Abstract
Background and purpose:
7-Ketocholesterol, an oxysterol present in atherosclerotic lesions, induces smooth muscle cell (SMC) death, thereby destabilizing plaques. Statins protect patients from myocardial infarction, though they induce SMC apoptosis. We investigated whether statins and 7-ketocholesterol exerted additive cell death effects.
Experimental approach:
Cultured rabbit aorta SMCs (passage 2–6) were exposed to 7-ketocholesterol with or without fluvastatin, simvastatin or pravastatin. Uptake of neutral red (NR), monolayer protein, cleavage of the pan-caspase substrate Asp-Glu-Val-Asp-rhodamine110, cell morphology (light and electron microscopy) and processing of microtubule-associated protein 1 light chain 3 (LC3, immunoblot) were determined.
Key results:
NR uptake declined upon 18 h exposure to 25 μM 7-ketocholesterol (−41±3%, n=13), 100 μM fluvastatin (−59%) or 30–100 μM simvastatin (−28 to −74%). Oxysterol and high statin concentrations exerted additive effects, but lower concentrations (fluvastatin 10–30 μM, simvastatin 1–10 μM) partly reversed viability loss. 7-Ketocholesterol caused intense cytoplasmic vacuolization, processing of LC3-I to LC3-II, but little caspase activation (increase 29.5%). Fluvastatin (10–100 μM, 70–545% increase) and simvastatin (3–100 μM 43–322% increase) induced caspase activation without LC3 processing, but failed to activate caspases in 7-ketocholesterol-treated SMCs. Pravastatin up to 100 μM was always inactive.
Conclusions and implications:
7-Ketocholesterol caused SMC death, mainly via autophagic vesicle formation with LC3 processing, whereas lipophilic statins evoked SMC apoptosis. Cell death following 7-ketocholesterol and low statin concentrations were not additive, presumably because the autophagic process interfered with statin-induced caspase activation. This further illustrates that drug effects in normal SMCs are not necessarily predictive for activities in atherosclerotic settings.
Keywords: atherosclerosis, autophagy, apoptosis, statins, fluvastatin, pravastatin, simvastatin, vascular smooth muscle cell, viability
Introduction
Atherosclerosis is a protracted inflammatory disease in the intima of large arteries, leading to the formation of atheromatous plaques. The typical lesion shows a necrotic core surrounded by a fibrous cap consisting of smooth muscle cells (SMCs) in a collagen–proteoglycan matrix (Virmani et al., 2000). A thin fibrous plaque atheroma is characterized by trimming of the fibrous cap that is infiltrated by macrophages and T cells with few SMCs, and an increase of extracellular lipids and necrotic core formation. Such plaques are vulnerable and at risk of rupture, thereby evoking atherothrombosis, myocardial infarction or stroke. Several factors have been proposed to increase the risk of plaque rupture, such as increased collagen degradation by matrix metalloproteinases secreted by macrophages, or decreased collagen biosynthesis because of suppression of SMC activity by interferon-γ, a cytokine produced by T cells.
The disappearance of SMCs from the fibrous cap through apoptosis or other forms of cell death could also jeopardize plaque stability (Clarke et al., 2006). SMC death is seen in the vicinity of macrophage-rich regions of human atherosclerotic plaques (Kockx et al., 1996, 1998). Factors that are known to kill SMCs can be macrophage derived (nitric oxide (NO), Fas ligand and tumour-necrosis factor-α) or products formed during oxidative modification of lipoproteins or cholesterol. Indeed, oxysterols, such as 7-ketocholesterol, may induce necrosis (Ghelli et al., 2002; Seye et al., 2004), apoptosis or type I cell death (Lizard et al., 1996, 1997; Nishio and Watanabe, 1996; Seye et al., 2004) or autophagy (Martinet et al., 2004) in vascular cells. Autophagic or type II cell death is a caspase-independent form of programmed cell death (Gozuacik and Kimchi, 2004; Ferraro and Cecconi, 2007). Processing of microtubule-associated protein I light chain 3 (LC3) from the cytoplasmic form (LC3-I) to a membrane-associated form (LC3-II) is essential for the formation of autophagic vesicles and leads to increased electrophoretic LC3 mobility on SDS-polyacrylamide gels (Gozuacik and Kimchi, 2004; Ferraro and Cecconi, 2007).
Generation of reactive oxidative species, mitochondrial stress, translocation of the pro-apoptotic protein Bax and release of cytochrome c have been implied in 7-ketocholesterol-induced SMC death (Lizard et al., 1998, 2000; Seye et al., 2004). Moreover, oxysterols may also reduce 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase activity, particularly when culturing mammalian cells with lipoprotein-deficient media (Schroepfer, 2000; Miguet et al., 2001). HMG-CoA reductase is the rate-limiting enzyme in the biosynthesis of cholesterol and of products involved in prenylation or farnesylation of several important membrane-bound proteins.
Statins inhibit HMG-CoA reductase as well, and several large clinical trials have documented that statins reduce death from coronary disease or stroke by 24–42% (Maron et al., 2000; Palinski and Napoli, 2002). In addition to lowering LDL cholesterol, the benefit has been attributed to several other effects of statins, such as improved plaque stability, endothelial cell function or antithrombotic and anti-inflammatory effects. However, lipophilic statins have also been reported to induce apoptotic death of vascular SMCs in culture (Baetta et al., 1997; Guijarro et al., 1998; Werner et al., 2004) and to render cells susceptible to induction of programmed cell death by Fas ligand (Knapp et al., 2000). These pro-apoptotic effects seem paradoxical, as SMC death is likely to increase the risk of plaque rupture. Therefore, this study investigated whether 7-ketocholesterol and statins exerted additive effects on SMC morphology, viability (uptake of neutral red; Babich and Borenfreund, 1990), cell loss (protein), mitochondrial function (reduction of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide; MTT), apoptotic processes (annexin V binding and caspase activation) or autophagy (autophagosome formation and conversion of LC3-I to LC3-II).
Methods
Smooth muscle cell culture
The studies were approved by the Ethical Committee of the University of Antwerp. SMCs were isolated from rabbit aortas by collagenase and elastase digestion and cultured in Ham F10 medium (Gibco Ltd, Paisley, UK) supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin 100 μ mL−1, streptomycin 100 μg mL−1 and gentamycin 100 μg mL−1; Seye et al., 2004). More than 95% of cells were α-SMC actin positive. SMCs (passage 2–6) were seeded in plates (Beckton Dickinson (BD) Biosciences, Erembodegem, Belgium) with 24 wells (50 000 cells) or 6 wells (150 000 cells, flow cytometry) in 3 mL Ham F10 with 10% FBS. After 48 h, the SMCs received 3 mL fresh medium with 2% FBS and 15 μL phosphate-buffered saline, 0.5% ethanol (control) or 6.25, 12.5, 25 or 50 μM 7-ketocholesterol with or without 10−8–10−4 M fluvastatin, simvastatin or pravastatin for 18 or 24 h.
Neutral red assay
Neutral red (300 μL, 0.1%) was added to the well 16 or 22 h after addition of 7-ketocholesterol. Two hours later, cells were examined by light microscopy. Thereafter medium and detached cells were removed, adherent cells were washed twice with phosphate-buffered saline, and neutral red was extracted with 150 μL NaH2PO4 (0.05 M) in 50% ethanol to measure absorbance at 540 nm (A540) in duplicate. Monolayer protein was precipitated with 5% trichloroacetic acid, dissolved in 1 mL NaOH (0.1 M) with 0.5% SDS to measure protein content in duplicate (BCA assay; Pierce, Perbio Science Belgium NV/SA, Erembodegem, Belgium).
Within each culture plate, the values of A540 were normalized to control (0 μM 7-ketocholesterol and 0 μM statin) according to the equation: A540sample (%)=A540sample/A540control × 100. Because the amount of neutral red in monolayers is determined by both cell mass and accumulation in individual cells (Babich and Borenfreund, 1990) (vide infra), a viability index was calculated: Viability=nmol neutral red·per μg protein, using a calibration curve (0–200 μM neutral red) to convert A540 into nmol neutral red.
Regression analysis showed that intracellular neutral red accumulation (nmol) was a linear function of cell number (range: 10 000–300 000): Neutral red (nmol)=4.1 (3.8–4.4)n × 10−3+8 (−13 to 29), with n=number of SMCs and (95% confidence intervals). Similarly, protein content of the monolayer was a linear function of SMC number: Protein (μg)=0.57 (0.50–0.63)n × 10−3+0.7 (−4.2 to 5.5). In contrast, the viability index (nmol·per μg protein) was independent of SMC number.
Mitochondrial function
To assess mitochondrial function, the NADH and NADPH-dependent reduction of MTT to formazan was measured during the final 4 h of the exposure to 7-ketocholesterol using the colorimetric MTT kit (Roche Applied Science 1465007, Vilvoorde, Belgium) (Pirillo et al., 1997). After solubilization of the formazan crystals, absorbance was measured at 540 nm.
Flow cytometry
Cells were collected using a brief trypsin treatment, and transferred into 5 mL FACS tubes in phosphate-buffered saline with 2% FBS. Apoptosis was detected with an annexin V-FITC and propidium iodide (PI) staining kit (BD Biosciences), always in the presence of 2% FBS. Cell samples (20 000 cells) were analysed on a FACSort cytometer using Cell Quest Pro software (BD Biosciences). All results were confirmed by manual counting of adherent SMCs using fluorescence microscopy.
Caspase assay
The conversion of the pan-caspase substrate Asp-Glu-Val-Asp-rhodamine 110 (DEVD-R110) was measured using the fluorimetric homogeneous caspase assay (Roche Applied Science, category no. 3005372). SMCs (50 000 cells) were seeded in two 24-well plates and exposed to 7-ketocholesterol for 18 h with or without statins. After removal of the medium, 100 μL lysis buffer containing 50 μM DEVD-R110 was added. The lysate was incubated at 37 °C and fluorescence of free rhodamine 110 (excitation filter 485 nm, emission filter 518 nm) was measured after 1 and 2 h. The difference between the two readings was converted to nmol rhodamine, using a calibration curve (0.03–1 μM rhodamine 110). The second 24-well plate was run in parallel to measure adherent SMC protein at 18 h. Caspase activity was expressed as nmol rhodamine per 110 h mg per protein, and compared with a lysate of camptothecin-treated apoptotic U937 cells (provided by the manufacturer).
Transmission electron microscopy
Smooth muscle cells were collected by trypsinization and fixed in 0.1 M sodium cacodylate-buffered (pH 7.4) 2.5% glutaraldehyde solution for 2 h, then rinsed (3 × 10 min) in 0.1 M sodium cacodylate-buffered (pH 7.4) 7.5% saccharose and postfixed in 1% OsO4 solution for 1 h. After dehydration in an ethanol gradient (70% ethanol (20 min), 96% ethanol (20 min) and 100% ethanol (2 × 20 min)), samples were embedded in Durcupan ACM (EMS, Hatfield, PA, USA). Ultrathin sections were stained with uranyl acetate and lead citrate. Sections were examined in a Philips CM 10 microscope (Philips, Eindhoven, The Netherlands) at 80 kV.
Plasmid construction and transient transfection of LC3
The eGFP coding sequence was excised from plasmid pEGFP-N3 (BD Biosciences Clontech, Palo Alto, CA, USA) by digestion with EcoRI and NotI. Vector ends were blunted with Klenow polymerase and self-ligated to produce plasmid pN3. Meanwhile, full-length cDNA encoding human LC3 protein was amplified by PCR from HepG2 cells using Pfu DNA polymerase (Stratagene, La Jolla, CA, USA) and primers 5′-CAAGATCTCGCGCGATGCCCTCMGACCGG-3′ and 5′-CAAAGCTTTCAGAAGCCGAAGGTTTCYTGGGAG-3′. The resultant PCR product was BglII/HindIII digested and cloned in the similarly opened pN3, to produce pHuLC3 as a final expression plasmid. Rabbit SMCs were transiently transfected with 5 μg pHuLC3 via Nucleofection technology (program U-25) using the Human AoSMC Nucleofector kit (Amaxa GmbH, Köln, Germany).
Western blot analysis of LC3
Smooth muscle cells were lysed in an appropriate volume of Laemmli sample buffer (Bio-Rad, Richmond, CA, USA). Cell lysates were then heat denatured for 4 min in boiling water and loaded on an SDS-polyacrylamide gel. After electrophoresis, proteins were transferred to an Immobilon-P Transfer Membrane (Millipore, Bedford, MA, USA) according to standard procedures. Membranes were blocked in TBS-T (Tris buffered saline containing 0.05% Tween-20) and 5% nonfat dry milk (Bio-Rad) for 1 h. After blocking, membranes were probed overnight at 4 °C with an anti-LC3 polyclonal antibody (raised against the synthetic peptide H2N-PSDRPFKQRRSFADC-CONH2; Eurogentec, Seraing, Belgium) in antibody dilution buffer (TBS-T containing 1% nonfat dry milk), followed by 1 h incubation with a peroxidase-conjugated secondary antibody (Dako, Glostrup, Denmark) at room temperature. Antibody detection was accomplished with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL, USA) using a Lumi-Imager (Roche Diagnostics, Mannheim, Germany).
Statistical analysis
Results are expressed as mean±s.e.mean. Treatment effects were evaluated using a factorial ANOVA with two factors: 7-ketocholesterol concentration and drug concentration. When an interaction between both factors was present, drug effects were analysed separately in the presence and absence of 7-ketocholesterol using a repeated measures ANOVA followed by Dunnett's multiple comparison test. A 5% level of significance was selected.
Materials
Fluvastatin was a gift from Novartis AG (Vilvoorde, Belgium), simvastatin from Merck Sharp & Dome (Brussels, Belgium) and pravastatin from Daiichi Sankyo Belgium BV (Louvain La Neuve, Belgium). 7-Ketocholesterol, mevalonolactone, farnesylpyrophosphate and geranylgeranylpyrophosphate were from Sigma (Bornem, Belgium). The lactone rings of simvastatin and mevalonolactone were opened with 1 N NaOH, followed by pH adjustment with HCl. 7-Ketocholesterol and simvastatin were dissolved in ethanol. All concentrations are given as final concentrations.
Results
Light microscopy
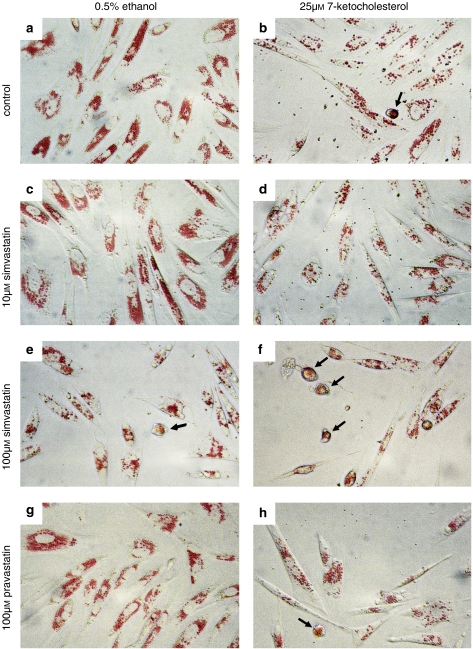
Smooth muscle cells showed a flat morphology and accumulated neutral red in cytoplasmic granules following 2 h incubation with the dye. After exposure to 25 μM 7-ketocholesterol for 18 h, the adherent SMCs contained fewer granules and showed highly variable signs of cell retraction, ranging from elongated to rounded shapes, while some cells were already floating. In globular cells, the granules seemed to merge (Figure 1). After exposure to 10 μM simvastatin, the density of neutral red granules was unaltered, but more spindle-shaped cells were present. At 100 μM, simvastatin caused variable degrees of cell detachment, ranging from elongated to globular cells containing fewer neutral red granules and floating cells. In combination with 7-ketocholesterol, 10 μM simvastatin seemed to attenuate SMC loss and to raise granular staining, whereas 100 μM simvastatin increased cell loss and frequency of spherical cells in comparison with SMCs treated with 7-ketocholesterol alone (Figure 1). The effects of fluvastatin on dye accumulation and SMC morphology were indistinguishable from those of simvastatin (results not shown), whereas even the highest concentration of pravastatin (100 μM) was without effect apart from increasing the frequency of elongated cells.
Figure 1.
Accumulation of neutral red in smooth muscle cells (SMCs) exposed to (a) 0.5% ethanol, (b) 25 μM 7-ketocholesterol, (c) 10 μM fluvastatin, (d) 7-ketocholesterol and 10 μM fluvastatin, (e) 100 μM simvastatin, (f) 7-ketocholesterol and 100 μM simvastatin, (g) 100 μM pravastatin or (h) 7-ketocholesterol and pravastatin for 18 h. The accumulation of neutral red in cytoplasmic granules (lysosomes) was suppressed by 7-ketocholesterol. The oxysterol-induced variable degrees of cell retraction with formation of spindle-shaped and round cells (arrows), in which granules seemed to merge. SMCs exposed to pravastatin or 10 μM simvastatin were indistinguishable from control, whereas 100 μM simvastatin caused cell retraction, rounding off (arrows) and cell loss. SMCs treated with pravastatin and 7-ketocholesterol were similar to SMCs exposed to 7-ketocholesterol alone, whereas 10 μM simvastin reduced and 100 μM increased the frequency of rounded SMCs.
Quantification of neutral red and SMC protein
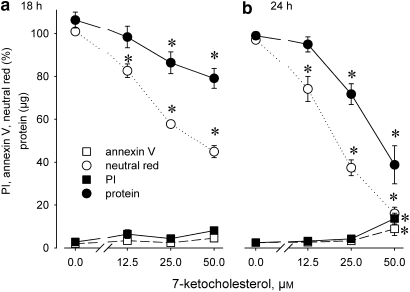
These qualitative observations were confirmed by quantitative assays. The neutral red and—to a lesser extent—protein content of adherent SMCs declined at increasing 7-ketocholesterol concentrations, and the gradual losses became more pronounced after 24 h exposure (Figure 2). At that time, adherent SMCs showed increased labelling of annexin V (annexin V positive and PI negative 4.6±0.9%) or PI (13.7±2.5%) at 50 μM 7-ketocholesterol. As neutral red accumulation decreased about 50% after 18 h exposure to 25 μM 7-ketocholesterol (Figure 2a), those conditions were selected for further experiments. Given that the neutral red content of monolayers was determined by dye content of individual cells and SMC mass (Figure 1), a viability index (neutral red expressed per mg protein) was determined.
Figure 2.
Concentration–response curve of neutral red accumulation (absorbance, %), protein content and percentages of adherent SMCs, which were positive for annexin V and propidium iodide (PI) after exposure to 7-ketocholesterol for (a) 18 h or (b) 24 h. Results shown as means±s.e.mean, n=4, *P<0.05 versus 0 μM, repeated measurements ANOVA, Dunnett's test.
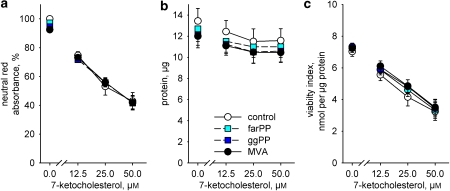
Figure 3 shows that none of the variables (neutral red accumulation, protein content or viability index) used to assess cell death were influenced by adding 100 μM mevalonic acid, 5 μM farnesylpyrophosphate or 5 μM geranylgeranylpyrophosphate to the SMCs cultured with 7-ketocholesterol.
Figure 3.
Exposure to 7-ketocholesterol for 18 h caused a concentration-dependent loss of (a) uptake of neutral red (P=0.001), (b) adherent protein (P=0.011) and (c) SMC viability (P=0.001). The effects of mevalonic acid (100 μM, MVA), farnesylpyrophosphate (5 μM, farPP) and geranylgeranylpyrophosphate (ggPP) or their interactions with 7-ketocholesterol were statistically not significant. Results analysed with a factorial ANOVA and shown as means±s.e.mean, n=4.
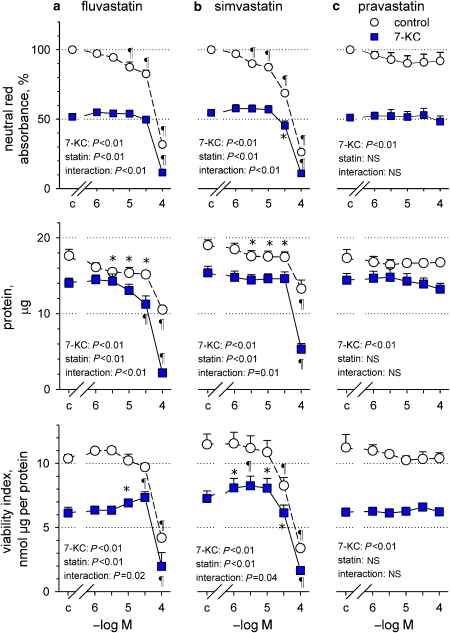
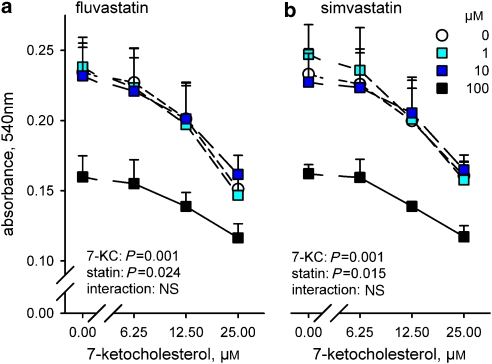
Low concentrations of fluvastatin and simvastatin (1–30 μM) caused a gradual decline of neutral red accumulation, which was mainly explained by modest cell loss, as indicated by the reduction of monolayer protein and unaltered viability index (Figure 4). Higher concentrations of fluvastatin (100 μM) and simvastatin (30–100 μM) caused more significant declines of dye accumulation by reducing both viability index and cell mass (protein content).
Figure 4.
Effects of increasing concentrations of fluvastatin (a), simvastatin (b) and pravastatin (c) on neutral red absorbance, protein content and viability index of adherent SMCs exposed to 0.5% ethanol (control) or 25 μM 7-ketocholesterol (7-KC) for 18 h. Results representative of three independent trials, shown as mean±s.e.mean, with fluvastatin n=4, simvastatin n=6 and pravastatin n=4. Significance of the effects of 7-ketocholesterol, statin and their interaction in a factorial ANOVA is shown. *P<0.05, ¶P<0.01, different from corresponding control without statin, repeated measurements ANOVA and Dunnett's test.
Interestingly, highly significant interactions were seen between effects of 7-ketocholesterol and those of fluvastatin or simvastatin. Lower concentrations of both statins did not reduce the dye or protein content of the monolayer any further and even raised the viability index. In contrast, at high concentrations both statins exerted additive effects with 7-ketocholesterol by further reducing dye accumulation, monolayer protein and viability index. Below 1 μM (0.01–1 μM), neither fluvastatin nor simvastatin influenced viability with or without oxysterol (results not shown). Pravastatin was without effect on viability in either control or oxysterol-treated SMCs (Figure 4).
Mitochondrial function
To test whether the differential effects of statins in control or 7-ketocholesterol-treated cells were due to an interference with mitochondrial function, the reduction of MTT by succinate dehydrogenase was assessed (Figure 5). Exposure to 7-ketocholesterol for 5 h led to a concentration-dependent decline of mitochondrial activity. At 100 μM, fluvastatin and simvastatin suppressed MTT reduction; their effect was additive with that of 7-ketocholesterol. Both statins were without effect at 1 or 10 μM, whereas pravastatin was inactive (results not shown).
Figure 5.
Reduction of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) in SMCs is suppressed by increasing concentrations of 7-ketocholesterol (7-KC) and by 100 μM fluvastatin (a) or 100 μM simvastatin (b). Results shown as mean absorbance±s.e.mean, n=9–10, except for 100 μM n=3. Significance of the effects of 7-ketocholesterol, statin and their interaction in a factorial ANOVA is shown.
Caspase activation
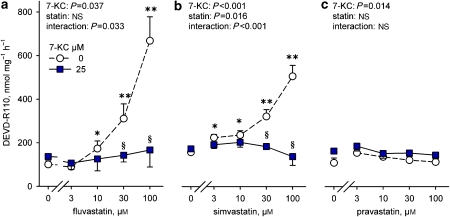
Conversion of the pan-caspase substrate DEVD-R110 increased slightly (29.5%) after 18 h exposure to 7-ketocholesterol (158±7 nmol per mg protein per h, n=12) compared with SMCs exposed to 0.5% ethanol (122±12 nmol per mg protein per h, n=12, P=0.015, paired Student's t-test). Fluvastatin (⩾10 μM) and simvastatin (⩾3 μM) raised hydrolysis of DEVD-R110 more significantly (Figure 6), although maximum activity was modest (18%, range: 4–34%) compared with the positive control of the assay (lysate of apoptotic U937 cells). Surprisingly, fluvastatin or simvastatin failed to activate caspases in SMCs exposed to 7-ketocholesterol. Pravastatin was inactive in either condition.
Figure 6.
Conversion of the pan-caspase substrate Asp-Glu-Val-Asp-rhodamine 110 (DEVD-R110) by adherent SMCs after 18 h exposure to fluvastatin, simvastatin or pravastatin with or without 25 μM 7-ketocholesterol (7-KC). Results shown as mean±s.e.mean, n=4. *P<0.05, **P<0.01 versus 0 μM statin, §P<0.05 different from 0 μM 7-ketocholesterol, repeated measurements ANOVA and Dunnett's test of logarithmically transformed values.
Electron microscopy
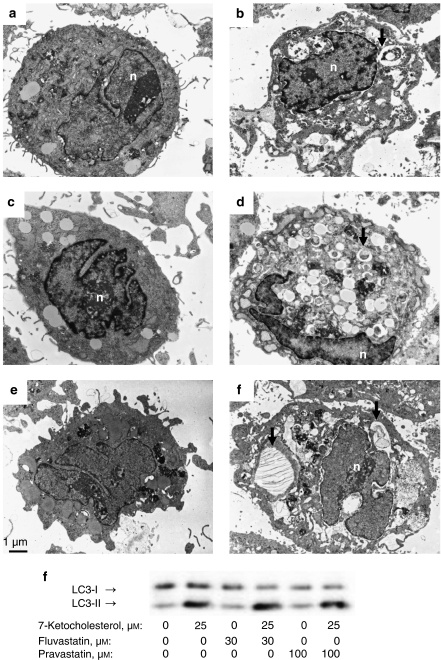
Transmission electron microscopy showed intense vacuolization in the cytoplasm of SMCs treated with 7-ketocholesterol alone or in combination with fluvastatin, pravastatin (Figure 7) or simvastatin for 18 h. Some of those cytoplasmic vesicles were filled with intracellular organelles. In contrast, fluvastatin, pravastatin (Figure 7) or simvastatin induced very little cytoplasmic vacuolization when compared with control cells.
Figure 7.
Transmission electron micrographs of SMCs exposed to (a) 0.5% ethanol, (b) 25 μM 7-ketocholesterol, (c) 30 μM fluvastatin, (d) the combination of 7-ketocholesterol and fluvastatin, (e) 100 μM pravastatin or (f) the combination of 7-ketocholesterol and pravastatin for 18 h. Cytoplasm of SMCs exposed to 7-ketocholesterol without, or with fluvastatin or pravastatin showed intense vacuolization with vesicles containing cytoplasmic material (arrows). Magnification, × 6610; n=nucleus. (g) Immunoblot analysis of conversion of LC3-I to LC3-II in SMCs exposed to 0 or 25 μM 7-ketocholesterol without, or with 30 μM fluvastatin or 100 μM pravastatin for 18 h. LC3, microtubule-associated protein I light chain 3.
LC3 processing
Recruitment of LC3 to autophagic membranes is the biochemical marker for autophagic vesicle formation. An increased electrophoretic mobility from the nonautophagic (LC3-I) form to the membrane recruited (LC3-II) form of LC3 was evident in SMCs transiently transfected with human LC3 and then exposed to 7-ketocholesterol in the absence or presence of fluvastatin or pravastatin, but not in SMCs treated with fluvastatin or pravastatin alone (Figure 7).
Discussion
The current study addressed the hypothesis that statins and 7-ketocholesterol, which are both known to induce SMC death, would exert additive cell death effects. Indeed, additive effects were seen with 7-ketocholesterol and high concentrations of fluvastatin and simvastatin. However, a more important finding was that, at lower and clinically more relevant concentrations, both statins did not increase oxysterol-induced SMC loss (protein) and even improved cell viability (uptake of neutral red). This was apparently explained by interactions between the different cell death pathways that were activated by 7-ketocholesterol (autophagy) and statins (apoptosis), as explained below.
7-Ketocholesterol-induced cell death in SMCs
7-Ketocholesterol caused early mitochondrial dysfunction, as indicated by decreased succinate dehydrogenase activity 6 h after exposure to the oxysterol. This was in line with previous data showing a reversible mitochondrial dysfunction with cytochrome c release in SMCs (Seye et al., 2004) and monocytes (Lizard et al., 2000; Miguet et al., 2001). The mitochondrial dysfunction was followed by decreased lysosomal accumulation of neutral red (viability index), followed by gradual cell retraction, rounding off and ultimately cell detachment (protein loss). Light microscopy revealed that the sensitivity of individual SMCs to the cytotoxic effects of 7-ketocholesterol was highly variable. The oxysterol-induced cytotoxicity was not due to suppression of HMG-CoA reductase activity in the current experimental setup, in which 2% FBS served as a source of cholesterol. Oxysterols may reduce HMG-CoA reductase activity under certain conditions, although 7-ketocholesterol and other major oxysterols present in oxidized LDL, showed only a low potency (Schroepfer, 2000). Indeed, intermediates of isoprenoid biosynthesis (mevalonic acid, farnesylpyrophosphate or geranylgeranylpyrophosphate) failed to improve the mitochondrial dysfunction or the viability loss in 7-ketocholesterol-treated SMCs.
Flow cytometry showed that a small percentage of the SMCs showed characteristics of necrosis (uptake of PI) or of apoptosis (annexin V labelling). The latter finding is in accordance with the observation that 4–5% of the adherent SMCs showed DNA fragmentation (TUNEL labelling) after 24 h exposure to 7-ketocholesterol (Seye et al., 2004). The modest increase of caspase activity also indicated that some SMCs were committed to undergo apoptotic or type I cell death.
However, when examined by transmission electron microscopy, many SMCs showed intense vacuolization, with appearance of double-membrane cytoplasmic vesicles, as shown previously in human vascular SMCs exposed to 7-ketocholesterol (Martinet et al., 2004). Formation of double-membrane cytoplasmic vesicles engulfing cytoplasm and/or cytoplasmic organelles such as mitochondria and endoplasmic reticulum is a hallmark of autophagy (Gozuacik and Kimchi, 2004; Ferraro and Cecconi, 2007). These autophagic vesicles and their contents are destroyed by the lysosomal system of the same cell. On light microscopic inspection of SMCs stained with neutral red, this was evident from the rarefaction and enlargement of the lysosomes, particularly in globular cells. Autophagy has multiple physiological functions, including protein degradation and organelle turnover and provision of amino acids and macromolecules necessary for survival under stress conditions. Increasing lines of evidence indicate that the same molecular mechanisms may be recruited by an alternative, caspase-independent form of programmed cell death, named autophagic type II cell death (Gozuacik and Kimchi, 2004; Ferraro and Cecconi, 2007). The microtubule-associated LC3 is essential for autophagic vesicle formation. When LC3 is cleaved at its C terminus, it is subsequently conjugated via an amide bond to the amino group of phosphatidylethanolamine. This conjugation converts the otherwise cytoplasmic form (LC3-I) to a tightly membrane-associated form (LC3-II), which is essential for autophagic vesicle formation. LC3 processing from LC3-I to LC3-II is thus a marker for autophagic vesicle formation (Gozuacik and Kimchi, 2004; Ferraro and Cecconi, 2007). Immunoblot analysis of the LC3-I to LC3-II conversion in SMCs exposed to 7-ketocholesterol confirmed the formation of autophagosomes. Taken together, the findings indicate that 7-ketocholesterol induces mitochondrial stress in rabbit aorta SMCs, gradually leading to autophagic (type II) cell death in most cells and apoptotic (type I) cell death in a minority of the cells.
SMC death induced by statins
In accordance with previous reports, fluvastatin- and simvastatin-induced cell death in SMCs (Baetta et al., 1997; Guijarro et al., 1998; Knapp et al., 2000; Sindermann et al., 2000). In agreement with those publications, SMC loss was mainly due to apoptosis, as indicated by the concentration-dependent activation of the caspase family of cysteine proteases. Owing to the highly variable sensitivity of individual SMCs (Figure 1), the activation occurred gradually in particular cells. The absence of autophagic vesicle formation (transmission electromicroscopy) and lack of LC3 processing (immunoblot) showed that autophagic cell death was a rare event, if occurring at all. Because the experiments were performed in the presence of 2% bovine fetal serum containing lipoproteins, it is unlikely that the statins caused cholesterol deprivation. Indeed, previous studies indicated that statin-induced apoptosis is most likely linked to suppression of prenylation of p21-Rho B or other proteins (Guijarro et al., 1998; Knapp et al., 2000; Takahashi et al., 2002), whereas high concentrations may evoke mitochondrial stress (Werner et al., 2004) and changes in calcium homeostasis (Tesfamariam et al., 1999).
In contrast to fluvastatin and simvastatin, pravastatin induced neither caspase activation nor changes in SMC morphology or viability. The lack of effect of pravastatin is in accordance with most studies on induction of apoptotic cell death by statins in vascular SMCs (Baetta et al., 1997; Guijarro et al., 1998; Knapp et al., 2000; Sindermann et al., 2000). Owing to its hydrophilic nature, pravastatin is a weak inhibitor of HMG-CoA reductase activity in rat (IC50 195 μM) or human (IC50 15 μM) vascular SMCs (Corsini et al., 1993; Bellosta et al., 1998). Biological effects of various statins in vascular SMCs are directly proportional to the IC50 of HMG-CoA reductase, but require 10–20-fold higher concentrations to suppress isoprenoid biosynthesis by more than 90% (Corsini et al., 1993; Bellosta et al., 1998). Hence, the highest concentration of pravastatin (100 μM) was apparently insufficient to affect viability of rabbit aorta SMCs.
Interactions between 7-ketocholesterol and statins
Interestingly, SMC death induced by 7-ketocholesterol was not raised further and the viability index was even improved by low statin concentrations that induced SMC loss by themselves. In previous (Knapp et al., 2000) and the present experiments, pravastatin was without effect, but a similar protection against 7-ketocholesterol-induced cell death has been described for pravastatin in human SMCs (Miyashita et al., 2002). The difference is not entirely clear, but different experimental conditions (10% FBS, prolonged exposure leading to more intracellular diffusion of the hydrophilic drug), another cell enumeration technique or greater sensitivity of human vascular SMCs to HMG-CoA inhibition by statins in comparison with rodent or rabbit cells (Corsini et al., 1993; Bellosta et al., 1998) could possibly explain the discrepancy.
7-Ketocholesterol and statins could interact at the level of the mitochondria, but early oxysterol-induced mitochondrial dysfunction, assessed by MTT reduction, was neither attenuated nor worsened by 1–10 μM fluvastatin or simvastatin. A striking finding was that fluvastatin and simvastatin failed to raise caspase activity in SMCs treated with 7-ketocholesterol, suggesting that activation of autophagic cell death processes interfered with the statin-induced apoptotic pathway. Indeed, recent lines of evidence indicate that interactions between both cell death programmes exist (Gozuacik and Kimchi, 2004). A study in breast cancer cells showed that autophagy delays apoptosis following DNA damage (Abedin et al., 2007). The mechanisms of this suppression are not yet understood, but it has been proposed that the engulfment of defective mitochondria by autophagosomes limits the release of pro-apoptotic factors, such as cytochrome c and apoptosis-inducing factor into the cytosol (Gozuacik and Kimchi, 2004; Ferraro and Cecconi, 2007). These findings confirm the general assumption that autophagy is an adaptive survival strategy that permits the cell to eliminate unwanted or unnecessary organelles and to recycle cellular components for reuse (Debnath et al., 2005). Only when autophagy is extensively triggered, will the cell die due to progressive degradation of cytoplasmic components.
At high concentrations, both statins and 7-ketocholesterol exerted additive effects on SMC death. This could be due to combined induction of mitochondrial dysfunction, as indicated by the assay of succinate dehydrogenase activity, which did not occur at lower statin concentrations. Additive effects have previously been reported for statins and apoptosis-inducing agents. In human rhabdomyosarcoma cells, high concentrations (⩾30 μM) of simvastatin induce Bax translocation and mitochondrial stress, followed by activation of caspases 9 and 3 and ultimately apoptotic cell death. In accordance with the present data on 7-ketocholesterol, these high statin concentrations exerted additive effects with doxorubicin-induced apoptosis in those cells (Werner et al., 2004). In addition, the SMC apoptosis induced by activation of cell death receptors by Fas ligand or cytokines (Knapp et al., 2000) was increased by a 24-h pretreatment with pharmacological concentrations of simvastatin or lovastatin, whereas pravastatin was inactive. The mechanisms were not completely understood, but upregulation of cell surface expression of Fas or the involvement of geranylgeranylated proteins in the assembly of the intracellular death-inducing signalling complex were proposed (Knapp et al., 2000). The main difference between those studies and the current data is that 7-ketocholesterol appeared to induce predominantly caspase-independent cell death pathways in rabbit (this study) and human vascular SMCs (Miguet-Alfonsi et al., 2002; Martinet et al., 2004).
In conclusion, our main finding is that vascular SMC death induced by an oxysterol, which was studied as a model for SMCs in an atherogenic setting, and cell death induced by relatively low concentrations of statins were not additive. This could be explained by the observation that autophagy, induced by 7-ketocholesterol, suppressed caspase activation leading to apoptotic cell death in statin-treated cells. The data further show that cell death processes induced by drugs in SMCs under standard conditions are not necessarily predictive for their effects on SMCs in an atherogenic setting.
Acknowledgments
We thank Hermine Fret, Dominique De Rijck, Lieve Svensson and Francis Terloo for their skilful technical assistance, and Liliane Van den Eynde for secretarial work. Wim Martinet and Dorien M Schrijvers are postdoctoral fellows of the Fund for Scientific Research—Flanders (Belgium) (FWO). This work was supported by FWO Grant 0627.06.
Abbreviations
- DEVD-R110
Asp-Glu-Val-Asp-rhodamine 110
- FBS
fetal bovine serum
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- LC3
microtubule-associated protein I light chain 3
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- PI
propidium iodide
- SMC
smooth muscle cell
Conflict of interest
The authors state no conflict of interest.
References
- Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007;14:500–510. doi: 10.1038/sj.cdd.4402039. [DOI] [PubMed] [Google Scholar]
- Babich H, Borenfreund E. Applications of the neutral red cytotoxicity assay to in vitro toxicology. Altern Lab Anim. 1990;18:129–144. [Google Scholar]
- Baetta R, Donetti E, Comparato C, Calore M, Rossi A, Teruzzi C, et al. Proapoptotic effect of atorvastatin on stimulated rabbit smooth muscle cells. Pharmacol Res. 1997;36:115–121. doi: 10.1006/phrs.1997.0211. [DOI] [PubMed] [Google Scholar]
- Bellosta S, Bernini F, Ferri N, Quarato P, Canavesi M, Arnaboldi L, et al. Direct vascular effects of HMG-CoA reductase inhibitors. Atherosclerosis. 1998;137 Suppl:S101–S109. doi: 10.1016/s0021-9150(97)00319-5. [DOI] [PubMed] [Google Scholar]
- Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- Corsini A, Mazzotti M, Raiteri M, Soma MR, Gabbiani G, Fumagalli R, et al. Relationship between mevalonate pathway and arterial myocyte proliferation: in vitro studies with inhibitors of HMG-CoA reductase. Atherosclerosis. 1993;101:117–125. doi: 10.1016/0021-9150(93)90107-6. [DOI] [PubMed] [Google Scholar]
- Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death. Autophagy. 2005;1:66–74. doi: 10.4161/auto.1.2.1738. [DOI] [PubMed] [Google Scholar]
- Ferraro E, Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Arch Biochem Biophys. 2007;462:210–219. doi: 10.1016/j.abb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Ghelli A, Porcelli AM, Zanna C, Rugolo M. 7-Ketocholesterol and staurosporine induce opposite changes in intracellular pH, associated with distinct types of cell death in ECV304 cells. Arch Biochem Biophys. 2002;402:208–217. doi: 10.1016/S0003-9861(02)00085-1. [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- Guijarro C, Blanco-Colio LM, Ortego M, Alonso C, Ortiz A, Plaza JJ, et al. 3-Hydroxy-3-methylglutaryl coenzyme a reductase and isoprenylation inhibitors induce apoptosis of vascular smooth muscle cells in culture. Circ Res. 1998;83:490–500. doi: 10.1161/01.res.83.5.490. [DOI] [PubMed] [Google Scholar]
- Knapp AC, Huang J, Starling G, Kiener PA. Inhibitors of HMG-CoA reductase sensitize human smooth muscle cells to fas-ligand and cytokine-induced cell death. Atherosclerosis. 2000;152:217–227. doi: 10.1016/s0021-9150(99)00462-1. [DOI] [PubMed] [Google Scholar]
- Kockx MM, De Meyer GRY, Bortier H, de Meyere N, Muhring J, Bakker A, et al. Luminal foam cell accumulation is associated with smooth muscle cell death in the intimal thickening of human saphenous vein grafts. Circulation. 1996;94:1255–1262. doi: 10.1161/01.cir.94.6.1255. [DOI] [PubMed] [Google Scholar]
- Kockx MM, De Meyer GRY, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97:2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- Lizard G, Deckert V, Dubrez L, Moisant M, Gambert P, Lagrost L. Induction of apoptosis in endothelial cells treated with cholesterol oxides. Am J Pathol. 1996;148:1625–1638. [PMC free article] [PubMed] [Google Scholar]
- Lizard G, Gueldry S, Sordet O, Monier S, Athias A, Miguet C, et al. Glutathione is implied in the control of 7-ketocholesterol-induced apoptosis, which is associated with radical oxygen species production. FASEB J. 1998;12:1651–1663. doi: 10.1096/fasebj.12.15.1651. [DOI] [PubMed] [Google Scholar]
- Lizard G, Miguet C, Bessede G, Monier S, Gueldry S, Neel D, et al. Impairment with various antioxidants of the loss of mitochondrial transmembrane potential and of the cytosolic release of cytochrome c occurring during 7-ketocholesterol-induced apoptosis. Free Radic Biol Med. 2000;28:743–753. doi: 10.1016/s0891-5849(00)00163-5. [DOI] [PubMed] [Google Scholar]
- Lizard G, Moisant M, Cordelet C, Monier S, Gambert P, Lagrost L. Induction of similar features of apoptosis in human and bovine vascular endothelial cells treated by 7-ketocholesterol. J Pathol. 1997;183:330–338. doi: 10.1002/(SICI)1096-9896(199711)183:3<330::AID-PATH933>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–213. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- Martinet W, De Bie M, Schrijvers DM, De Meyer GR, Herman AG, Kockx MM. 7-Ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:2296–2301. doi: 10.1161/01.ATV.0000146266.65820.a1. [DOI] [PubMed] [Google Scholar]
- Miguet C, Monier S, Bettaieb A, Athias A, Besséde G, Laubriet A, et al. Ceramide generation occurring during 7β-hydroxycholesterol- and 7-ketocholesterol-induced apoptosis is caspase independent and is not required to trigger cell death. Cell Death Differ. 2001;8:83–99. doi: 10.1038/sj.cdd.4400792. [DOI] [PubMed] [Google Scholar]
- Miguet-Alfonsi C, Prunet C, Monier S, Bessede G, Lemaire-Ewing S, Berthier A, et al. Analysis of oxidative processes and of myelin figures formation before and after the loss of mitochondrial transmembrane potential during 7beta-hydroxycholesterol and 7-ketocholesterol-induced apoptosis: comparison with various pro-apoptotic chemicals. Biochem Pharmacol. 2002;64:527–541. doi: 10.1016/s0006-2952(02)01110-3. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Ozaki H, Koide N, Otsuka M, Oyama T, Itoh Y, et al. Oxysterol-induced apoptosis of vascular smooth muscle cells is reduced by HMG-CoA reductase inhibitor, pravastatin. J Atheroscler Thromb. 2002;9:65–71. doi: 10.5551/jat.9.65. [DOI] [PubMed] [Google Scholar]
- Nishio E, Watanabe Y. Oxysterols induced apoptosis in cultured smooth muscle cells through CPP32 protease activation and bcl-2 protein downregulation. Biochem Biophys Res Commun. 1996;226:928–934. doi: 10.1006/bbrc.1996.1452. [DOI] [PubMed] [Google Scholar]
- Palinski W, Napoli C. Unraveling pleiotropic effects of statins on plaque rupture. Arterioscler Thromb Vasc Biol. 2002;22:1745–1750. doi: 10.1161/01.atv.0000038754.39483.cd. [DOI] [PubMed] [Google Scholar]
- Pirillo A, Jacoviello C, Longoni C, Radaelli A, Catapano AL. Simvastatin modulates the heat shock response and cytotoxicity mediated by oxidized LDL in cultured human endothelial smooth muscle cells. Biochem Biophys Res Commun. 1997;231:437–441. doi: 10.1006/bbrc.1997.6117. [DOI] [PubMed] [Google Scholar]
- Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- Seye CI, Knaapen MWM, Daret D, Desgranges C, Herman AG, Kockx MM, et al. 7-Ketocholesterol induces reversible cytochrome c release in smooth muscle cells in absence of mitochondrial swelling. Cardiovasc Res. 2004;64:144–153. doi: 10.1016/j.cardiores.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Sindermann JR, Fan L, Weigel KA, Troyer D, Muller JG, Schmidt A, et al. Differences in the effects of HMG-CoA reductase inhibitors on proliferation and viability of smooth muscle cells in culture. Atherosclerosis. 2000;150:331–341. doi: 10.1016/s0021-9150(99)00393-7. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ogata Y, Okazaki H, Takeuchi K, Kobayashi E, Ikeda U, et al. Fluvastatin enhances apoptosis in cytokine-stimulated vascular smooth muscle cells. J Cardiovasc Pharmacol. 2002;39:310–317. doi: 10.1097/00005344-200202000-00018. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Frohlich BH, Gregg RE. Differential effects of pravastatin, simvastatin, and atorvastatin on Ca2+ release and vascular reactivity. J Cardiovasc Pharmacol. 1999;34:95–101. doi: 10.1097/00005344-199907000-00016. [DOI] [PubMed] [Google Scholar]
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- Werner M, Sacher J, Hohenegger M. Mutual amplification of apoptosis by statin-induced mitochondrial stress and doxorubicin toxicity in human rhabdomyosarcoma cells. Br J Pharmacol. 2004;143:715–724. doi: 10.1038/sj.bjp.0705928. [DOI] [PMC free article] [PubMed] [Google Scholar]