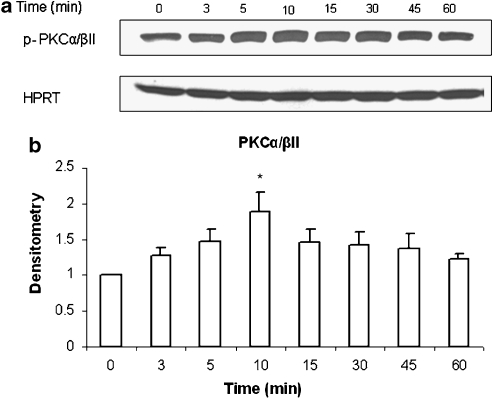
Pancreatic acinar cells were treated with 1 μM SP or vehicle (saline) for 0, 3, 5, 10, 15, 30, 45 and 60 min at 37 °C. Cells were then lysed, and cell proteins were subjected to western blot analysis. As shown in Figures 1a and b, SP-induced phosphorylation of PKCα/βII (about a twofold increase) in pancreatic acini, which was evident as from 3 min and increased in a time-dependent manner up to 10 min, followed by a time-dependent decrease until 60 min. Densitometric analysis of western blot experiments revealed a significant increase in phosphorylation of PKCα/βII at 10 min when compared to 0 min control. No significant change was detected in the housekeeping protein HPRT. No significant change was observed when pancreatic acinar cells were treated with the vehicle at different time points.
Figure 1.
SP induces phosphorylation of PKCα/βII in a time dependent manner. Freshly isolated pancreatic acini were incubated with either 1 μM SP or vehicle (saline) for 0, 3, 5, 10, 15, 30, 45 and 60 min at 37 °C. The cells were lysed, and cell proteins were subjected to western blot analysis using antibodies against (a) phospho-PKCα/βII and HPRT. (b) Densitometric analysis of western blot experiments from pancreatic acini. The results are representative of three independent (n=3) experiments. Results shown are the means+s.e. *P⩽0.05 when compared with 0 min control.
SP-induced [Ca2+]i is involved in activation of PKCα/βII, ERK and JNK
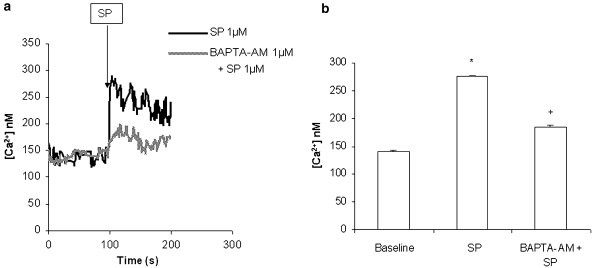
Our data in Figures 2a and b shows that SP stimulation rapidly triggered calcium release from internal stores in pancreatic acinar cells. However, when these cells were pretreated for 30 min with 1 μM BAPTA-AM, a calcium-specific chelator, the increase in [Ca2+]i was inhibited (Figure 2).
Figure 2.
SP induces mobilization of [Ca2+]i in pancreatic acinar cells. Freshly isolated pancreatic acini were pre-incubated with either 1 μM BAPTA-AM or vehicle (DMSO) for 30 min. The cells were then rinsed two times with calcium assay buffer and then loaded with 1 mg mL−1 Fura2-AM. After removal of excess reagents, the cells were re-suspended in calcium assay buffer and warmed to 37 °C in the cuvette; after the baseline reading was obtained, the cells were stimulated by the addition of 1 μM SP. Fluorescence was measured at 340 and 380 nm. (a) is a representative example. The results shown in (b) are representative of three independent (n=3) experiments. Results shown are the means+s.e. *P⩽0.05 when compared with control, +P⩽0.05 when compared with SP.
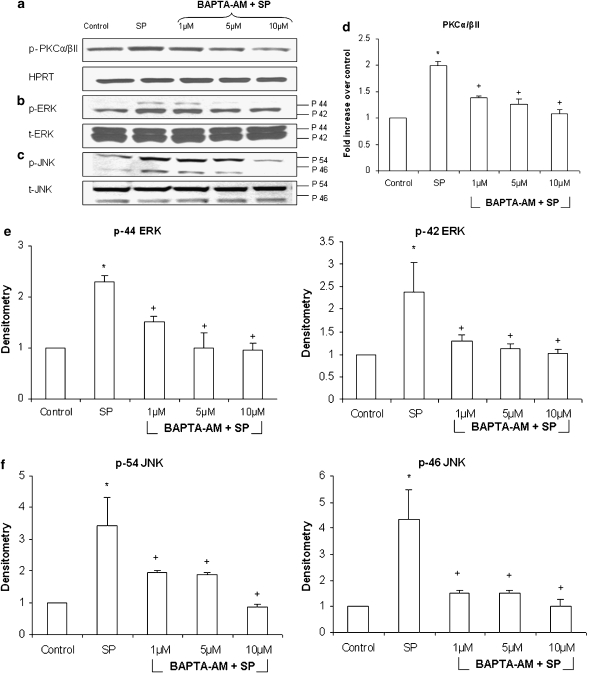
Stimulation of pancreatic acinar cells with 1 μM SP significantly upregulated phosphorylation of PKCα/βII, ERK and JNK by more than twofold. Pancreatic acinar cells were pretreated for 30 min with different concentration of BAPTA-AM, ranging from 1 to 10 μM, followed by stimulation with 1 μM of SP for 10 min. Cells were then lysed, and cell proteins were subjected to western blot analysis. As shown in Figures 3a–c, BAPTA-AM dose-dependently decreased phosphorylation of PKCα/βII, ERK and JNK in pancreatic acinar cells when compared to SP only stimulated cells. No significant change was detected in the housekeeping protein HPRT, total ERK and total JNK.
Figure 3.
SP-induced [Ca2+]i is involved in activation of PKCα/βII, ERK and JNK. Freshly isolated pancreatic acini were pre-incubated with either different concentrations of BAPTA-AM, ranging from 1 to 10 μM, or vehicle (DMSO) for 30 min followed by stimulation with 1 μM of SP for 10 min. Cells were then lysed, and cell proteins were subjected to western blot analysis using antibodies against (a) phospho-PKCα/βII, HPRT (b) phospho-ERK, total ERK (c) phospho-JNK and total JNK. The phosphorylated subunits such as p-PKCα/βII, p-44 ERK, p-42 ERK, p-54 JNK and p-46 JNK have been quantified. Densitometric analysis of western blot experiments was performed and the group data from three independent preparations (n=3) are presented in (d–f). Results shown are the means+s.e. *P⩽0.05 when compared with control, +P⩽0.05 when compared with SP. p-PKCα/βII is phosphorylated protein kinase C α/βII, HPRT is hypoxanthine-guanine phosphoribosyl transferase, p-ERK is phosphorylated extracellular signal-regulated kinase, t-ERK is total extracellular signal-regulated kinase, p-JNK is phosphorylated Jun N-terminal kinase and t-JNK is total Jun N-terminal kinase.
Role of PLC in SP-induced activation of PKCα/βII, ERK and JNK in pancreatic acinar cells
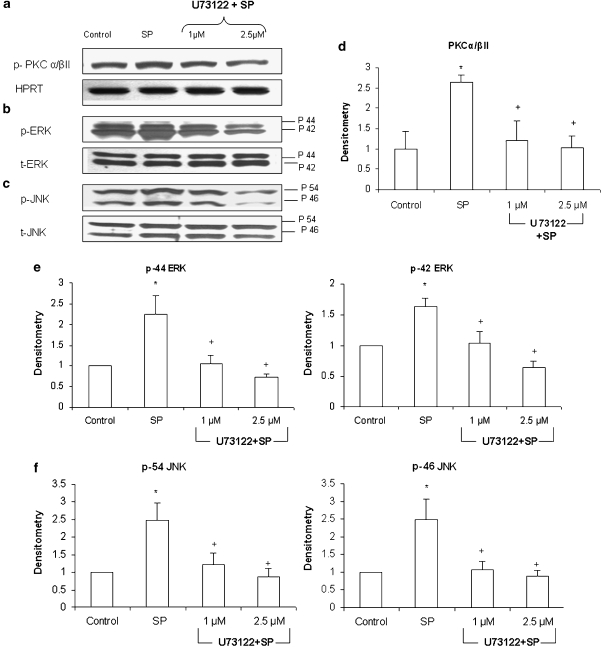
Pancreatic acini were pretreated with the selective PLC inhibitor, U73122, at 1, 2.5 μM for 30 min followed by stimulation with 1 μM of SP for 10 min. Cells were then lysed, and cell proteins were subjected to western blot analysis. As shown in Figure 4. U73122 dose-dependently decreased phosphorylation of PKCα/βII, ERK and JNK in pancreatic acinar cells when compared with SP only stimulated cells. No significant change was detected in the housekeeping protein HPRT, total ERK and total JNK.
Figure 4.
SP-induced activation of PKCα/βII, ERK and JNK is mediated by PLC in pancreatic acinar cells. Freshly isolated pancreatic acini were pre-incubated with either U73122, at 1, 2.5 μM or vehicle (DMSO) for 30 min at 37 °C followed by stimulation with 1 μM SP for 10 min at 37 °C. Cells were subsequently lysed, and cell proteins were subjected to western blot analysis using antibodies against (a) PKCα/βII, HPRT, (b) phospho-ERK, total ERK, (c) phospho-JNK and total JNK. The phosphorylated subunits such as p-PKCα/βII, p-44 ERK, p-42 ERK, p-54 JNK and p-46 JNK have been quantified. Densitometric analysis of western blot experiments were performed and the group data from three independent preparations (n=3) are presented in (d–f). Results shown are the means+s.e. *P⩽0.05 when compared with control, +P⩽0.05 when compared with SP. p- PKCα/βII is phosphorylated protein kinase C α/βII, HPRT is hypoxanthine-guanine phosphoribosyl transferase, p-ERK is phosphorylated extracellular signal-regulated kinase, t-ERK is total extracellular signal-regulated kinase, p-JNK is phosphorylated Jun N-terminal kinase and t-JNK is total Jun N-terminal kinase.
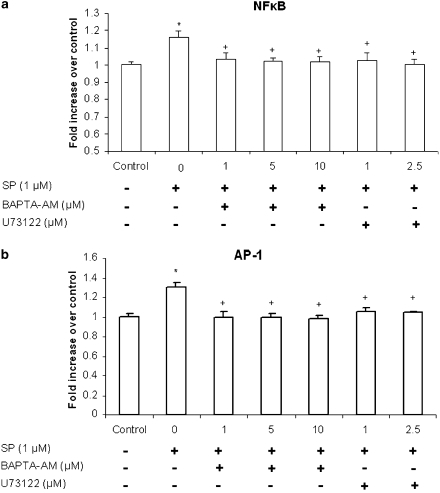
PLC and Ca2+ are involved in SP-induced NFκB p65 and AP-1 c-Jun activation in pancreatic acinar cells
Pancreatic acinar cells were preincubated for 30 min with either BAPTA-AM, ranging from 1 to 10 μM, or U73122, at 1, 2.5 μM followed by stimulation with 1 μM of SP for 45 min. NF-κB p65 and AP-1 c-Jun DNA-binding assay revealed that treatment with SP led to a notable increase in the activity of NF-κB p65 and AP-1 c-Jun, in pancreatic acinar cell. As shown in Figures 5a and b, pretreatment with either BAPTA-AM or U73122 attenuated SP-induced DNA-binding activity of NF-κB p65 and AP-1 c-Jun when compared with SP only treated cells.
Figure 5.
PLC and Ca2+ are involved in SP-induced NF-κB p65 and AP-1 c-Jun activation in pancreatic acinar cells. Freshly isolated pancreatic acini were pre-incubated with BAPTA-AM, ranging from 1 to 10 μM, or U73122 at 1, 2.5 μM or vehicle (DMSO) followed by stimulation with 1 μM of SP for 45 min. The cells were separated from incubation medium by centrifugation. The pellet (cells) was used for (a) NF-κB and (b) AP-1 nuclear extraction. NF-κB p65 and AP-1 c-Jun DNA-binding assays were then carried out. The results are representative of three independent (n=3) experiments. Results shown are the means+s.e. *P⩽0.05 when compared with control, +P⩽0.05 when compared with SP.
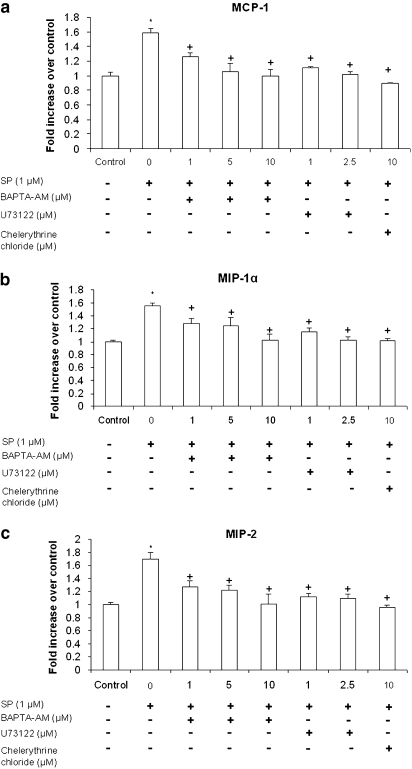
PLC, Ca2+ and PKC are involved in the secretion of several pro-inflammatory chemokines in pancreatic acinar cells
Treatment of pancreatic acini with 1 μM SP resulted in enhanced expression of pro-inflammatory chemokines MCP-1, MIP-1α and MIP-2. Pancreatic acinar cells were pretreated with BAPTA-AM, ranging from 1 to 10 μM, or U73122, at 1, 2.5 μM or chelerythrine chloride at 10 μM for 30 min followed by stimulation with 1 μM of SP for 45 min. The protein levels of these chemokines were determined by ELISA. Figures 6a–c shows that pretreatment with either BAPTA-AM or U73122 markedly decreased MCP-1, MIP-1α and MIP-2 production in a dose-dependent manner when compared with SP only treated cells. Furthermore, treatment with PKC inhibitor chelerythrine chloride significantly decreased the secretion of MCP-1, MIP-1α and MIP-2 in pancreatic acinar cells. The negative control in which pancreatic acini were pretreated with 1 μM BAPTA-AM or 1 μM U73122 or 10 μM chelerythrine chloride for 30 min followed by stimulation with placebo (saline) for 45 min had no significant effect on chemokine production when compared with unstimulated controls (data not shown). These results demonstrated that PLC, Ca2+ and PKC were involved in the production of several chemokines in pancreatic acinar cells.
Figure 6.
PLC, Ca2+ and PKC are involved in the secretion of several pro-inflammatory chemokines in pancreatic acinar cells. Freshly isolated pancreatic acini were pre-incubated with BAPTA-AM, ranging from 1 to 10 μM, or U73122 at 1, 2.5 μM or chelerythrine chloride at 10 μM or vehicle (DMSO) for 30 min followed by stimulation with 1 μM of SP for 45 min. The supernatant was used to measure (a) MCP-1, (b) MIP-1α and (c) MIP-2 levels by ELISA. The results are representative of three independent (n=3) experiments. Results shown are the means+s.e. *P⩽0.05 when compared with control, +P⩽0.05 when compared with SP. Che is chelerythrine chloride.
Discussion and conclusions
Phospholipase C-dependent signaling pathways have been implicated in the development of acute and chronic inflammatory responses in vivo (Hou et al., 2004). Studies have shown that bombesin stimulation of pancreatic acinar cells leads to activation of PLC. PLC then hydrolyzes membrane phospholipid, phosphatidylinositol 4,5-bisphosphate, to generate two intracellular messengers, diacylglycerol and inositol triphosphate, which in turn, mediate the activation of protein kinase C (PKC) and intracellular Ca2+ release, respectively (Schulz et al., 1999; Wu et al., 2000; Hou et al., 2004). In the present study, we have investigated the role of PLC in SP-NK1 receptor-induced chemokine production in pancreatic acinar cells using U73122. U73122 is a membrane-permeable aminosteroid PLC inhibitor. It was reported to selectively inhibit the PLC-dependent process in human platelets and neutrophils and was thus proven to be useful in evaluating the role of PLC in cell activation (Bleasdale et al., 1990; Smith et al., 1990, 1996; Stam et al., 1998). Inhibition of PLC significantly decreased SP-induced phosphorylation of Ca2+-dependent PKCα/βII, ERK and JNK. Previous studies have shown that in PLC-deficient mice, there was a reduction in chemoattractant-induced phosphorylation of JNK and MAPK in murine neutrophils (Li et al., 2000). Moreover, in our study U73122 significantly inhibited SP-induced activation of NF-κB p65 and AP-1 c-Jun and the secretion of MCP-1, MIP-1α and MIP-2 in pancreatic acinar cells.
Acute pancreatitis was found to be associated with hypercalcaemia in humans (Frick et al., 1987) and in experimental animal models, acute pancreatitis is induced by bolus infusions (intravenous injection) of calcium (Mithofer et al., 1995). It has been shown that hypercalcaemia induces a secretory block and accumulation of digestive zymogens within the pancreatic acinar cell (Frick et al., 1995). It is generally believed that ectopic activation of digestive enzymes, particularly intracellular trypsinogen activation, is an early event in the pathophysiology of acute pancreatitis (Mithofer et al., 1995; Frick et al., 1997). In the current study, we were interested in elucidating the mechanisms by which SP-induced [Ca2+]i elevation leads to production of pro-inflammatory mediators. We have shown that SP elevates [Ca2+]i in pancreatic acinar cells which is in accordance with previous studies conducted in various cells expressing the SP receptor (Gallacher et al., 1990; Mochizuki-Oda et al., 1993; Ansel et al., 1996). Moreover, treatment with BAPTA-AM significantly attenuated the increase in [Ca2+]i in pancreatic acinar cells. BAPTA-AM, the membrane-permeable form of BAPTA, is hydrolyzed by cytosolic esterases and is trapped intracellularly as the active Ca2+ chelator BAPTA and is widely used as an intracellular calcium sponge (Harrison and Bers, 1987; Smith et al., 1992; Ahluwalia et al., 2001; Kim et al., 2002). BAPTA-AM significantly attenuated phosphorylation of PKCα/βII, ERK and JNK, subsequently leading to a decrease in activation of NF-κB p65, AP-1 c-Jun and production of MCP-1, MIP-1α and MIP-2 in pancreatic acinar cells.
The PKC superfamily is classified into three subfamilies based on their domain structure and their ability to respond to calcium and diacylglycerol (Newton and Johnson, 1998). In this study, we have focused on the Ca2+-dependent conventional PKCα/βII. SP induces a time-dependent increase in phosphorylation of PKCα/βII in pancreatic acinar cells. The increase in phosphorylation was blocked by pretreatment with either U73122 or BAPTA-AM. Moreover, chelerythrine chloride abolished the increase in SP-induced production of chemokines MCP-1, MIP-1α and MIP-2 in pancreatic acinar cells. Studies have shown that chelerythrine chloride specifically blocked PKC phosphorylation and it has been shown to inhibit PKCα and PKCβ translocation from the cytosol to the membrane in isolated ileal synaptosomes (Herbert et al., 1990; Chao et al., 1998).
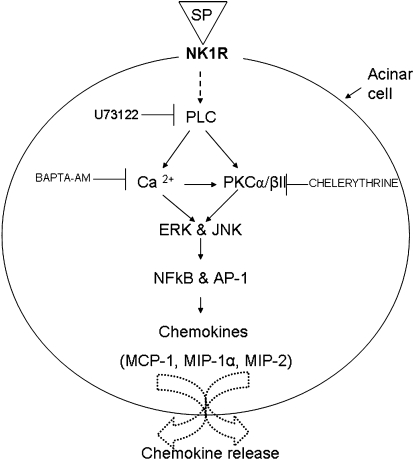
In conclusion, SP stimulates pancreatic acinar cells to release chemokines MCP-1, MIP-1α and MIP-2 through PLC-dependent mechanisms. SP induces an increase in [Ca2+]i which results in the phosphorylation of PKCα/βII, ERK and JNK; consequently leading to the activation of NF-κB p65, AP-1 c-Jun and ultimately to chemokine production. On the basis of our results, we have proposed a signalling cascade (see Figure 7) that mediates SP/NK1 receptor-induced chemokine secretion in mouse pancreatic acinar cells. Apart from being involved in trypsinogen activation (Mithofer et al., 1995; Frick et al., 1997), an increase in [Ca2+]i also entailed elevated production of chemokines which play a critical role in the pathogenesis of acute pancreatitis. In light of this study, we propose that drugs targeting the SP-calcium-mediated signalling pathways could prove beneficial in the treatment of acute pancreatitis.
Figure 7.
A schematic representation of the signalling cascade that mediates SP/NK1 receptor-induced chemokine production in pancreatic acinar cells. SP/NK1 receptors induce the activation of PLC which mediates the activation of PKCα/βII and also increases intracellular mobilization of Ca2+ in pancreatic acinar cells. Increased [Ca2+]i induces the phosphorylation of PKCα/βII, MAPkinases ERK and JNK. It also mediates the activation of transcription factors NF-κB p65 and AP-1 c-Jun and secretion of chemokines MCP-1, MIP-1α and MIP-2. PKCα/βII is also involved in the production of MCP-1, MIP-1α and MIP-2 in pancreatic acinar cells. SP is substance P, NK1R is neurokinin 1 receptor, PLC is phospholipase C, PKCα/βII is protein kinase C α/βII, Ca2+ is calcium, MAPkinases is mitogen-activated protein kinases, ERK is extracellular signal-regulated kinase, JNK is Jun N-terminal kinase. NF-κB is nuclear factor kappa B, AP-1 is activator protein-1, MCP-1 is monocyte chemoattractant protein-1, MIP-1α is macrophage inflammatory protein-1 α and MIP-2 is macrophage inflammatory protein-2.
Acknowledgments
We give our thanks to Mei Leng Shoon and Moizza Mansoor for technical assistance. This work was supported by the R-184-000-069-305 and Bridging funds R-184-000-139-101.
Abbreviations
- AP
acute pancreatitis
- AP-1
activator protein-1
- [Ca2+]i
intracellular calcium concentration
- DMSO
dimethyl sulphoxide
- p-ERK
phosphorylated extracellular signal-regulated kinase
- t-ERK
total extracellular signal-regulated kinase
- HPRT
hypoxanthine-guanine phosphoribosyl transferase
- IL-8
interleukin-8
- p-JNK
phosphorylated Jun N-terminal kinase
- t-JNK
total Jun N-terminal kinase
- MAPKs
mitogen-activated protein kinases
- MCP-1
monocyte chemoattractant protein-1
- MIP-1α
macrophage inflammatory protein-1 alpha
- MIP-2
macrophage inflammatory protein-2
- NK1
neurokinin 1
- NF-κB
nuclear factor kappa B
- PKCα/βII
protein kinase C α/βII
- PLC
phospholipase C
- SP
substance P
Conflict of interest
The authors state no conflict of interest.
References
- Ahluwalia JP, Topp JD, Weirather K, Zimmerman M, Stamnes M. A role for calcium in stabilizing transport vesicle coats. J Biol Chem. 2001;276:34148–34155. doi: 10.1074/jbc.M105398200. [DOI] [PubMed] [Google Scholar]
- Ansel JC, Kaynard AH, Armstrong CA, Olerud J, Bunnett N, Payan D. Skin-nervous system interactions. J Invest Dermatol. 1996;106:198–204. doi: 10.1111/1523-1747.ep12330326. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Brady M, Kang YK, Costello E, Newton DJ, Christmas SE, et al. MCP-1 but not CINC synthesis is increased in rat pancreatic acini in response to cerulein hyperstimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G77–G85. doi: 10.1152/ajpgi.00031x.2002. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci USA. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Slavin J, Cao Y, Basbaum AI, Neoptolemos JP. Preprotachykinin-A gene deletion protects mice against acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2003;284:G830–G836. doi: 10.1152/ajpgi.00140.2002. [DOI] [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, et al. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, et al. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci USA. 1994;91:8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MD, Chen IS, Cheng JT. Inhibition of protein kinase C translocation from cytosol to membrane by chelerythrine. Planta Med. 1998;64:662–663. doi: 10.1055/s-2006-957545. [DOI] [PubMed] [Google Scholar]
- Frick TW, Fernandez-del Castillo C, Bimmler D, Warshaw AL. Elevated calcium and activation of trypsinogen in rat pancreatic acini. Gut. 1997;41:339–343. doi: 10.1136/gut.41.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick TW, Fryd DS, Sutherland DE, Goodale RL, Simmons RL, Najarian JS. Hypercalcemia associated with pancreatitis and hyperamylasemia in renal transplant recipients. Data from the Minnesota randomized trial of cyclosporine versus antilymphoblast azathioprine. Am J Surg. 1987;154:487–489. doi: 10.1016/0002-9610(87)90260-1. [DOI] [PubMed] [Google Scholar]
- Frick TW, Mithofer K, Fernandez-del Castillo C, Rattner DW, Warshaw AL. Hypercalcemia causes acute pancreatitis by pancreatic secretory block, intracellular zymogen accumulation, and acinar cell injury. Am J Surg. 1995;169:167–172. doi: 10.1016/s0002-9610(99)80127-5. [DOI] [PubMed] [Google Scholar]
- Gallacher DV, Hanley MR, Petersen OH, Roberts ML, Squire-Pollard LG, Yule DI. Substance P and bombesin elevate cytosolic Ca2+ by different molecular mechanisms in a rat pancreatic acinar cell line. J Physiol. 1990;426:193–207. doi: 10.1113/jphysiol.1990.sp018133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Bers DM. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987;925:133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hou C, Kirchner T, Singer M, Matheis M, Argentieri D, Cavender D. In vivo activity of a phospholipase C inhibitor, 1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole -2,5-dione (U73122), in acute and chronic inflammatory reactions. J Pharmacol Exp Ther. 2004;309:697–704. doi: 10.1124/jpet.103.060574. [DOI] [PubMed] [Google Scholar]
- Kim YM, Kim YM, Lee YM, Kim HS, Kim JD, Choi Y, et al. TNF-related activation-induced cytokine (TRANCE) induces angiogenesis through the activation of Src and phospholipase C (PLC) in human endothelial cells. J Biol Chem. 2002;277:6799–6805. doi: 10.1074/jbc.M109434200. [DOI] [PubMed] [Google Scholar]
- Lau HY, Wong FL, Bhatia M. A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochem Biophys Res Commun. 2005;327:509–515. doi: 10.1016/j.bbrc.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- Lieb K, Fiebich BL, Berger M, Bauer J, Schulze-Osthoff K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- Melendez AJ, Ibrahim FB. Antisense knockdown of sphingosine kinase 1 in human macrophages inhibits C5a receptor-dependent signal transduction, Ca2+ signals, enzyme release, cytokine production, and chemotaxis. J Immunol. 2004;173:1596–1603. doi: 10.4049/jimmunol.173.3.1596. [DOI] [PubMed] [Google Scholar]
- Mithofer K, Fernandez-del Castillo C, Frick TW, Lewandrowski KB, Rattner DW, Warshaw AL. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology. 1995;109:239–246. doi: 10.1016/0016-5085(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Mochizuki-Oda N, Nakajima Y, Nakanishi S, Ito S. Substance P-induced elevation of intracellular calcium in transfected Chinese hamster ovary cells: role of inositol trisphosphate. Regul Pept. 1993;46:450–452. doi: 10.1016/0167-0115(93)90116-p. [DOI] [PubMed] [Google Scholar]
- Mochizuki-Oda N, Nakajima Y, Nakanishi S, Ito S. Characterization of the substance P receptor-mediated calcium influx in cDNA transfected Chinese hamster ovary cells. A possible role of inositol 1,4,5-trisphosphate in calcium influx. J Biol Chem. 1994;269:9651–9658. [PubMed] [Google Scholar]
- Newton AC, Johnson JE. Protein kinase C: a paradigm for regulation of protein function by two membrane-targeting modules. Biochim Biophys Acta. 1998;1376:155–172. doi: 10.1016/s0304-4157(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Parry GC, Mackman N. A set of inducible genes expressed by activated human monocytic and endothelial cells contain kappa B-like sites that specifically bind c-Rel-p65 heterodimers. J Biol Chem. 1994;269:20823–20825. [PubMed] [Google Scholar]
- Patto RJ, Vinayek R, Jensen RT, Gardner JD. Carbachol does not downregulate substance P receptors in pancreatic acini. Pancreas. 1992;7:447–452. doi: 10.1097/00006676-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Ramnath RD, Sun J, Adhikari S, Bhatia M. Effect of MAP kinases on chemokine synthesis induced by substance P in mouse pancreatic acinar cells. J Cell Mol Med. 2007;11:1326–1341. doi: 10.1111/j.1582-4934.2007.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnath RD, Bhatia M. Substance P treatment stimulates chemokine synthesis in pancreatic acinar cells via the activation of NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1113–G1119. doi: 10.1152/ajpgi.00177.2006. [DOI] [PubMed] [Google Scholar]
- Schulz I, Krause E, Gonzalez A, Gobel A, Sternfeld L, Schmid A. Agonist-stimulated pathways of calcium signaling in pancreatic acinar cells. Biol Chem. 1999;380:903–908. doi: 10.1515/BC.1999.111. [DOI] [PubMed] [Google Scholar]
- Sjodin L, Gylfe E. A selective and potent antagonist of substance P receptors on pancreatic acinar cells. Biochem Int. 1992;27:145–153. [PubMed] [Google Scholar]
- Smith JB, Selak MA, Dangelmaier C, Daniel JL. Cytosolic calcium as a second messenger for collagen-induced platelet responses. Biochem J. 1992;288 Part 3:925–929. doi: 10.1042/bj2880925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Justen JM, McNab AR, Rosenbloom CL, Steele AN, Detmers PA, et al. U-73122: a potent inhibitor of human polymorphonuclear neutrophil adhesion on biological surfaces and adhesion-related effector functions. J Pharmacol Exp Ther. 1996;278:320–329. [PubMed] [Google Scholar]
- Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- Stam JC, Michiels F, van der Kammen RA, Moolenaar WH, Collard JG. Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Bhatia M. Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2007;292:G143–G153. doi: 10.1152/ajpgi.00271.2006. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Otani H, Bao L, Mikami Y, Yasukura T, Ninomiya T, et al. Intracellular signaling pathway of substance P-induced superoxide production in human neutrophils. Eur J Pharmacol. 1996;299:187–195. doi: 10.1016/0014-2999(95)00816-0. [DOI] [PubMed] [Google Scholar]
- Thurston G, Baluk P, Hirata A, McDonald DM. Permeability-related changes revealed at endothelial cell borders in inflamed venules by lectin binding. Am J Physiol. 1996;271:H2547–H2562. doi: 10.1152/ajpheart.1996.271.6.H2547. [DOI] [PubMed] [Google Scholar]
- Wu D, Huang CK, Jiang H. Roles of phospholipid signaling in chemoattractant-induced responses. J Cell Sci. 2000;113 Part 17:2935–2940. doi: 10.1242/jcs.113.17.2935. [DOI] [PubMed] [Google Scholar]
- Zhi L, Leung BP, Melendez AJ. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by TNFalpha in primary human monocytes. J Cell Physiol. 2006;208:109–115. doi: 10.1002/jcp.20646. [DOI] [PubMed] [Google Scholar]