Abstract
Background and purpose:
Clinical studies have demonstrated significant reductions in allergen-induced nasal symptoms of atopic rhinitis subjects by CysLT1 antagonists, including neuronally mediated symptoms such as sneeze, itch and reflex hypersecretion. Here, we test the hypothesis that cysteinyl leukotrienes activate and/or alter the activity of nasal nociceptive (capsaicin-sensitive) sensory neurones.
Experimental approach:
Using retrograde tracer (DiI), we labelled guinea-pig trigeminal sensory neurones that projected fibres to the nasal mucosa. Single-neurone reverse transcriptase (RT)-PCR was used to evaluate CysLT receptor gene expression. The effect of cysteinyl leukotrienes on individual nasal sensory nerve activity was assessed in Ca2+ assays and whole-cell gramicidin-perforated patch-clamp studies.
Key results:
Nasal C-fibre neurones express CysLT1 but not CysLT2 mRNA. LTD4 and LTC4 increased intracellular [Ca2+]free in a population of capsaicin-sensitive trigeminal nerves, an effect blocked by the CysLT1 antagonist ICI198615. In current clamp mode, LTD4 had no effect on resting membrane potential. However, LTD4 significantly increased electrical excitability (action potential discharge during current pulses) threefold in capsaicin-sensitive nasal neurones, which was inhibited by CysLT1 antagonists ICI198615 and montelukast. LTD4 had no effect on electrical excitability in capsaicin-insensitive neurones. Finally, LTD4 significantly augmented histamine-induced responses in capsaicin-sensitive neurones as measured by increased action potential discharge, peak frequency and membrane depolarization.
Conclusions and implications:
LTD4, likely through CysLT1 receptors, directly increases the excitability of capsaicin-sensitive guinea-pig nasal trigeminal neurones, demonstrating a novel mechanism for the actions of cysteinyl leukotrienes and potentially explains the effectiveness of CysLT1 antagonists in treating nasal allergen-induced neuronal symptoms.
Keywords: CysLT1 antagonist, allergic rhinitis, trigeminal, nasal, nerve, leukotriene, excitability, histamine, single-cell RT-PCR
Introduction
Cysteinyl leukotrienes (leukotriene (LT) C4, LTD4 and LTE4) are potent inflammatory mediators commonly associated with allergic disease (Orange and Austen, 1969). Cysteinyl leukotrienes have been measured in nasal lavages following allergen challenge (Miadonna et al., 1987; Terada et al., 1989) and have come into focus with regards to their contribution to symptom generation in allergic rhinitis (Howarth, 2000; Peters-Golden and Henderson, 2005). Pharamcological studies have shown that exogenous cysteinyl leukotrienes can cause nasal blockage, but fail to cause rhinorrhea, itching or sneezing (Bisgaard et al., 1986; Miadonna et al., 1987; Okuda et al., 1988). It may therefore be surprising that CysLT1 antagonists have demonstrated significant reductions in allergen-induced hypersecretion, nasal itching and sneezing (Donnelly et al., 1995; Philip et al., 2002; Chervinsky et al., 2004; Patel et al., 2005; Virchow and Bachert, 2006). This paradox that CysLT1 antagonism may inhibit neuronal symptoms of rhinitis, yet the agonist alone has little effect, is addressed in this study.
The symptoms of sneezing, itch (or pruritus) and reflex hypersecretion (major contributor of rhinorrhea) are contingent upon the activation of nasal sensory nerves. These neurones, with their cell bodies situated in the trigeminal ganglion, densely innervate the nasal mucosa at their peripheral endings and terminate centrally in the trigeminal nucleus of the brain stem. Electrical and chemical activation of nasal trigeminal neurones has been shown to cause brain stem c-FOS production, brain stem neurotransmitter release and electrical activity in conjunction with sneezing, itch and hypersecretion (Batsel and Lines, 1975; Richardson and Peatfield, 1981; Lucier and Egizii, 1989; Anton et al., 1991; Schaible et al., 1997). It is clear that sensory neurones in both visceral and somatosensory systems, including the respiratory tract, are not uniform (Lawson et al., 1993; Schmelz et al., 2003; Taylor-Clark and Undem, 2006). Sensory neurones are categorized according to their action potential conduction velocities: fast A-fibres tending to be low threshold mechanosensors, which do not discriminate noxious stimuli and slow C-fibres that respond to a variety of noxious stimuli, such as temperature, pH, algogens and inflammatory mediators. It is thought that the airway neurones responsible for the defensive reflexes (for example, hypersecretion) are the nociceptor C-fibre neurones, whereas non-nociceptive nerves conduct information regarding stretch, airflow and touch (Canning, 2002; Carr and Undem, 2003; Tai and Baraniuk, 2003). Almost all mammalian nociceptive C-fibre neurones express TRPV1 channels, the receptor for capsaicin, the pungent component in chili peppers. We have previously shown that nasal trigeminal sensory neurones can be divided into two subgroups depending on their response to capsaicin (Taylor-Clark et al., 2005). Thus, using capsaicin sensitivity we are able to identify those neurones likely to be involved with the initiation of the nasal symptoms of sneezing, pruritus and reflex hypersecretion.
In this study, experiments were designed to test the hypothesis that guinea-pig nasal trigeminal neuronal activity is modulated by cysteinyl leukotrienes. Our data indicate that LTD4 alone failed to independently activate nasal sensory nerves, but it consistently increased the excitability of capsaicin-sensitive nerves, thus potentiating neuronal responses to nonspecific electrical stimulation and to specific stimulation with histamine.
Methods
All experiments were approved by the Johns Hopkins Animal Care and Use Committee. In total, 50 male Hartley guinea-pigs (200–400 g; Hilltop Laboratory Animals Inc., Scottsdale, PA, USA) were used in this study. Guinea-pigs were housed in-house in a light-controlled room (light: 0700–2100 h, dark: 2100–0700 h) kept at 23 °C and at a relative humidity of 40%, supplied with pathogen-free air, and food and water ad libitum. All drug/molecular target nomenclature used conforms with BJP's Guide to Receptors and Channels (Alexander et al., 2007).
Nasal trigeminal neurones—retrograde tracing and dissociation
Nasal afferent neurones were retrogradely labelled as previously described (Taylor-Clark et al., 2005). Briefly, under anaesthesia (50 mg kg−1 of ketamine and 2.5 mg kg−1 of xylazine; i.p.), 30 μL DiI (DiC18(3)) solution (2%, in dimethylsulphoxide) was instilled into the right nostril and the guinea-pigs were placed in a supine position leaning slightly to their right side. Procedure was repeated the next day for left nostril instillation. Fourteen days after labelling, animals were killed and the trigeminal ganglia were removed and cleared of adhering tissue. Trigeminal afferent cell bodies were enzymatically dissociated (2 mg mL−1 of collagenase type-1A and 2 mg mL−1 of dispase II in 2 mL of Ca2+-, Mg2+-free Hanks' balanced salt solution) for 50 min at 37 °C) and plated onto poly-D-lysine (0.1 mg mL−1)-coated coverslips as previously described. Neurone-attached coverslips were flooded with L-15 medium containing 10% fetal bovine serum and used within 24 h.
Single-cell RT-PCR on nasal trigeminal neurones
First-strand cDNA was synthesized from single nasal-labelled trigeminal neurones by using the SuperScript III CellsDirect cDNA Synthesis System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations.
Cell picking
Coverslips of retrogradely labelled, dissociated neurones were constantly perfused by Locke's solution and identified by using fluorescence microscope. Single cells were harvested into a glass pipette (tip diameter 50–150 μm) pulled with a micropipette puller (model P-87; Sutter Instruments Company, Novato, CA, USA) by applying negative pressure. The pipette tip was then broken in a PCR tube containing resuspension buffer (1 μL) and RNAse inhibitor (RNAseOUT, 2 U μL−1), immediately snap frozen and stored on dry ice. A sample of the bath solution from the vicinity of a labelled neurone was collected from each coverslip for no-template experiments (bath control).
RT-PCR
Samples were defrosted, lysed (10 min at 75 °C) and treated with DNAseI. Then, poly(dT) and random hexamer primers (Roche Applied Bioscience, Indianapolis, IN, USA) were added. Half of the volume was reverse transcribed by adding Superscript III RT for cDNA synthesis, whereas water was added to the remaining sample, which was used in the following as RNA control.
PCR
The PCR reaction mix (final volume of 20 μL) contained 0.5 U HotStar Taq polymerase (Qiagen, Valencia, CA, USA) supplemented with 2.5 mM MgCl2, PCR buffer, dNTPs, custom synthesized primers for β-actin (sense: 5′-TGGCTACAGTTTCACCACCA-3′, antisense: 5′-GGAAGGAGGGCTGGAAGA-3′; GenBank/EMBL/DDBJ accession no. AF508792; calculated product length 212 bp), TRPV1 receptor (sense: 5′-GTCGTCGCCTATTGATTGCT-3′, antisense: 5′-AGAGCTTGAGTGGCTTCTCG-3′; GenBank/EMBL/DDBJ accession no. AY729017; calculated product length 167 bp), CysLT1 receptor (sense 5′-GAAACTGGAAATCCGACAATACC-3′, antisense: 5′-CACAATAGATCAGCCACAGCTAAA-3′; GenBank/EMBL/DDBJ accession no. AY236968; calculated product length 218 bp), or CysLT2 receptor (sense 5′-GTCTGAACCGAAGAACAGCA-3′, antisense 5′-GATAAGGCAGGAAGCACAGG-3′; GenBank/EMBL/DDBJ accession no. AY236969; calculated product length 260 bp) (all purchased from Invitrogen), and 3 μL of each sample (cDNA, RNA control or bath control, respectively). After an initial activation step at 95 °C for 15 min, samples were amplified by 45 cycles (β-actin and TRPV1) or 50 cycles (CysLT1 and CysLT2) of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 1 min followed by a final extension at 72 °C for 10 min. Products were then visualized in ethidum bromide-stained 1.5% agarose gels.
Intracellular calcium measurement
Coverslips were loaded with Fura-2 AM (8 μM) in L-15 media containing 20% fetal bovine serum and incubated for 40 min at 37 °C. Coverslips were placed in a custom-made chamber that was superfused with Locke solution (composition (mM): 136 NaCl, 5.6 KCl, 1.2 MgCl2, 2.2 CaCl2, 1.2 NaH2PO4, 14.3 NaHCO3 and 10 dextrose (gassed with 95% O2–5% CO2, pH 7.3–7.4, 336.8 mOs mol L−1)) at 35 °C for 15 min before the experiment by an infusion pump (4 mL min−1). LTC4 concentrations were stabilized by performing experiments using Locke solution containing a serine–borate complex (0.5 mM L-serine and 1 mM sodium tetraborate (Hisamatsu et al., 1996)) that inhibits γ-glutamyltranspeptidase, the enzyme responsible for the conversion of LTC4 into LTD4.
Changes in intracellular [Ca2+]free were measured by digital microscope (Universal; Carl Zeiss Inc., Thornwood, NY, USA) equipped with in-house equipment for ratiometric recording of single cells. The field of cells was monitored every 6 s by sequential dual excitation, 352 and 380 nm, and the analysis of the image ratios used methods previously described to calculate changes in intracellular [Ca2+]free (MacGlashan, 1989; Taylor-Clark et al., 2005). Neurones were exposed to drug treatments (vehicle, LTD4 (from 1 to 500 nM) or LTC4 (from 10 to 500 nM)) for 60 s (in the presence or absence of the CysLT1 antagonist ICI198615 (1 μM) or the phospholipase C inhibitor U-73122 (10 μM)), followed by capsaicin (60 s, 1 μM), KCl (30 s, 75 mM) and ionomycin (30 s, 1 μM). KCl was used as an indicator of voltage-sensitive cells, and the ionomycin was used to obtain a maximal response. Between each stimulus the cells were continuously washed with fresh buffer.
For the analysis of Fura-2 AM loaded cells, the measurement software converted ratiometric information to intracellular [Ca2+]free using a default set of Tsien parameters (Grynkiewicz et al., 1985) particular to this instrumentation and a broad selection of cells. We did not specifically calibrate the relationship between ratiometric data and absolute calcium concentration, choosing instead to use the default parameters provided and relate all measurements to the peak ionomycin response in each viable cell. If a cell lacked a robust response ([Ca2+]free>400 nM) to ionomycin (1 μM) or produced a mean response to KCl (75 mM) less than 40% peak ionomycin response, it was not included in the analysis. Mean apparent [Ca2+]free response to ionomycin of included neurones was 1313 nM (s.d. of 305 nM). A specific neurone was considered to have responded to a drug treatment (for example, cysteinyl leukotriene or capsaicin) if the mean response over the 60 s of drug treatment was greater than 2 s.d. above the mean baseline response (60 s immediately before drug treatment).
Whole-cell patch clamp
Patch-clamp experiments were performed on DiI-labelled dissociated trigeminal neurones from a total of 32 nasally labelled animals. DiI-labelled cells were clearly identifiable using fluorescent microscope (excitation band 500–570 nm, emission band 555–655 nm). To maintain intracellular signal pathways, a gramicidin-perforated whole-cell patch-clamp technique was employed using Multiclamp 700A amplifier and Axograph 4.9 software. The pipette (1.5–3MΩ) was filled with a pipette solution composed of (mM) 140 KCl, 1 CaCl2, 2 MgCl2, 10 HEPES, 11 EGTA and 10 dextrose; titrated to pH 7.3 with KOH; 320 mOs mol L−1. Gramicidin was dissolved in dimethylsulphoxide and mixed with the pipette solution for a final concentration of 2–3.5 μg mL−1 (0.1–0.175% dimethylsulphoxide) just before each recording. After forming a gigaohm seal, cell membrane potential was held at −60 mV and the pipette capacitance was measured. Recordings were made when the series resistance was less than 30 MΩ. In current clamp mode the pipette capacitance was neutralized by 90% of measured value. During the experiments, the cells were continuously superfused (6 mL min−1) with Locke solution at 35 °C.
For each neurone, the action potential threshold was determined in current clamp mode using stepwise current pulses (10–100 pA and if required 100–600 pA). The threshold current required to elicit action potential discharge was then used for the current clamp electrical excitability studies, in which each neurone was subjected to three 500-ms current pulses 5 s apart. The magnitude of the pulses was calibrated for each neurone to be (1) the threshold current, (2) 2 × threshold current and (3) 3 × threshold current. The electrical excitability protocol was performed three times: control, in the presence of and following a 30-s pretreatment with LTD4 (500 nM), and a final control following 2 min of washout.
In current clamp studies, the number of action potentials elicited were counted by the Axograph 4.9 software, and if required, the peak frequency was calculated as the maximum number of action potentials elicited in 1-s bins. In voltage clamp studies, the inward current was normalized to the capacitance of the neurone. Data are expressed as current density (pA pF−1).
In the experiments investigating action potential waveform and input resistance neurones were held in current clamp mode at −60 mV and subjected to alternating 1-Hz, 1-nA, 1-ms depolarizing pulses and 1-Hz, 10-pA, 100-ms hyperpolarizing pulses for 90 s (such that at depolarizing pulses occurred at t=1, 2, 3 s…, and so on, and hyperpolarizing pulses occurred at 1.5, 2.5, 3.5 s…, and so on). LTD4 (500 nM) was applied at t=30 for 40 s. Input resistance was determined by calculating the difference in the membrane potential (mV) at rest and during 10 pA hyperpolarizing pulse (during steady state only) and applying Ohm's law. Action potential overshoot was determined as the highest point on the upstroke of the elicited action potential and the afterhyperpolarization was calculated as the difference between the resting membrane potential and the lowest point on the downward slope of the action potential. The rise rate of the action potential upstroke (ΔV/Δt) was determined between 50 and 80% of the upstroke, calculated by dividing the magnitude of the potential between 50 and 80% of the upstroke (ΔV) by the time taken for the potential to rise from 50 to 80% of its maximum (Δt). Mean data at t=5–25 (control) were compared with mean data at t=40–60 (LTD4) for all biophysical analyses.
Statistical analysis
All data are expressed as mean±s.e.m. Paired and unpaired t-tests were used for statistical analysis when appropriate, and a P-value less than 0.05 was taken as a significant difference. Nonlinear regression for curve fitting was performed using Prism 4 software.
Drug preparations
All test drugs were made up in Locke solution just before experimentation. Histamine diphosphate, capsaicin and ionomycin were purchased from Sigma-Aldrich (St Louis, MO, USA). Montelukast, LTD4 and LTC4 were purchased from Cayman Chemicals (Ann Arbor, MI, USA). DiI (DiC18(3)) and Fura-2 AM were purchased from Molecular Probes (Eugene, OR, USA).
Results
Detection of cysteinyl leukotriene receptor mRNA in individual nasal-specific trigeminal neurones
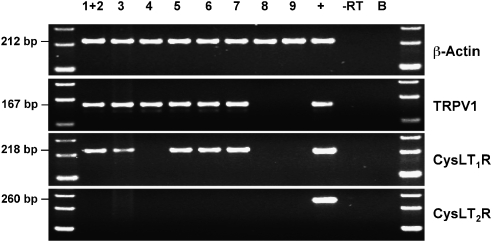
Single-cell reverse transcriptase (RT)-PCR was performed on dissociated trigeminal neurones that project fibres to the nasal mucosa (DiI-labelled), using specific primers for β-actin, TRPV1 (capsaicin receptor), CysLT1 and CysLT2 (Figure 1). All nine nasal neurones contained mRNA encoding for β-actin, with seven of these positive for TRPV1 mRNA (indicating they are likely nociceptive C-fibre neurones). Six out of seven TRPV1 mRNA-positive nasal neurones also contained mRNA encoding for the CysLT1 receptor. The two TRPV1 mRNA-negative neurones did not express CysLT1 mRNA. None of the nasal neurones expressed CysLT2 receptor mRNA, although the whole trigeminal ganglia (used as a positive control) demonstrated CysLT2 receptor mRNA expression. Neither the collected buffer, nor the PCR of single neurones without reverse transcription, revealed specific mRNA expression.
Figure 1.
Expression of β-actin, TRPV1, CysLT1 and CysLT2 mRNA in nasal trigeminal neurones. Single-cell reverse transcriptase (RT)-PCR of dissociated neurones (neurones numbered 1–9). cDNA obtained from a whole trigeminal ganglion served as positive control (+). Samples from nasal neurones in which the RT was omitted (−) served as negative control. B=bath control (bath solution was used as a template).
Actions of cysteinyl leukotrienes on cytosolic calcium in capsaicin-sensitive trigeminal neurones
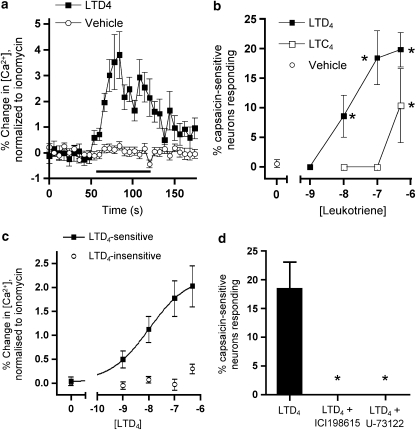
The increase in cytosolic calcium was used to monitor the direct response of capsaicin-sensitive trigeminal neurones to LTC4 and LTD4. These experiments were performed on neurones taken from animals that had not been nasally labelled with DiI and so are likely to include neurones innervating a wide variety of facial tissues, not just the nasal mucosa. Capsaicin (1 μM) activated 607 out of 2620 trigeminal neurones (89 experiments); mean increase of responders to capsaicin and KCl was 51.3±2.9 and 40.3±2.4% of maximum ionomycin response, respectively. The percentage of capsaicin-sensitive trigeminal neurones was similar to previous studies measuring changes in cytosolic calcium (Taylor-Clark et al., 2005). Cysteinyl leukotrienes stimulated calcium increase in a subset of capsaicin-sensitive trigeminal neurones. As with other GPCR agonists such as histamine acting through the H1 receptor (Taylor-Clark et al., 2005), the leukotriene-induced increase was readily quantifiable, but much less than that observed with capsaicin in this assay. A 60-s application of 100 nM LTD4 activated 18.5% of capsaicin-sensitive neurones (data from 25 experiments), with a typical calcium response reaching a peak of approximately 3–4% of ionomycin maximum within 20 s, a plateau phase for 40 s before a drop back towards baseline within approximately 20 s after washout (P<0.001 compared with baseline; Figure 2a). Vehicle failed to increase calcium compared with baseline (P>0.3). The percentage of capsaicin-sensitive neurones responding to LTD4 (1, 10, 100 or 500 nM) and LTC4 (10, 100 or 500 nM) increased significantly (P<0.05) in a concentration-dependent manner, reaching a maximum of approximately 20% of capsaicin-sensitive neurones (Figure 2b), with LTD4 being more potent than LTC4. As would be expected, not only was the percentage of neurones that responded concentration-dependent, the magnitude of the effect in the responding neurones was also concentration-dependent, with an EC50 for LTD4 of 10.5 nM (Figure 2c).
Figure 2.
LTD4 increases [Ca2+] in a subset of capsaicin-sensitive trigeminal neurones. (a) Normalized calcium response of LTD4-responsive capsaicin-sensitive trigeminal neurones to 100 nM LTD4 (black squares) (n=19 neurones), compared with capsaicin-sensitive neurone response to buffer (open circles) (n=282 neurones). Black line denotes drug treatment. (b) Percentage of capsaicin-sensitive trigeminal neurones responding to single doses of LTD4 (black squares) and LTC4 (open squares) (n=4–6 experiments, 32–140 neurones). *Significant increase compared with vehicle (open circles) (P<0.05). (c) Normalized calcium responses of capsaicin-sensitive trigeminal neurones to increasing doses of LTD4 (0–500 nM). Responses of LTD4-sensitive neurones (black squares, n=41 neurones) were fitted by nonlinear regression. Neurones were included in the entire analysis if they were sensitive to one or more doses of LTD4. Responses of LTD4-insensitive neurones (open circles, n=129 neurones) were unable to be fitted. (d) LTD4 (100 nM) response in capsaicin-sensitive trigeminal neurones abolished by ICI198615 (1 μM, n=13 experiments, 36 neurones) and by U-73122 (10 μM, n=4 experiments, 21 neurones). *Significant decrease in LTD4 response after pretreatment with inhibitor (P<0.05). All data are mean±s.e.m.
Further experiments were designed to investigate the mechanism of LTD4-induced calcium increases in capsaicin-sensitive trigeminal neurones. Neurones were pretreated for 5 min with the selective CysLT1 antagonist ICI198615 (1 μM), before 100 nM LTD4 challenge. ICI198615 abolished the LTD4-induced increase in cytosolic calcium in capsaicin-sensitive trigeminal neurones (P<0.05) (Figure 2d). In separate experiments, neurones were pretreated for 10 min with U-73122 (10 μM), an inhibitor of phospholipase C, before 100 nM LTD4 challenge. U-73122 also abolished the LTD4-induced increase in cytosolic calcium in capsaicin-sensitive trigeminal neurones (P<0.05) (Figure 2d). Neither ICI198615 nor U-73122 blocked the response in these neurones to capsaicin (data not shown). Finally, neurones were pretreated for 15 min with Locke solution containing 5 mM EDTA (a calcium chelator) and 0 mM calcium before 100 nM LTD4 challenge. The neurones were then perfused for 10 min with normal Locke solution before 1 μM capsaicin challenge. Four out of 19 capsaicin-sensitive neurones (21%) responded to LTD4 in the absence of extracellular calcium (data not shown).
LTD4 increases nasal neuronal excitability to current pulses
Given that changes in cytosolic calcium do not necessarily indicate changes in neuronal electrical activity, we further investigated the role of LTD4 on neuronal responses in whole-cell patch-clamp recordings, focusing on those neurones that project fibres to the nasal mucosa (DiI-labelled). A gramicidin-perforated patch protocol was used to minimize the loss of potentially crucial intracellular mediators and proteins. Calcium imaging data had demonstrated that 500 nM LTD4 was a near maximal CysLT1 receptor stimulus of trigeminal neurones, both in terms of percentage of responders and the magnitude of calcium increases in responding neurones. In addition, LTD4-induced increases in calcium were relatively stable between 20 and 50 s of application. Thus, the effect of LTD4 on nasal neuronal excitability was investigated after 30 s of 500 nM LTD4 exposure.
Current clamp recordings of nasal-specific trigeminal neurones showed that LTD4 (500 nM) significantly depolarized very few neurones (3 out of 55). Given their relative rareness, we decided not to study these neurones further and they were discarded from any other studies or analysis.
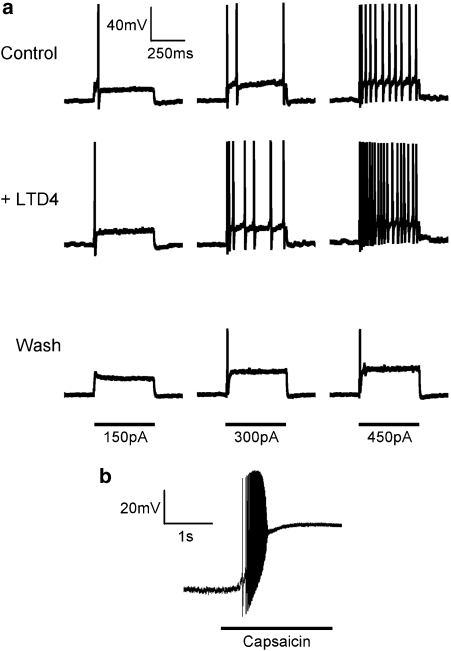
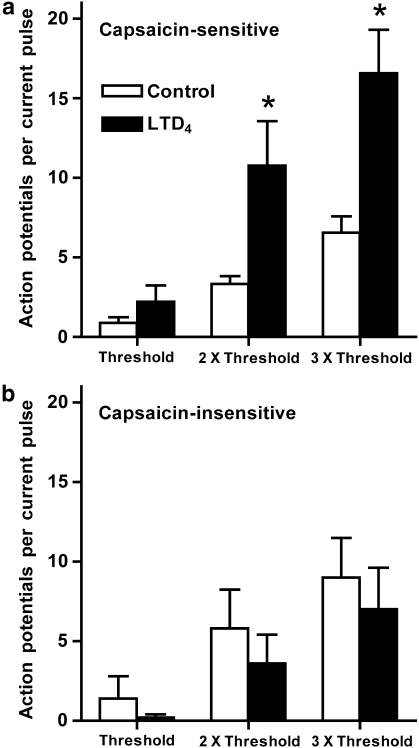
To test the effect of LTD4 on the electrical excitability of nasal-specific neurones, we employed a three 500 ms current pulse protocol (current required to elicit action potential firing (threshold), 2 × threshold and 3 × threshold), which was performed three times; the first as a control, the second in the presence of LTD4 (following 30 s pretreatment) and a third following 2 min of washout. At the end of the experiment the neurone was challenged with capsaicin (1 μM). In all 14 neurones a positive relationship between current pulse strength and action potential discharge was apparent, although it appeared that the number of action potentials elicited by these peri-threshold current pulses tended to decrease over time (35% reduction after 10 min, this did not reach significance (P>0.1))—likely due to a decrease in seal resistance caused by gramicidin-induced membrane perforation. In eight out of nine capsaicin-sensitive neurones LTD4 (500 nM) increased the number of action potentials elicited by the three current pulses (to 303±65% of control) (Figures 3 and 4), and this increase was statistically significant for the 2 × threshold and 3 × threshold pulses (P<0.05). The potentiating effect of LTD4 was reversible and following a 2 min washout the action potential discharge was at or below control responses. In addition, LTD4 significantly increased spontaneous action potential discharge (those not recorded during current pulses) in seven out of nine capsaicin-sensitive neurones (control mean number of spontaneous action potentials 9.2±8.9, following LTD4 mean number of spontaneous action potentials 168±72 (P<0.05)). Spontaneous activity was observed directly only after neurones were treated with the current pulses. LTD4 failed to increase electrical excitability in all five capsaicin-insensitive nasal-specific neurones (Figure 4).
Figure 3.
Whole-cell current-clamp responses of a capsaicin-sensitive nasal-specific neurone (representative trace). (a) Response to three current injections (150, 300 and 450 pA) before, during and 2 min after LTD4 (500 nM) treatment. Neurone held at −60 mV. Action potential firing threshold for this neurone was previously determined to be 150 pA. (b) The same neurone then responded robustly to capsaicin (1 μM).
Figure 4.
Excitability of nasal-specific capsaicin-sensitive sensory neurones to current pulses (white bars) is increased by LTD4 (500 nM, black bars). Threshold current pulse calibrated for each neurone to equal the lowest current that initiates action potentials. (a) Capsaicin-sensitive neurones (n=9). (b) Capsaicin-insensitive neurones (n=5). *Significant increase in number of action potentials elicited during current pulse with LTD4 treatment (P<0.05).
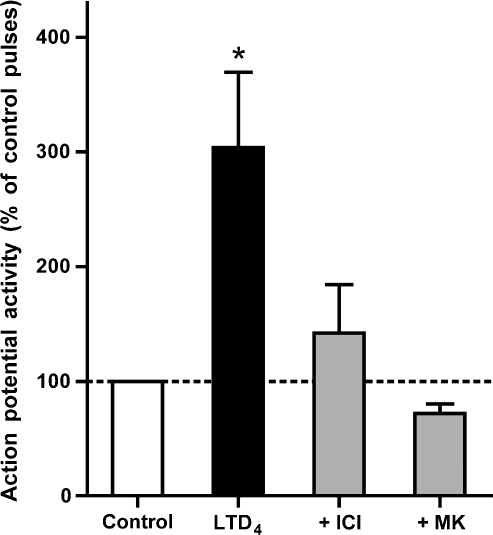
The effect of LTD4 on electrical excitability in nasal-specific neurones was tested in the presence of CysLT1 receptor antagonists montelukast and ICI198615. In the presence of montelukast (1 μM), LTD4 (500 nM) failed to increase the electrical excitability in all five capsaicin-sensitive nasal-specific neurones tested. LTD4 (500 nM) also failed to significantly increase the electrical excitability of capsaicin-sensitive nasal-specific neurones when studied in the presence of ICI198615 (1 μM, n=8) (Figure 5).
Figure 5.
Augmentation of current pulse-induced action potential discharge by LTD4 (500 nM, n=9) in capsaicin-sensitive nasal neurones is inhibited by ICI198615 (ICI, 1 μM, n=8) and montelukast (MK, 1 μM, n=5). Current-induced action potentials in the presence of LTD4 were summed and expressed as a percentage of controls. *Significant increase in action potential discharge elicited in the presence of LTD4 (P<0.05).
LTD4 increases nasal neuronal excitability to histamine
Given that LTD4 increased neuronal sensitivity to current pulse injections, we predicted that LTD4 would increase neuronal sensitivity to substances that induce neuronal activity. Due to its relevance to allergen-induced nasal symptoms (Taylor-Clark and Foreman, 2005), we compared the histamine-induced responses of nasal-specific capsaicin-sensitive neurones with and without LTD4 pretreatment. Our previous study had shown that histamine responses (which are sensitive to the H1 antagonist diphenhydramine) are only observed in nasal-specific neurones that also respond to capsaicin (Taylor-Clark et al., 2005). Preliminary data indicated that histamine (10 μM) caused depolarization of nasal-specific capsaicin-sensitive neurones, often leading to action potential discharge. Histamine (100 μM) caused even greater depolarizations and action potential discharge (data not shown), indicating that 10 μM was a submaximal dose. Consecutive histamine challenges led to some degree of desensitization (data not shown) and thus much of the data were unpaired.
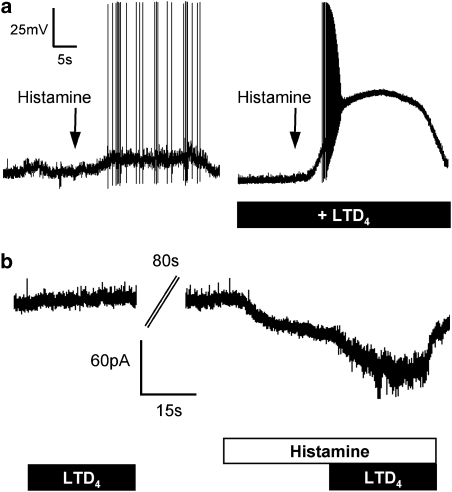
In whole-cell current-clamp experiments, histamine (10 μM) depolarized seven out of eight capsaicin-sensitive neurones and induced action potentials in six of these (Figure 6a). In the presence of, and following 30 s pretreatment with, LTD4 (500 nM), histamine (10 μM) depolarized eight out of eight capsaicin-sensitive neurones and induced action potentials in all eight neurones (Figure 6a). In the presence of LTD4, the peak action potential discharge frequency, the total number of action potentials and the maximum depolarization caused by histamine were all significantly increased (P<0.05) (Table 1). These increases were not due to an additive effect of the LTD4 and histamine, rather the neurones in the presence of LTD4 were hyperexcitable.
Figure 6.
Histamine-induced responses of capsaicin-sensitive nasal-specific neurones to histamine (10 μM) are augmented by LTD4 (500 nM). (a) Representative traces of current clamp responses of neurones challenged with histamine (left) and following pretreatment with LTD4 (right). Both neurones held at −60 mV. (b) Voltage clamp responses of a neurone challenged with LTD4 alone, histamine alone and histamine plus LTD4 (representative trace).
Table 1.
LTD4 increases histamine-induced responses in capsaicin-sensitive nasal-specific neurones
| Histamine (10 μM) | LTD4 (500 nM) | Histamine+LTD4 | P-value | |
|---|---|---|---|---|
| Current clamp | ||||
| Peak frequency (1 s bins) | 9 (±4) | 0.0 | 30 (±6) | 0.005* |
| Action potentials | 63 (±35) | 0.0 | 157 (±40) | 0.049* |
| Depolarization (mV) | 14 (±3) | 0.9 (±0.5) | 33 (±7) | 0.007* |
| Voltage clamp | ||||
| Inward current (pA) | 28 (±4.9) | 8.3 (±8.3) | 176 (±73) | 0.042** |
| Current density (pA pF−1) | 1.4 (±0.3) | 0.4 (±0.4) | 8.3 (±3.6) | 0.041** |
*Significant increase in histamine responses in the presence of LTD4 (histamine (n=8), LTD4 alone and in combination with histamine (n=7)).
**Significant increase in histamine-induced current in the presence of LTD4 (n=7).
LTD4-induced hyperexcitability to histamine was also observed in paired whole-cell voltage-clamp experiments. Nasal-specific capsaicin-sensitive neurones (mean capacitance 23 pF) were treated with LTD4 for 30 s, washed for 2 min, and then challenged with histamine (10 μM) for 60 s. Halfway through the histamine challenge, LTD4 (500 nM) was added to the buffer. LTD4 alone produced little or no inward current. Histamine-induced inward currents were significantly greater following the addition of LTD4 (P<0.05, n=7) (Figure 6b; Table 1).
LTD4-induced effects on nasal neuronal biophysical properties
Results indicating that LTD4 can augment action potential discharge to current injections suggest that, downstream of leukotriene receptor activation, the biophysical properties of the neurone are being altered in such a way that promotes electrical hyperexcitability. In current clamp mode, characteristics of the action potential waveform and the membrane resistance were investigated with alternating 1-Hz, 1-nA, 1-ms depolarizing pulses and 1-Hz, 10-pA, 100-ms hyperpolarizing pulses, respectively. As seen in Table 2, LTD4 had no effect on resting membrane resistance, nor did LTD4 alter the magnitude of the action potential overshoot or afterhyperpolarization. However, LTD4 caused a significant increase in the rate of the action potential upstroke in capsaicin-sensitive nasal neurones (P<0.001, n=5).
Table 2.
Biophysical properties of capsaicin-sensitive nasal neurones
| Membrane potential (mV) | Membrane resistance (Ω) | Overshoot (mV) | Action potential rise rate (mV.ms−1) | AHP (ΔmV) | |
|---|---|---|---|---|---|
| Control | −61.6 (±0.9) | 298 (±8) | 35.8 (±0.3) | 536 (±3) | −16.2 (±0.1) |
| LTD4 | −61.5 (±0.9) | 292 (±8) | 35.3 (±0.1) | 580 (±5)* | −16.3 (±0.1) |
Abbreviation: AHP, after hyperpolarization.
*Significant difference in biophysical property after application of LTD4 (500 nM) (P<0.001, n=5).
Discussion
Our data demonstrate that cysteinyl leukotrienes directly interact with CysLT receptors on guinea-pig trigeminal neurones that innervate the nasal mucosa. The consequences of activation of these receptors include an increase in cytosolic calcium and increased electrical excitability of capsaicin-sensitive (presumably nociceptive) sensory neurones. Evidence of receptor mRNA expression, agonist potency ratios and the effectiveness of selective antagonists support the hypothesis that the CysLT receptor involved in these responses is the CysLT1 receptor.
Nasal trigeminal nerve activity is responsible for the initiation of sensations (for example, itch) and central reflexes (for example, sneeze, increase in parasympathetic-mediated hypersecretion) from the nasal mucosa. It is likely that the majority of these defensive responses are mediated by nociceptive sensory neurones (Stjarne et al., 1989; Silver et al., 1991; Namimatsu et al., 1992; Konno et al., 1995; Stjarne et al., 1998). Nociceptive fibres in mammals respond to the TRPV1 agonist capsaicin and often contain neuropeptides (such as substance P and CGRP). Consistent with previous reports of capsaicin-sensitivity in nasal neurones (Taylor-Clark et al., 2005; Damann et al., 2006), we detected expression of TRPV1 mRNA in the majority of nasal sensory nerves. CysLT1 receptor mRNA was coexpressed with TRPV1 mRNA in six of seven nasal neurones, and appeared to be lacking in nasal neurones that did not express TRPV1 mRNA. CysLT2 receptor mRNA was not detected in any nasal neurones, but was detected in the whole-ganglia preparation—suggesting either that non-nasal neurones may express the CysLT2 receptor or, more likely, the expression of CysLT2 comes from non-neuronal tissue such as endothelial or immune cells (Brink et al., 2003; Steinke and Borish, 2004).
The Fura-2AM calcium assay is a useful high-throughput assay for determining direct neuronal sensitivity to certain stimuli, in particular stimuli that open plasma membrane calcium-permeable channels (for example, TRPV1 agonists) and stimuli that activate Gq coupled receptors (for example, CysLT1 agonists). LTD4 caused an increase in cytosolic calcium in approximately 20% of trigeminal capsaicin-sensitive neurones. This effect was blocked by ICI198615, a selective inhibitor of CysLT1 receptors, and by U-73122, an inhibitor of phospholipase C, but was unaffected by removing extracellular calcium. Overall, the data suggest that LTD4 increases cytosolic calcium by the release of calcium from intracellular stores under the control of phospholipase C-mediated mechanisms and downstream of CysLT1 receptor activation. The magnitude of the LTD4-induced increase in calcium was modest compared with capsaicin, although it was similar to other Gq coupled receptor-mediated calcium increases reported in similar neurones (Nicolson et al., 2002; Taylor-Clark et al., 2005). LTD4-induced responses were near maximal at 100 and 500 nM, which is consistent with LTD4 activation of CysLT1 receptors (Sarau et al., 1999).
The trigeminal ganglion not only contains neurones that innervate the nasal mucosa but also neurones that innervate a wide range of tissues, both visceral and somatosensory, throughout the head. LTD4 activated approximately 20% of guinea-pig trigeminal capsaicin-sensitive neurones (represents approximately 4–5% of the total trigeminal neurones), a figure comparable with the 23% of guinea-pig trigeminal capsaicin-sensitive neurones that responded to histamine (Taylor-Clark et al., 2005). Given that nasal-specific capsaicin-sensitive neurones account for approximately 2% of the total trigeminal population (Taylor-Clark et al., 2005), it is probable that non-nasal trigeminal neurones are also influenced by LTD4.
Increases in cytosolic calcium, as measured in the Fura-2AM calcium assay, do not necessarily indicate changes in neuronal electrical activity (for example, action potential initiation). Such activity/excitability can be assessed in whole-cell patch-clamp experiments. In our patch-clamp studies of nasal-specific guinea-pig trigeminal neurones, capsaicin activated a high percentage (∼90%) of nasal trigeminal neurones, which is consistent with our RT-PCR data and our previous studies (Taylor-Clark et al., 2005), indicating that the capsaicin receptor TRPV1 is present on a much higher percentage of trigeminal neurones innervating the nasal mucosa compared with the overall trigeminal sensory nerve population (20–30%). Capsaicin-sensitive and capsaicin-insensitive dissociated nasal-specific neurones likely correspond to nociceptive and non-nociceptive nerve fibres in the nasal mucosa (Taylor-Clark et al., 2005). Interestingly, LTD4 increased the excitability of nearly all capsaicin-sensitive neurones, while having no effect on the excitability of capsaicin-insensitive neurones. The increase in excitability was observed as an increase in action potential discharge in response to current injections. It can be anticipated that this type of LTD4-induced nonselective increase in sensory neuroexcitability would cause a significant increase in sensory input from nasal nociceptive fibres in vivo in response to any stimuli capable of inducing depolarizing currents. In support of this, we found that LTD4 caused a threefold increase in histamine-induced depolarization, action potential discharge number and frequency and a fivefold increase in histamine-induced inward current.
Cysteinyl leukotrienes activate both CysLT1 and CysLT2 receptors in the nanomolar range (Brink et al., 2003). The finding that two structurally unrelated CysLT1 receptor antagonists, montelukast and ICI198615 (similar in structure to zafirlukast), blocked the LTD4-induced responses demonstrate that, consistent with the single-cell RT-PCR data, the receptor involved is definitely not CysLT2 and is likely CysLT1. Recently, a deorphanized receptor, GPR17, has also been shown to be activated by nanomolar concentrations of cysteinyl leukotrienes (Ciana et al., 2006). At the present time, it is difficult to pharmacologically distinguish CysLT1 from GPR17. Montelukast has been shown to antagonize the actions of cysteinyl leukotrienes at GPR17 in a range similar to its antagonism at CysLT1 receptors (Ciana et al., 2006). The affinity of ICI198615 for GPR17 is unknown as is the guinea-pig gene for GPR17. The LTD4/LTC4 potency ratio, however, may provide some insight regarding this issue. LTD4 was more potent than LTC4 in increasing cytosolic calcium in trigeminal capsaicin-sensitive neurones. This is consistent with CysLT1 receptors where LTD4 is approximately 10 times more potent than LTC4 (Sarau et al., 1999), but inconsistent with GPR17 receptors where the limited information available indicates that LTC4 is 10 times more potent than LTD4 (Ciana et al., 2006). More definitive support for or against our hypothesis that the CysLT1 receptor is responsible for increasing the excitability of guinea-pig nasal trigeminal neurones must await the development of more selective pharmacological tools.
The mechanism by which LTD4 increased the response of the nasal nociceptive neurones to histamine has not been worked out. LTD4 increased the neurones' response to electrical current injection as well as to histamine. It is clear, therefore, that the effect extends beyond an interaction specifically at the histamine H1 receptor. LTD4 did not evoke a depolarization (or an inward current) of the nociceptor membrane. This means that, by contrast to histamine and capsaicin, LTD4, although a sensory neuromodulator, is not a direct activator of the neurones. All nasal neurones that were modulated by LTD4 were also capsaicin-sensitive (that is, expressed TRPV1 channels). A direct role of TRPV1 in the LTD4-induced hyperexcitability is unlikely as although some lipoxygenase products (such as 12-(S)-HPETE and LTB4) have been shown to gate TRPV1 channels, cysteinyl leukotrienes are not able to do so (Hwang et al., 2000). Neuronal hyperexcitability can be induced by an increase in membrane resistance, often the result of the closing of certain potassium channels (or leak currents). This increase in resistance would lead to a greater depolarization for a given activating stimulus (for example, injected current), thus increasing action potential discharge. However, data presented here suggest that LTD4 did not alter the input resistance of nasal neurones. Instead, LTD4 increased the rate of the action potential upstroke, potentially permitting a faster frequency of action potential discharge. Although we have not determined the molecular identity of the channels taking part in the action potential that have been modulated by LTD4, it is likely to be some form of voltage-gated sodium channel. Both TTX-sensitive and TTX-resistant currents have been detected in nasal neurones (Damann et al., 2006) and further studies are required to confirm the specific target(s) of LTD4-induced neuromodulation and their control by pathways downstream of CysLT1 activation (for example, phospholipase C and PKC).
A modulation of voltage-gated ion channel function is unlikely to account, however, for the augmentation of the histamine-induced inward current as seen in voltage clamp experiments. All histamine receptors are G protein-coupled, thus histamine can only evoke an inward current in nasal capsaicin-sensitive neurones by gating an ion channel downstream of histamine receptor activation. It is likely that LTD4 augments histamine-induced inward current by modulating this (as yet) unknown mechanism.
Cysteinyl leukotrienes have been shown to increase the airways response to histamine in asthmatics (Arm et al., 1988). The present data suggest that this could be secondary to a leukotriene-induced increase in the sensory neuro-excitability such that the histamine-induced reflex bronchoconstriction is increased. Although this study focused on nasal nociceptors, previous studies have shown that airway vagal sensory neurones are also modulated by CysLT1 receptors (McAlexander et al., 1998).
Clinical trials have demonstrated no ‘neuronal' responses to LTD4 nasal challenge (for example, sneezing, itching, reflex hypersecretion) (Bisgaard et al., 1986), whereas allergen-induced ‘neuronal' responses have been reduced with CysLT1 antagonists (Donnelly et al., 1995; Philip et al., 2002; Chervinsky et al., 2004; Patel et al., 2005; Virchow and Bachert, 2006). These clinical pharmacological findings are consistent with a sensitizing action of LTD4. On the basis of our results, LTD4 would not be expected to initiate nerve activity on its own. However, in the presence of other neural activating stimuli (for example, temperature changes, air-born irritants, anosmotic solutions and allergy-associated mediators such as histamine and bradykinin) LTD4 would be expected to augment the nerve activity and reflex responses.
In conclusion, we present new evidence that LTD4 is able to increase the excitability of nociceptor-like neurones, likely through a CysLT1-dependent mechanism. It is possible that cysteinyl leukotrienes, when present in the nasal mucosa, may contribute to nerve activity in nasal diseases and so contribute to symptom generation.
Acknowledgments
This study was supported in part by the National Institutes of Health (Bethesda, MD, USA), and by a research grant from the Investigator-Initiated Studies Program of Merck & Co. Inc. (West Point, PA, USA). The opinions in this paper are those of the authors and do not necessarily represent those of Merck & Co.
Abbreviations
- DiI
DiC18(3)
- LT
leukotriene
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels, 2nd edition (2007 Revision) Br J Pharmacol. 2007;150 Suppl 1:S1. doi: 10.1038/sj.bjp.0707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton F, Herdegen T, Peppel P, Leah JD. c-FOS-like immunoreactivity in rat brainstem neurons following noxious chemical stimulation of the nasal mucosa. Neuroscience. 1991;41:629–641. doi: 10.1016/0306-4522(91)90355-r. [DOI] [PubMed] [Google Scholar]
- Arm JP, Spur BW, Lee TH. The effects of inhaled leukotriene E4 on the airway responsiveness to histamine in subjects with asthma and normal subjects. J Allergy Clin Immunol. 1988;82:654–660. doi: 10.1016/0091-6749(88)90979-7. [DOI] [PubMed] [Google Scholar]
- Batsel HL, Lines AJ. Neural mechanisms of sneeze. Am J Physiol. 1975;229:770–776. doi: 10.1152/ajplegacy.1975.229.3.770. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Olsson P, Bende M. Effect of leukotriene D4 on nasal mucosal blood flow, nasal airway resistance and nasal secretion in humans. Clin Allergy. 1986;16:289–297. doi: 10.1111/j.1365-2222.1986.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, et al. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- Canning BJ. Neurology of allergic inflammation and rhinitis. Curr Allergy Asthma Rep. 2002;2:210–215. doi: 10.1007/s11882-002-0021-2. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8:291–301. doi: 10.1046/j.1440-1843.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- Chervinsky P, Philip G, Malice MP, Bardelas J, Nayak A, Marchal JL, et al. Montelukast for treating fall allergic rhinitis: effect of pollen exposure in 3 studies. Ann Allergy Asthma Immunol. 2004;92:367–373. doi: 10.1016/S1081-1206(10)61576-1. [DOI] [PubMed] [Google Scholar]
- Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damann N, Rothermel M, Klupp BG, Mettenleiter TC, Hatt H, Wetzel CH. Chemosensory properties of murine nasal and cutaneous trigeminal neurons identified by viral tracing. BMC Neurosci. 2006;7:46. doi: 10.1186/1471-2202-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly AL, Glass M, Minkwitz MC, Casale TB. The leukotriene D4-receptor antagonist, ICI 204,219, relieves symptoms of acute seasonal allergic rhinitis. Am J Respir Crit Care Med. 1995;151:1734–1739. doi: 10.1164/ajrccm.151.6.7767514. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hisamatsu K, Ganbo T, Nakazawa T, Nakajima M, Goto R, Murakami Y. Effect of leukotriene C4 exposure on ciliated cells of the nasal mucosa. Prostaglandins. 1996;51:69–79. doi: 10.1016/0090-6980(95)00156-5. [DOI] [PubMed] [Google Scholar]
- Howarth PH. Leukotrienes in rhinitis. Am J Respir Crit Care Med. 2000;161:S133–S136. doi: 10.1164/ajrccm.161.supplement_1.ltta-26. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno A, Nagata H, Nomoto M, Motosugi H, Terada N. Role of capsaicin-sensitive trigeminal nerves in development of hyperreactive nasal symptoms in guinea pig model of nasal allergy. Ann Otol Rhinol Laryngol. 1995;104:730–735. doi: 10.1177/000348949510400912. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull. 1993;30:239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- Lucier GE, Egizii R. Characterization of cat nasal afferents and brain stem neurones receiving ethmoidal input. Exp Neurol. 1989;103:83–89. doi: 10.1016/0014-4886(89)90189-1. [DOI] [PubMed] [Google Scholar]
- MacGlashan D., Jr Single-cell analysis of Ca++ changes in human lung mast cells: graded vs. all-or-nothing elevations after IgE-mediated stimulation. J Cell Biol. 1989;109:123–134. doi: 10.1083/jcb.109.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlexander MA, Myers AC, Undem BJ. Inhibition of 5-lipoxygenase diminishes neurally evoked tachykinergic contraction of guinea pig isolated airway. J Pharmacol Exp Ther. 1998;285:602–607. [PubMed] [Google Scholar]
- Miadonna A, Tedeschi A, Leggieri E, Lorini M, Folco G, Sala A, et al. Behavior and clinical relevance of histamine and leukotrienes C4 and B4 in grass pollen-induced rhinitis. Am Rev Respir Dis. 1987;136:357–362. doi: 10.1164/ajrccm/136.2.357. [DOI] [PubMed] [Google Scholar]
- Namimatsu A, Go K, Tanimoto H, Okuda M. Mechanism of nasal secretion mediated via nerve reflex in guinea pigs and evaluation of antiallergic drugs. Int Arch Allergy Immunol. 1992;97:139–145. doi: 10.1159/000236109. [DOI] [PubMed] [Google Scholar]
- Nicolson TA, Bevan S, Richards CD. Characterisation of the calcium responses to histamine in capsaicin-sensitive and capsaicin-insensitive sensory neurones. Neuroscience. 2002;110:329–338. doi: 10.1016/s0306-4522(01)00561-9. [DOI] [PubMed] [Google Scholar]
- Okuda M, Watase T, Mezawa A, Liu CM. The role of leukotriene D4 in allergic rhinitis. Ann Allergy. 1988;60:537–540. [PubMed] [Google Scholar]
- Orange RP, Austen KF. Slow reacting substance of anaphylaxis. Adv Immunol. 1969;10:105–144. doi: 10.1016/s0065-2776(08)60416-2. [DOI] [PubMed] [Google Scholar]
- Patel P, Philip G, Yang W, Call R, Horak F, LaForce C, et al. Randomized, double-blind, placebo-controlled study of montelukast for treating perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95:551–557. doi: 10.1016/S1081-1206(10)61018-6. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Henderson WR., JrThe role of leukotrienes in allergic rhinitis Ann Allergy Asthma Immunol 200594609–618.quiz 618–20, 669 [DOI] [PubMed] [Google Scholar]
- Philip G, Malmstrom K, Hampel FC, Weinstein SF, LaForce CF, Ratner PH, et al. Montelukast for treating seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial performed in the spring. Clin Exp Allergy. 2002;32:1020–1028. doi: 10.1046/j.1365-2222.2002.01422.x. [DOI] [PubMed] [Google Scholar]
- Richardson PS, Peatfield AC. Reflexes concerned in the defence of the lungs. Bull Eur Physiopathol Respir. 1981;17:979–1012. [PubMed] [Google Scholar]
- Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ, et al. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol Pharmacol. 1999;56:657–663. doi: 10.1124/mol.56.3.657. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Ebersberger A, Peppel P, Beck U, Messlinger K. Release of immunoreactive substance P in the trigeminal brain stem nuclear complex evoked by chemical stimulation of the nasal mucosa and the dura mater encephali—a study with antibody microprobes. Neuroscience. 1997;76:273–284. doi: 10.1016/s0306-4522(96)00353-3. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Silver WL, Farley LG, Finger TE. The effects of neonatal capsaicin administration on trigeminal nerve chemoreceptors in the rat nasal cavity. Brain Res. 1991;561:212–216. doi: 10.1016/0006-8993(91)91597-t. [DOI] [PubMed] [Google Scholar]
- Steinke JW, Borish L. Leukotriene receptors in rhinitis and sinusitis. Curr Allergy Asthma Rep. 2004;4:217–223. doi: 10.1007/s11882-004-0029-x. [DOI] [PubMed] [Google Scholar]
- Stjarne P, Lundblad L, Lundberg JM, Anggard A. Capsaicin and nicotine-sensitive afferent neurones and nasal secretion in healthy human volunteers and in patients with vasomotor rhinitis. Br J Pharmacol. 1989;96:693–701. doi: 10.1111/j.1476-5381.1989.tb11870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjarne P, Rinder J, Heden-Blomquist E, Cardell LO, Lundberg J, Zetterstrom O, et al. Capsaicin desensitization of the nasal mucosa reduces symptoms upon allergen challenge in patients with allergic rhinitis. Acta Otolaryngol. 1998;118:235–239. doi: 10.1080/00016489850154955. [DOI] [PubMed] [Google Scholar]
- Tai CF, Baraniuk JN. A tale of two neurons in the upper airways: pain versus itch. Curr Allergy Asthma Rep. 2003;3:215–220. doi: 10.1007/s11882-003-0042-5. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark T, Foreman J. Histamine-mediated mechanisms in the human nasal airway. Curr Opin Pharmacol. 2005;5:214–220. doi: 10.1016/j.coph.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark T, Undem BJ. Transduction mechanisms in airway sensory nerves. J Appl Physiol. 2006;101:950–959. doi: 10.1152/japplphysiol.00222.2006. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Kollarik M, MacGlashan DW, Jr, Undem BJ. Nasal sensory nerve populations responding to histamine and capsaicin. J Allergy Clin Immunol. 2005;116:1282–1288. doi: 10.1016/j.jaci.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Terada N, Ando H, Ito E, Sugiyama T, Yoshino Y, Togawa K, et al. [Nasal allergy and leukotriene. 2. Kinetics of peptide leukotrienes and inflammatory cells in nasal lavage fluid after antigen challenge] Nippon Jibiinkoka Gakkai Kaiho. 1989;92:1337–1344. doi: 10.3950/jibiinkoka.92.1337. [DOI] [PubMed] [Google Scholar]
- Virchow JC, Bachert C. Efficacy and safety of montelukast in adults with asthma and allergic rhinitis. Respir Med. 2006;100:1952–1959. doi: 10.1016/j.rmed.2006.02.026. [DOI] [PubMed] [Google Scholar]