Abstract
Background and purpose:
We have recently shown that the phytocannabinoid Δ9-tetrahydrocannabivarin (Δ9-THCV) and the CB1 receptor antagonist AM251 increase inhibitory neurotransmission in mouse cerebellum and also exhibit anticonvulsant activity in a rat piriform cortical (PC) model of epilepsy. Possible mechanisms underlying cannabinoid actions in the CNS include CB1 receptor antagonism (by displacing endocannabinergic tone) or inverse agonism at constitutively active CB1 receptors. Here, we investigate the mode of cannabinoid action in [35S]GTPγS binding assays.
Experimental approach:
Effects of Δ9-THCV and AM251 were tested either alone or against WIN55,212-2-induced increases in [35S]GTPγS binding in mouse cerebellar and PC membranes. Effects on non-CB receptor expressing CHO-D2 cell membranes were also investigated.
Key results:
Δ9-THCV and AM251 both acted as potent antagonists of WIN55,212-2-induced increases in [35S]GTPγS binding in cerebellar and PC membranes (Δ9-THCV: pA2=7.62 and 7.44 respectively; AM251: pA2=9.93 and 9.88 respectively). At micromolar concentrations, Δ9-THCV or AM251 alone caused significant decreases in [35S]GTPγS binding; Δ9-THCV caused larger decreases than AM251. When applied alone in CHO-D2 membranes, Δ9-THCV and AM251 also caused concentration-related decreases in G protein activity.
Conclusions and implications:
Δ9-THCV and AM251 act as CB1 receptors antagonists in the cerebellum and PC, with AM251 being more potent than Δ9-THCV in both brain regions. Individually, Δ9-THCV or AM251 exhibited similar potency at CB1 receptors in the cerebellum and the PC. At micromolar concentrations, Δ9-THCV and AM251 caused a non-CB receptor-mediated depression of basal [35S]GTPγS binding.
Keywords: Δ9-tetrahydrocannabivarin; WIN55,212-2; AM251; CB1 receptor antagonist; mouse cerebellum; mouse piriform cortex
Introduction
The cannabinoid G protein-coupled receptors CB1 and CB2 are targets for endocannabinoids, exogenous synthetic compounds and phytocannabinoids derived from Cannabis sativa (Howlett et al., 2002). Plant-derived Δ9-tetrahydrocannabivarin (Δ9-THCV) is the propyl analogue of the CB1 receptor partial agonist Δ9-tetrahydrocannabinol. However, Δ9-THCV receptor pharmacology is not yet fully defined, with diverse tissue- and ligand-dependent actions and, importantly, concentration-dependent agonist and antagonist effects (Pertwee, 2008). We have recently conducted the first in vitro electrophysiological study investigating the functional effects of Δ9-THCV in the CNS. Δ9-THCV and the selective CB1 antagonist N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-multipyrazole-3-carboxamide (AM251) increased inhibitory neurotransmission between interneurones and Purkinje cells in the mouse cerebellum (Ma et al., 2008). In addition, Δ9-THCV and AM251 both exhibited anticonvulsant activity in an Mg2+-free rat piriform cortical (PC) brain slice model of epilepsy (Weston et al., 2006). These data showed that Δ9-THCV and AM251 acted in the opposite direction to the CB receptor agonist (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate (WIN55,212-2), which suggests a mechanism by which CB1 receptor antagonists act either via blockade of endocannabinergic tone or by inverse agonism at constitutively active CB1 receptors. CB1 receptor antagonists have been shown to reduce basal [35S]guanosine-5′-O-(3-thiotriphosphate) ([35S]GTPγS) binding with high potency (EC50 1–5 nM) in recombinant expression systems (Landsman et al., 1997; MacLennan et al., 1998), also supporting inverse agonist properties. In contrast, Savinainen et al. (2003) reported that rimonabant and AM251 exhibit no CB1 receptor inverse agonism in [35S]GTPγS binding assays in cerebellar membranes; this group instead propose that suppression of basal G protein activity by these compounds is due to blockade of adenosine A1 receptors.
Δ9-THCV has recently been reported to act as a CB1 and CB2 receptor antagonist in [35S]GTPγS binding assays in whole mouse brain membranes and recombinant cells respectively (Thomas et al., 2005; Pertwee et al., 2007). However, differences in CB receptor/G protein coupling between distinct brain regions have been reported (Breivogel et al., 1997). Moreover, specific differences in CB receptors between cerebellum and cortex have been demonstrated; cerebellar membranes from CB1 receptor knockout mice (cnr1−/−) lacked significant cannabinoid binding, whereas cortical membranes retained significant binding and G protein turnover under the same conditions (Breivogel et al., 1997). Recent functional studies have shown that Δ9-THCV and its synthetic analogue O-4394 both behaved as antagonists in mouse-isolated vas deferens and also in antinociceptive and hypothermia tests in vivo (Pertwee et al., 2007). In contrast, micromolar concentrations of Δ9-THCV inhibited electrically evoked responses in vas deferens, reportedly by a non-CB1 receptor-mediated mechanism (Thomas et al., 2005).
Here, we sought to resolve these issues and to extend our electrophysiological studies by determining the concentration dependency of the effects of cannabinoids in the cerebellum and PC, the two distinct brain regions that exhibit high CB1 receptor expression (Herkenham et al., 1991; Glass et al., 1997; Tsou et al., 1998). Using [35S]GTPγS binding assays, we show that Δ9-THCV and AM251 act as highly potent CB1 receptor antagonists in mouse cerebellum and PC. At concentrations ⩾10 μM, Δ9-THCV and AM251 cause non-CB1 receptor-mediated decreases in G protein turnover by an, as yet, unknown mechanism.
Methods
Membrane preparation
Mice were humanely killed by cervical dislocation and decapitated in line with UK Home Office procedures (Animals (Scientific Procedures) Act 1986) and associated guidelines for the humane use of experimental animals. Cerebellar and PC tissue was dissected from the brains of male TO mice (3–5 weeks old, 10–20 g) and stored separately at −80 °C until use. Tissue from each region was suspended in a membrane buffer (containing (in mM) Tris-HCl 50, MgCl2 5, EDTA 2 and 0.5 mg mL−1 fatty acid-free BSA and Complete protease inhibitor (Roche, Mannheim, Germany); pH 7.4) and was then homogenized using an Ultra-Turrax blender (Labo Moderne, Paris, France). Homogenates were centrifuged at 1000 g for 10 min and supernatants decanted and retained. Resulting pellets were re-homogenized and centrifugation was repeated as before. Combined supernatants were then centrifuged at 39 000 g for 30 min in a high-speed Sorvall centrifuge and remaining pellets resuspended in membrane buffer and protein content determined by the Lowry method (Lowry et al., 1951). All procedures were carried out on ice and all centrifugations performed at 4 °C. Membranes derived from Chinese hamster ovary (CHO) cells transfected to express the human dopamine D2short receptor (CHO-D2 cells) were prepared as previously described (Wilson et al., 2001).
[35S]GTPγS binding assays
Assays were carried out in triplicate on a minimum of three separate occasions in assay buffer containing (in mM) HEPES 20, MgCl2 3, NaCl 60, EGTA 1 and 0.5 mg mL−1 fatty acid-free BSA; pH 7.4. All stock solutions of drugs and membrane preparations were diluted in assay buffer immediately prior to use and stored on ice prior to incubation. Assay tubes contained a final volume of 1 mL and guanosine 5′-diphosphate (GDP) at a final concentration of 10 μM, together with either drugs at the desired final concentration, vehicle at an equivalent concentration or additional assay buffer to determine basal binding. Assays were initiated by addition of 10 μg membrane protein from cerebellum or PC (or 20 μg membrane protein from CHO-D2 cells). Assays were incubated for 30 min at 30 °C prior to addition of [35S]GTPγS to a final concentration of 0.1 nM. Assays were terminated after a further 30-min incubation at 30 °C by rapid filtration through Whatman GF/C filters using a Brandell cell harvester, followed by three washes with ice-cold phosphate-buffered saline to remove unbound radioactivity. Filters were incubated for 2 h in 2 mL scintillation fluid, and radioactivity was quantified by liquid scintillation spectrometry. In further studies investigating the effects of the adenosine A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) on cannabinoid action, membrane suspensions were incubated for 30 min at 30 °C with or without DPCPX (1 μM) and returned to ice prior to use.
Data analysis and statistical procedures
Data and statistical analyses were performed using GraphPad Prism v4.03 (GraphPad San Diego, CA, USA). Concentration–response data were analysed using a sigmoidal concentration–response model or linear regression and compared using an F-test to select the appropriate model. On this basis, best fits to sigmoidal curves were obtained with Hill slopes of unity and no other constraints (that is at the top or bottom of curves) were applied. For curves showing no clear concentration-related increases, a linear regression was performed to determine if slopes significantly differed from zero. [35S]GTPγS binding was expressed as percentage increase in radioactivity (measured as d.p.m.) in the presence of drugs relative to basal levels of binding according to
Here, we define basal d.p.m. as the radioactivity measured in conditions of no agonist stimulation, in the presence of 10 μM GDP (established by determining a GDP dependency curve for [GDP] 10 pM–100 μM in triplicate on four separate occasions for each membrane preparation) and 0.1 nM GTPγS. In our experiments, basal binding was 3711±194 d.p.m. (n=22) in cerebellum and 5497±353 d.p.m. (n=22) in PC. In the presence of 10 000-fold excess of cold GTPγS, nonspecific binding was 726±67 d.p.m. (n=9) in cerebellum and 892±69 d.p.m. (n=9) in PC.
Values for EC50 and log (dose ratio=1) for Schild analyses were derived from fitted curves to mean data; Schild plots were analysed using linear regression, and pA2 (negative logarithm of the concentration of antagonist causing a dose ratio=2) values determined. Data for Δ9-THCV and AM251 with and without DPCPX were analysed using a non-parametric Mann–Whitney U-test. All data presented are means and s.e.mean from a minimum of three independent experiments.
Drugs and chemicals
The following agents were used: WIN55,212-2, AM251, DPCPX, adenosine-5-N-ethylcarboxamide (NECA) (Tocris, Bristol, UK); dopamine hydrochloride (Sigma-Aldrich, Poole, UK); Complete mini EDTA-free protease inhibitor cocktail tablets (Roche, Mannaheim, Germany); [35S]GTPγS (GE Healthcare, Amersham, UK); GDP (ICN Biomedicals, Hampshire, UK); Ultima Gold scintillation fluid (Perkin Elmer, Cambridge, UK); all other reagents were obtained from Fisher Scientific, Loughborough, UK. Δ9-THCV was generously supplied by GW Pharmaceuticals (Porton Down, UK). CHO-D2 cell membrane preparations were generously provided by Dr E Kara and Professor P Strange (University of Reading). WIN55,212-2, AM251 and NECA were dissolved in dimethyl sulphoxide, and DPCPX in ethanol and stored at −20 °C prior to use. Dimethyl sulphoxide and ethanol were present at a maximum final concentration of 0.1%; solvent, applied alone at equivalent experimental concentrations, had no effect on [35S]GTPγS binding (for example, Figures 3a and b). Δ9-THCV was supplied as a 63 mM stock solution in ethanol and stored at 4 °C prior to use. Dopamine was diluted in assay buffer containing dithiothreitol to a final concentration of 0.1 mM immediately prior to use, to prevent oxidation of dopamine.
Results
Effects of cannabinoids on [35S]GTPγS binding in cerebellar and PC membranes
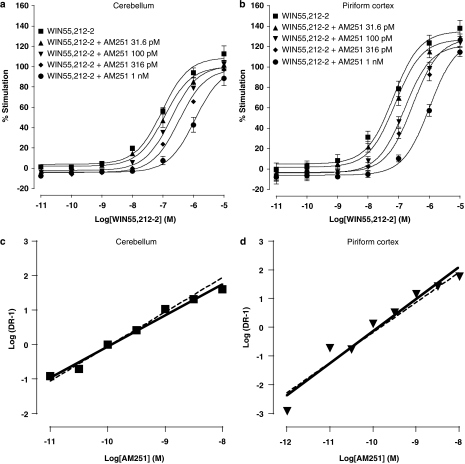
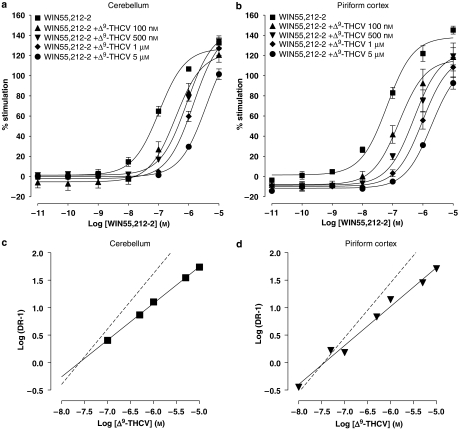
The effects of the synthetic CB1 receptor antagonist AM251 and the phytocannabinoid Δ9-THCV on agonist-induced percentage stimulation of [35S]GTPγS binding were compared in mouse cerebellar and PC membranes. Basal GTPγS binding differed between cerebellar (3711±194 d.p.m.) and PC (5497±353 d.p.m.) membranes (P<0.001; n=22). We first confirmed the presence of functional CB receptors in the distinct brain regions. Accordingly, WIN55,212-2 (10 pM–10 μM) caused an increase in percentage stimulation of [35S]GTPγS binding in cerebellar and PC mouse brain membranes (Figures 1a, b, 2a, b and 3a, b) with an EC50 of 62 nM (n=8) and 96 nM (n=6) respectively. WIN55,212-2 agonist effects in both cerebellar and PC membranes were antagonized by the standard CB1 antagonist AM251 (1 pM–10 nM, Figures 1a and b) and Δ9-THCV (100 nM–1 μM, Figures 2a and b). Mean group data were subsequently used to perform Schild analyses (Figure 1c and d; 2c and d), and the values derived are shown in Table 1. From these data, it is clear that AM251 and Δ9-THCV both exhibit potent antagonism of WIN55,212-2 in cerebellar and PC membranes. In both brain regions tested, AM251 was more than 200-fold more potent as an antagonist than Δ9-THCV.
Figure 1.
Log concentration–response curves for WIN55,212-2 (10 pM–10 μM) effects on percentage stimulation of [35S]GTPγS binding in the presence of AM251 (31.6 pM–1 nM) in (a) cerebellar (n=4 separate experiments) and (b) PC (n=3 separate experiments) membranes. Plot symbols show mean percentage increase in [35S]GTPγS binding±s.e.mean (n=minimum three trials per agonist concentration used). Note the progressive rightward shifts in WIN55,212-2 concentration–response curves in both cerebellar and PC membranes induced by increasing concentrations of AM251, consistent with an antagonistic effect. Schild plots were subsequently constructed for antagonism of WIN55,212-2 by AM251 in (c) cerebellar and (d) PC membranes, yielding slope and pA2 values given in Table 1. Slopes of unity (dotted lines) are shown for reference. AM251, N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-multipyrazole-3-carboxamide; PC, piriform cortex; [35S]GTPγS, [35S]guanosine-5′-O-(3-thiotriphosphate); WIN55,212-2, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate.
Figure 2.
Log concentration–response curves for WIN55,212-2 (10 pM–10 μM) effects on percentage stimulation of [35S]GTPγS binding in the presence of Δ9-THCV (100 nM–5 μM) in (a) cerebellar and (b) PC membranes (both n=3). Plot symbols show mean percentage increase in [35S]GTPγS binding±s.e.mean (n=minimum three trials per agonist concentration used). Note the progressive rightward shifts in WIN55,212-2 concentration–response curves in both cerebellar and PC membranes induced by increasing concentrations of Δ9-THCV, consistent with an antagonistic effect. Schild plots were subsequently constructed for antagonism of WIN55,212-2 by Δ9-THCV in (c) cerebellar and (d) PC membranes, yielding slope and pA2 values given in Table 1. Slopes of unity (dotted lines) are shown for reference. PC, piriform cortex; WIN55,212-2, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate; Δ9-THCV, Δ9-tetrahydrocannabivarin.
Figure 3.
Log concentration–response curves for WIN55,212-2 (10 pM–10 μM), AM251 and Δ9-THCV (10 pM–100 μM for both) effects on percentage stimulation of [35S]GTPγS binding in (a) cerebellar (WIN55,212-2, n=8; AM251, n=6 and Δ9-THCV, n=7) and (b) PC (WIN55,212-2, n=6; AM251, n=7 and Δ9-THCV, n=6) membranes. AM251, N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-multipyrazole-3-carboxamide; PC, piriform cortex; [35S]GTPγS, [35S]guanosine-5′-O-(3-thiotriphosphate); WIN55,212-2, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate; Δ9-THCV, Δ9-tetrahydrocannabivarin.
Table 1.
Schild analysis values for WIN55,212-2 vs AM251 and vs Δ9-THCV in mouse cerebellum and piriform cortex (PC)
|
AM251 |
Δ9-THCV |
|||
|---|---|---|---|---|
| Cerebellum | PC | Cerebellum | PC | |
| Slope | 0.90 | 1.05 | 0.67 | 0.71 |
| pA2 | 9.93 | 9.88 | 7.62 | 7.44 |
Abbreviations: AM251, N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-multipyrazole-3-carboxamide; PC, piriform cortex; WIN55,212-2, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate; Δ9-THCV, Δ9-tetrahydrocannabivarin.
The analysis was performed using mean dose ratios from n=4 (cerebellum) and n=3 (PC) experiments. The slope of Schild plots for AM251 approximates to unity, suggesting competitive antagonism of WIN55,212-2 by AM251. The slope of Schild plots for Δ9-THCV does not approach unity implying other/additional mechanisms of action.
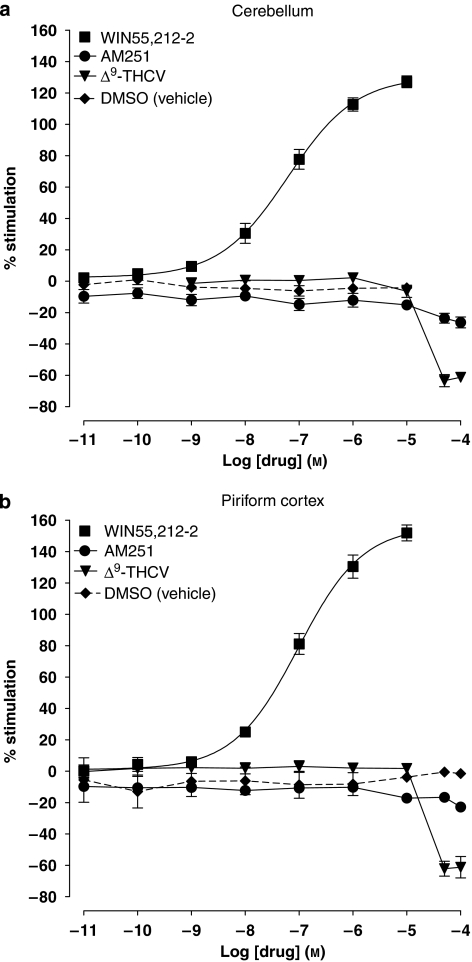
We next examined the effects of AM251 and Δ9-THCV alone on [35S]GTPγS binding to either cerebellar (Figure 3a) or PC membranes (Figure 3b). At concentrations below 10 μM, AM251 or Δ9-THCV alone showed no significant concentration-dependent effects on [35S]GTPγS binding to either cerebellar or PC membranes (determined by linear regression; slopes did not significantly deviate from zero). At concentrations above 10 μM, AM251 or Δ9-THCV caused a decrease in [35S]GTPγS binding. These decreases were significantly greater for Δ9-THCV than for AM251 in both cerebellar (P<0.001) and PC membranes (P<0.005). We examined the effect of these higher concentrations (≥10 μM) of AM251 or Δ9-THCV in greater detail, and concentration-related responses for percentage decreases in [35S]GTPγS binding are summarized as bar graphs for AM251 (Figure 4a) and Δ9-THCV (Figure 4b). The magnitude of the decrease in [35S]GTPγS binding for either AM251 or Δ9-THCV alone did not significantly differ between these two brain areas at all concentrations (AM251: P>0.05; Δ9-THCV: P>0.05; PC vs cerebellum).
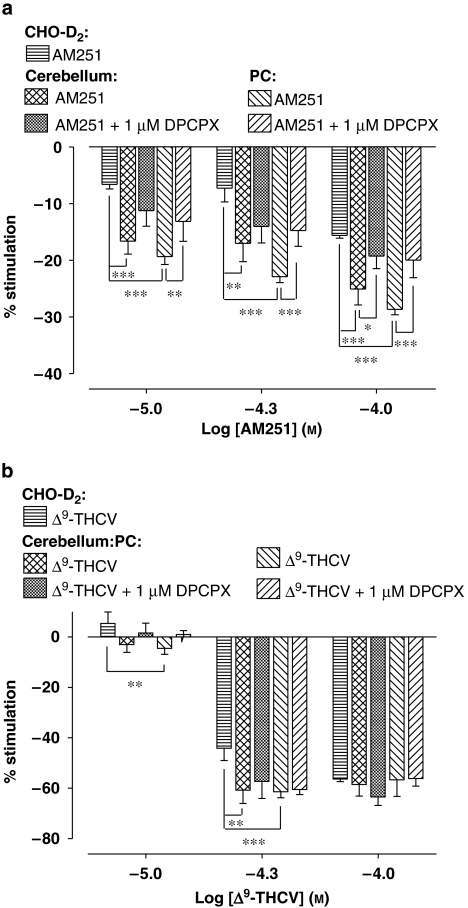
Figure 4.
Bar charts summarizing effects of (a) AM251 and (b) Δ9-THCV (10–100 μMfor both) on percentage stimulation of [35S]GTPγS binding in cerebellar, PC and CHO-D2 cell membranes in the absence or presence of the selective adenosine A1 antagonist, DPCPX (1 μM). AM251-induced depression of [35S]GTPγS binding was significantly less in CHO-D2 membranes vs PC and cerebellar membranes at all concentrations. Δ9-THCV-induced depression of [35S]GTPγS binding was significantly less in CHO-D2 membranes vs PC (10 and 50 μM) and cerebellar (50 μM only) membranes. At 100 μM Δ9-THCV, there were no significant differences in binding between CHO-D2, cerebellar and PC (P>0.05 for both) membranes. AM251-induced decreases in [35S]GTPγS binding were significantly attenuated by DPCPX in PC (all concentrations) and cerebellar (only at 100 μM) membranes. DPCPX had no significant effects on Δ9-THCV responses in each membrane preparation (b; P>0.2). Significance levels were tested using non-parametric Mann–Whitney U-tests and are shown as *P<0.05, **P<0.01 and ***P<0.001. Minimum n=3 for each experiment in each membrane preparation. AM251, N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-multipyrazole-3-carboxamide; DPCPX, 8-cyclopentyl-1,3-dipropylxanthine; PC, piriform cortex; Δ9-THCV, Δ9-tetrahydrocannabivarin.
AM251 has previously been proposed to block adenosine A1 receptors in cerebellar membranes (Savinainen et al., 2003). To investigate potential adenosine A1 receptor contributions to observed decreases in [35S]GTPγS binding, the effects of the selective adenosine A1 receptor antagonist DPCPX (1 μM final concentration) on concentration-related responses for AM251 and Δ9-THCV in cerebellar, PC and CHO-D2 cell membranes were investigated. As expected from a previous report by Savinainen et al. (2003), DPCPX alone inhibited basal [35S]GTPγS binding in the cerebellum (control=4010±502 d.p.m.; with 1 μM DPCPX=3360±426 d.p.m.; n=7; P<0.05). DPCPX also inhibited basal binding in PC (control=6170±720 d.p.m.; with 1 μM DPCPX=5378±667 d.p.m.; n=7; P<0.01), but not in CHO-D2 membranes (control=3942±218 d.p.m.; with 1 μM DPCPX=3815±223 d.p.m.; n=5; P=0.07). In these and other experiments, we controlled for any effects on basal binding by expressing results as percentage stimulation over basal levels. In cerebellar membranes, DPCPX caused a significant attenuation of AM251-induced decreases in [35S]GTPγS binding only at the highest AM251 concentration tested (100 μM) (Figure 4a). In PC membranes, DPCPX caused a significant attenuation of AM251-induced decreases in [35S]GTPγS binding at all concentrations tested (Figure 4a). Thus, effects of DPCPX were significantly more pronounced in PC than in cerebellar membranes, suggesting greater adenosine A1 receptor involvement in the PC. In contrast, DPCPX had no significant affect on Δ9-THCV-induced decreases in [35S]GTPγS binding in cerebellar or PC membranes (Figure 4b), suggesting that adenosine A1 receptors do not contribute to observed Δ9-THCV effects under these conditions.
Effects of cannabinoids on [35S]GTPγS binding in CHO-D2 membranes
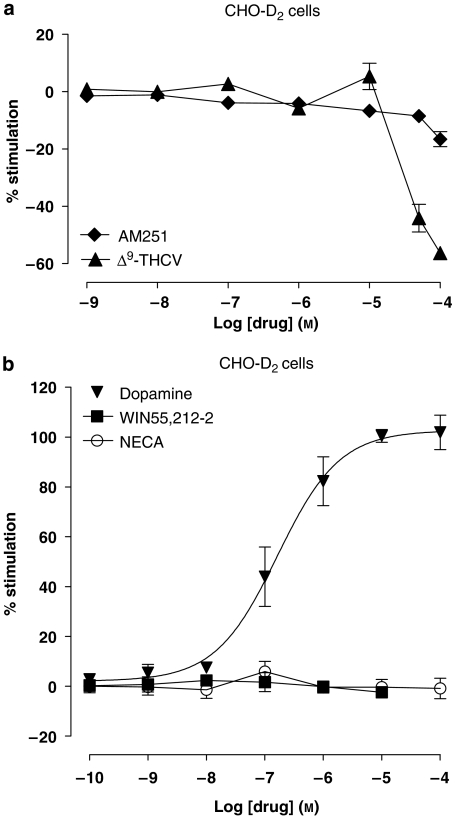
To further investigate the role of CB receptors in decreases of [35S]GTPγS binding caused by micromolar concentrations of AM251 and Δ9-THCV, concentration–response curves were also constructed for either AM251 or Δ9-THCV alone in membranes prepared from non-CB receptor-expressing CHO-D2 cells. AM251- and Δ9-THCV-induced decreases in [35S]GTPγS binding were still observed in CHO-D2 cell membranes at concentrations >10 μM (Figure 5a). AM251-induced decreases in [35S]GTPγS binding were significantly less in CHO-D2 membranes than in cerebellar and PC membranes at all concentrations tested (Figure 4a). These data suggest that a component of AM251-mediated decrease in [35S]GTPγS binding is present in cerebellar and PC, but not CHO-D2, membranes, and this component may correlate with the adenosine A1 receptor-mediated effects described above. No significant differences in binding between CHO-D2, cerebellar and PC membranes were seen at the highest Δ9-THCV concentration used (100 μM) (Figure 4b). At lower Δ9-THCV concentrations, some significant differences in [35S]GTPγS binding were seen. Δ9-THCV-induced decreases were significantly lower in CHO-D2 vs PC (at 10 and 50 μM) and CHO-D2 vs cerebellum (at 50 μM only) (Figure 4b). These data suggest that there may be a small additional component for low-micromolar Δ9-THCV-mediated effects on [35S]GTPγS binding in cerebellar and PC membranes compared to CHO-D2 membranes. This may be due to block of (as yet unidentified) G protein-coupled receptor(s) or may reflect inherent differences between brain-derived and cultured cell membranes. CHO-D2 cell membranes lacked responses to WIN55,212-2 (100 pM–10 μM) or to the mixed adenosine receptor agonist NECA (100 pM–100 μM) in concentration–response experiments (Figure 5b). Consequently, neither CB nor adenosine receptor-mediated events explain the observed AM251/Δ9-THCV-induced decreases in [35S]GTPγS binding seen in CHO-D2 cell membranes. Dopamine (100 pM–100 μM) caused clear concentration-related responses in CHO-D2 membranes (Figure 5b), confirming the presence of functional human dopamine D2short G protein-coupled receptors and the validity of the [35S]GTPγS binding assay in these membranes.
Figure 5.
Log concentration–response curves for (a) AM251 (1 nM–10 μM) and Δ9-THCV (1 nM–100 μM) on percentage stimulation of [35S]GTPγS binding in CHO-D2 cell membranes (both, n=3 separate experiments). (b) Agonist log concentration–response curves for dopamine (100 pM–100 μM), WIN55,212-2 (100 pM–10 μM) and NECA (100 pM–100 μM) effects on percentage stimulation of [35S]GTPγS binding in CHO-D2 cell membranes (all, n=3 separate experiments). Dopamine had an agonist concentration–response relationship at D2short receptors (EC50 value=164 nM; n=3); following linear regression, curves for WIN55,212-2 and NECA were found not to differ significantly from zero, indicating a lack of CB and adenosine receptor-mediated effects respectively. AM251, N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-multipyrazole-3-carboxamide; [35S]GTPγS, [35S]guanosine-5′-O-(3-thiotriphosphate); WIN55,212-2, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate; Δ9-THCV, Δ9-tetrahydrocannabivarin.
Overall, we demonstrate a concentration-dependent effect for Δ9-THCV and AM251 on [35S]GTPγS binding, in addition to potent CB1 receptor antagonist effects. Our data are consistent with micromolar concentrations of these compounds also exerting effects via a non-CB1 receptor mechanism in cerebellar and PC (and also CHO-D2) membranes. We further demonstrate that these findings cannot be fully explained by an action at adenosine A1 receptors.
Discussion and conclusion
Δ9-THCV and AM251 act as potent antagonists at CB1 receptors in cerebellum and PC
The present study shows that the phytocannabinoid Δ9-THCV and the biarylpyrazole compound AM251 act as potent CB1 receptor antagonists in [35S]GTPγS binding assays in mouse cerebellar and PC membranes. Schild plots, constructed using a range of antagonist concentrations with the CB receptor agonist WIN55,212-2, showed that AM251 was a more potent antagonist at CB1 receptors than Δ9-THCV in both cerebellar and PC membranes. Interestingly, although the slope of the Schild plot for AM251 approximated unity, this value was significantly lower for Δ9-THCV. AM251 action was consistent with surmountable, competitive antagonism; however, Δ9-THCV values may reflect a different mechanism of interaction between Δ9-THCV and CB1 receptors. A Schild plot slope of less than 1 is typically interpreted as a deviation from simple competitive antagonism, such as binding to more than one receptor or allosteric interactions. With regard to the former, Δ9-THCV has been reported to act also as a competitive CB2 receptor antagonist (Thomas et al., 2005). Moreover, CB2 receptor immunohistochemical labelling has recently been described in the cerebellar molecular layer (Ashton et al., 2006). However, we have observed that the selective CB2 agonist JWH-133 (10 μM) has no effect on inhibitory synaptic transmission in the mouse cerebellum (YL Ma and GJ Stephens, unpublished data), consistent with a lack of functionally coupled CB2 receptors in the cerebellar membrane preparation used here. CB1 receptors have also been suggested to possess allosteric binding sites, the occupation of which can modulate ligand affinity for orthosteric sites (Price et al., 2005). However, the effects of Δ9-THCV or AM251 action in such systems remain to be elucidated. Moreover, there is some suggestion of reductions in Emax (for example, in Figure 2b), consistent with potential allosteric effect in the PC. However, curves do not fully plateau at the concentration used (due to maximum dilutions of dimethyl sulphoxide not permitting the testing of higher concentrations), and thus experiments with more potent agonists are needed to fully investigate effects on Emax.
Δ9-THCV has a diverse receptor pharmacology (Pertwee, 2008). We confirmed that Δ9-THCV was a potent antagonist in both cerebellar and PC membranes. This is of importance as regional differences in CB receptor/G protein coupling within the CNS have been reported (Breivogel et al., 1997); moreover, differences in basal GTPγS binding between cerebellar and PC membranes were seen here. The pA2 values for Δ9-THCV derived in the present study (cerebellum: 7.62; PC: 7.44) compare reasonably well with reported data for Δ9-THCV and the synthetic analogue O-4394 in whole mouse brain membrane (apparent KB=82–93 nM and Ki=47–75 nM, Thomas et al., 2005; Pertwee et al., 2007). Potential differences may well reflect the use of membranes from specific brain regions here as Δ9-THCV is reported to display tissue-specific effects (Pertwee, 2008). Another potential confounder is GDP concentration (10 μM here vs 30 μM in Thomas et al., 2005), as increased GDP levels have been shown to lead to lower agonist potency in GTPγS binding assays (McLoughlin and Strange, 2000). In functional studies, Δ9-THCV was reported to be a more potent antagonist of agonist-stimulated contraction of the vas deferens, with apparent KB vs different agonists of 1.5–10 nM (Thomas et al., 2005); these experiments were performed at 37 °C in comparison to GTPγS binding studies at 30 °C (as in the present study). It may be that receptor populations reported here have some similarity with CB1 receptors in the vas deferens; however, WIN55,212-2 and CP55940 inhibited electrically evoked contractions of vas deferens not only via CB1 receptors, but also by activating non-CB1 targets (Thomas et al., 2005). Moreover, there may be important differences between peripheral and central CB receptor signalling, such as the tonic activity of the endocannabinoid system (for example, our electrophysiological experiments suggest a prominent endocannabinoid tone in the cerebellum (Ma et al., 2008)).
Effects of micromolar Δ9-THCV and AM251 concentrations on G protein turnover
Although our data suggest that Δ9-THCV and AM251 act as CB1 receptor antagonists, we would argue against inverse agonist effects and thus constitutive CB1 receptor activity. We found decreases in basal [35S]GTPγS binding for Δ9-THCV and AM251 at concentrations >10 μM; in particular, Δ9-THCV produced large depressions at higher concentrations. We propose that such decreases are mediated by CB1 receptor-independent mechanisms, as Δ9-THCV and AM251 also decreased [35S]GTPγS binding in CHO-D2 cell membranes. CHO-D2 membranes lacked responses to WIN55,212-2, confirming that CHO cell membranes do not express significant levels of CB receptors. The CHO-D2 cells used stably expressed the human dopamine D2short receptor, and agonist-stimulated G protein turnover in these cells was confirmed by dopamine action. There is some evidence for convergence in CB1 and D2 receptor signal transduction pathways (Meschler and Howlett, 2001); however, our data suggest that the prototypic CB receptor agonist WIN55 had no effect on GTPγS binding in CHO-D2 membranes, arguing against any cross talk in signalling pathways between CB and D2 receptors here. Decreases in [35S]GTPγS binding have been widely reported in studies using micromolar concentrations of the CB1 receptor antagonist rimonabant (Breivogel et al., 1998; Sim-Selley et al., 2001; Ooms et al., 2002), consistent with an inverse agonist action. Similarly, micromolar concentrations of the phytocannabinoid cannabidiol were recently reported to decrease [35S]GTPγS binding to mice whole brain membranes (Thomas et al., 2007). Cannabidiol-induced decreases in basal GTPγS binding were retained in cnr1−/− mice; in contrast, cannabidiol effects were no longer seen in untransfected CHO cell membranes (Thomas et al., 2007). It was proposed that cannabidiol inhibits GTP binding through one or more CB1 receptor-independent mechanisms. Such reports suggest diversity in phytocannabinoid receptor pharmacology (Pertwee, 2008). Moreover, in the study by Thomas et al. (2007), rimonabant-induced decreases in [35S]GTPγS binding were absent in cnr1−/− mice whole brain membranes. Interestingly, a previous study using cnr1−/− mice reported that cerebellar membranes lacked any significant [3H]WIN55,212-2 or [3H]rimonabant binding and WIN55,212-2-stimulated [35S]GTPγS binding, whereas cortical membranes retained significant binding and G protein turnover (Breivogel et al., 2001). The latter study is consistent with the presence of distinct forms of G protein-coupled CB receptors in mouse cerebellum and in cortex. It also substantiates previous work highlighting regional differences in CB receptor expression in the rodent brain (Breivogel et al., 1997), and it is possible that reported differences in cannabinoid effects in cnr1−/− mice are due to the use of whole brain vs specific region membrane preparations.
Our data suggest that Δ9-THCV has a differential, concentration-dependent effect on [35S]GTPγS binding, acting as a potent CB1 receptor antagonist, but also having non-CB1 effects at higher micromolar concentrations. The significance of these results is emphasized by recent reports demonstrating that Δ9-THCV has distinct, concentration-related functional effects. Thus, Δ9-THCV acted as a potent antagonist of agonist-induced inhibition of electrically evoked contractions in the vas deferens, conversely having an agonist action at micromolar concentrations (EC50=13 μM, Thomas et al., 2005). These responses were most likely mediated by a non-CB1 receptor. Moreover, Δ9-THCV acted as a CB1 antagonist in vivo (preventing agonist-induced hypothermia and antinociception) at doses <3 mg kg−1 i.v., while having agonist effects at higher (>10 mg kg−1) i.v. doses (Pertwee et al., 2007). The molecular basis of cannabinoid action at non-CB1 receptor targets requires further elucidation.
In addition to an antagonist action at CB1 receptors, AM251 has also been proposed to suppress basal G protein activity by blocking adenosine A1 receptors in cerebellar membranes (Savinainen et al., 2003). In the present study, AM251-induced decreases in G protein turnover were significantly attenuated by the adenosine A1 receptor antagonist DPCPX at all AM251 concentrations tested in PC membranes, but only at the highest AM251 concentration tested (100 μM) in cerebellar membranes. Hence, our data are consistent with an adenosine A1 receptor-mediated component of AM251 action that is more pronounced in PC than in cerebellar membranes. In marked contrast, Δ9-THCV-induced decreases in G protein turnover were not significantly affected by DPCPX, suggesting a lack of involvement of adenosine A1 receptors in Δ9-THCV-mediated responses. A recent study also reported that DPCPX did not affect cannabidiol-induced depression of [35S]GTPγS binding in mouse whole brain membranes (Thomas et al., 2007). A remaining possibility is that decreases in G protein activity caused by micromolar concentrations of cannabinoids may be due to direct membrane effects. For example, cannabinoids are highly lipophilic compounds and may partition into the lipid bilayer to alter membrane fluidity (Lawrence and Gill, 1975; Howlett et al., 1989; Bloom et al., 1997) and hence to affect behaviour mediated by bilayer-embedded proteins, such as G protein-coupled receptors.
Functional significance of Δ9-THCV and AM251 action in the cerebellum and PC
The present study complements our recent in vitro electrophysiological studies showing that Δ9-THCV and AM251 act on CB1 receptors at interneurone–Purkinje cell synapses to increase inhibitory GABA release (Ma et al., 2008) and, also, to exert anticonvulsive activity in a PC model of epilepsy (Weston et al., 2006). In experiments using brain slices, it is necessary to use relatively high drug concentrations to elicit measurable responses (due to factors including lipophilicity of cannabinoids discussed above); this makes comparisons between effective concentrations difficult. However, taken together with the [35S]GTPγS binding studies presented here, our electrophysiological data are consistent with a mechanism of action whereby Δ9-THCV (and AM251) acts as CB1 receptor antagonist to displace endocannabinergic-mediated inhibition of transmitter (GABA) release. In contrast, [35S]GTPγS binding data are not consistent with Δ9-THCV and AM251 acting as inverse agonists at CB1 receptors.
Phytocannabinoids have received considerable attention as potential therapeutic agents. Data from our recent studies suggest that these cannabinoids could have a therapeutic role in the treatment of pathophysiological hyperexcitability disorders associated with either the cerebellum, such as cerebellar ataxia (Smith and Dar, 2007), or the PC, such as cortical epilepsies (Whalley et al., 2004). Δ9-THCV is present in natural cannabis; thus, the chemical or cultivar isolation of phytocannabinoids offers a unique opportunity to isolate compounds with a selective pharmacological profile. The synthetic CB1 receptor antagonist rimonabant is currently marketed as an antiobesity agent, with a number of other potential therapeutic applications (Bifulco et al., 2007); in the future, natural, potent CB1 receptor antagonists such as Δ9-THCV may achieve similar clinical significance.
Acknowledgments
We would like to thank GW Pharmaceuticals for the gift of Δ9-THCV and Dr E Kara and Professor P Strange (School of Pharmacy, University of Reading) for providing CHO-D2 cell membrane preparations. We would also like to thank Professor P Strange for useful discussion and critical comment on the manuscript. This work was supported by a University of Reading Research Endowment Trust Fund award to ID and The Wellcome Trust (GJS).
Abbreviations
- AM251
N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-multipyrazole-3-carboxamide
- CHO
Chinese hamster ovary
- Δ9-THCV
Δ9-tetrahydrocannabivarin
- DMSO
dimethyl sulphoxide
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- GDP
guanosine 5′-diphosphate
- [35S]GTPγS
[35S]guanosine-5′-O-(3-thiotriphosphate)
- NECA
adenosine-5-N-ethylcarboxamide
- PC
piriform cortex
- WIN55,212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
References
- Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett. 2006;396:113–116. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Bifulco M, Grimaldi C, Gazzerro P, Pisanti S, Santoro A. Rimonabant: just an antiobesity drug? Current evidence on its pleiotropic effects. Mol Pharmacol. 2007;71:1445–1456. doi: 10.1124/mol.106.033118. [DOI] [PubMed] [Google Scholar]
- Bloom AS, Edgemond WS, Moldvan JC. Nonclassical and endogenous cannabinoids: effects on the ordering of brain membranes. Neurochem Res. 1997;22:563–568. doi: 10.1023/a:1022413901857. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Breivogel CS, Selley DE, Childers SR. Cannabinoid receptor agonist efficacy for stimulating [35S]GTPγS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J Biol Chem. 1998;273:16865–16873. doi: 10.1074/jbc.273.27.16865. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Sim LJ, Childers SR. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neurosci. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Groen BG, Lynn AB, De Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res. 1991;552:301–310. doi: 10.1016/0006-8993(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Scott DK, Wilken GH. Regulation of adenylate cyclase by cannabinoid drugs. Insights based on thermodynamic studies. Biochem Pharmacol. 1989;38:3297–3304. doi: 10.1016/0006-2952(89)90628-x. [DOI] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- Lawrence DK, Gill EW. The effects of delta1-tetrahydrocannabinol and other cannabinoids on spin-labeled liposomes and their relationship to mechanisms of general anesthesia. Mol Pharmacol. 1975;11:595–602. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Ma YL, Weston SE, Whalley BJ, Stephens GJ. The phytocannabinoid Δ9-tetrahydrocannabivarin (Δ9-THCV) modulates inhibitory neurotransmission in the cerebellum. Br J Pharmacol. 2008;154:204–215. doi: 10.1038/bjp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan SJ, Reynen PH, Kwan J, Bonhaus DW. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br J Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin DJ, Strange PG. Mechanisms of agonism and inverse agonism at serotonin 5-HT1A receptors. J Neurochem. 2000;74:347–357. doi: 10.1046/j.1471-4159.2000.0740347.x. [DOI] [PubMed] [Google Scholar]
- Meschler JP, Howlett AC. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacol. 2001;40:918–926. doi: 10.1016/s0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Ooms F, Wouters J, Oscari O, Happaerts T, Bouchard G, Carrupt PA, et al. Exploration of the pharmacophore of 3-alkyl-5-arylimidazolidinediones as new CB1 cannabinoid receptor ligands and potential antagonists: synthesis, lipophilicity, affinity, and molecular modeling. J Med Chem. 2002;45:1748–1756. doi: 10.1021/jm010896y. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, et al. The psychoactive plant cannabinoid, Δ9-tetrahydrocannabinol, is antagonized by Δ8- and Δ9-tetrahydrocannabivarin in mice in vivo. Br J Pharmacol. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, Niemi R, Jarvinen T, Laitinen JT. An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. Br J Pharmacol. 2003;140:1451–1419. doi: 10.1038/sj.bjp.0705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Brunk LK, Selley DE. Inhibitory effects of SR141716A on G-protein activation in rat brain. Eur J Pharmacol. 2001;414:135–143. doi: 10.1016/s0014-2999(01)00784-1. [DOI] [PubMed] [Google Scholar]
- Smith AD, Dar MS. Behavioral cross-tolerance between repeated intracerebellar nicotine and acute 9-THC-induced cerebellar ataxia: role of cerebellar nitric oxide. J Pharmacol Exp Ther. 2007;322:243–253. doi: 10.1124/jpet.107.120634. [DOI] [PubMed] [Google Scholar]
- Thomas A, Baillie G, Philips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:917–926. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, et al. Evidence that the plant cannabinoid Δ9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol. 2005;146:917–926. doi: 10.1038/sj.bjp.0706414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neurosci. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Weston S, Williamson E, Constanti A, Stephens GJ, Whalley BJ.Tetrahydrocannabivarin exhibits anticonvulsant effects in a piriform cortical brain slice model of epileptiform activity Proceedings of the British Pharmacological Society 2006. at
- Whalley BJ, Wilkinson JD, Williamson EM, Constanti A. A novel component of cannabis extract potentiates excitatory synaptic transmission in rat olfactory cortex in vitro. Neurosci Lett. 2004;365:58–63. doi: 10.1016/j.neulet.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Wilson J, Lin H, Fu D, Javitch JA, Strange PG. Mechanisms of inverse agonism of antipsychotic drugs at the D2 dopamine receptor: use of a mutant D2 dopamine receptor that adopts the activated conformation. J Neurochem. 2001;77:493–504. doi: 10.1046/j.1471-4159.2001.00233.x. [DOI] [PubMed] [Google Scholar]