Abstract
Background and purpose:
Increased activity of the Na+/H+-exchanger (NHE-1) in heart failure underlies raised [Na+]i causing disturbances of calcium handling. Inhibition of NHE-1, initiated at the onset of pressure/volume overload, prevents development of hypertrophy, heart failure and remodelling. We hypothesized that chronic inhibition of NHE-1, initiated at a later stage, would induce regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling.
Experimental approach:
Development of heart failure in rabbits was monitored electrocardiographically and echocardiographically, after one or three months. Cardiac myocytes were also isolated. One group of animals were treated with cariporide (inhibitor of NHE-1) in the diet after one month. Cytoplasmic calcium, sodium and action potentials were measured with fluorescent markers and sarcoplasmic reticulum calcium content by rapid cooling. Calcium after-transients were elicited after rapid pacing. Sodium channel current (INa) was measured using patch-clamp techniques.
Key results:
Hypertrophy and heart failure developed after one month and progressed during the next two months. After one month, dietary treatment with cariporide was initiated. Two months of treatment reduced hypertrophy and heart failure, duration of action potential QT-interval and QRS, and restored sodium and calcium handling and the incidence of calcium after-transients. In cardiac myocytes, parameters of INa were not changed by cariporide.
Conclusion and implications:
In rabbit hearts with hypertrophy and signs of heart failure one month after induction of pressure/volume overload, two months of dietary treatment with the NHE-1 inhibitor cariporide caused regression of hypertrophy, heart failure and ionic and electrophysiological remodelling.
Keywords: heart failure, regression, arrhythmias, remodelling, sodium, calcium (cellular), Na/H exchanger, Na/Ca exchanger, SR function, Na channel
Introduction
Electrophysiological and ionic remodelling predisposes patients with heart failure to an increased risk for sudden arrhythmic death (Janse, 2004). During hypertrophy and heart failure, the activity of the Na+/H+ exchanger-1 (NHE-1) becomes upregulated. Increased activity of NHE-1 results in elevated [Na+]i and consequently leads to increased [Ca2+]i (Baartscheer et al., 2003a; Chahine et al., 2005). Several studies support the contention that the increase of [Na+]i and [Ca2+]i can signal development of hypertrophy and cardiac remodelling (Cingolani and Ennis, 2007). Prolonged cardiac hypertrophy is a significant risk factor for development of heart failure and fatal arrhythmias. Therefore, inhibition of NHE-1 activity might favorably interfere with [Na+]i and [Ca2+]i homoeostasis and prevent hypertrophic remodelling and prove antiarrhythmic. We previously reported that acute inhibition of NHE-1 in isolated left ventricular myocytes of rabbits reversed ionic remodelling (Baartscheer et al., 2003b). We also reported that dietary cariporide treatment, initiated at induction of volume and pressure overload, greatly reduced hypertrophy and prevented the development of heart failure as well as cellular ionic and electrical remodelling (Baartscheer et al., 2005). Other studies also found beneficial effects of chronic inhibition of NHE-1, initiated at a very early stage, on fibrosis, hypertrophy and survival rates after myocardial infarction (Baartscheer, 2006).
From a clinical point of view, it is of great relevance to determine whether chronic inhibition of NHE-1, initiated once hypertrophy has already been established, is able to reverse or stabilize hypertrophy, development of heart failure and cellular remodelling after these changes have been established. In this respect, it has been shown that NHE-1 inhibition in rats reduces hypertrophy and increases contractility when started 2 or 4 weeks after myocardial infarction (Chen et al., 2004) and that inhibition of NHE-1 in spontaneously hypertensive rats reverses hypertrophy (Camillión de Hurtado et al., 2002).
So far, no data on the reversibility of ionic and electrophysiological remodelling by inhibition of NHE-1 are available. We hypothesize that chronic inhibition of NHE-1, initiated after the establishment of hypertrophy and heart failure, can normalize ionic and electrophysiological remodelling in concert with regression of the signs of hypertrophy and congestive heart failure. To this purpose, we studied rabbits with heart failure that received chronic treatment with cariporide after the development of electrophysiological and ionic changes due to heart failure.
Methods
This study was approved by the local institutional ethical committee. Animal care and handling conformed to the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Design of the study
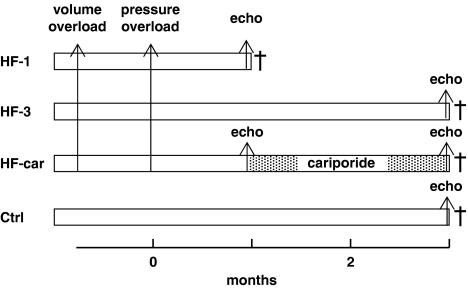
Figure 1 shows a diagram of the experimental protocol. Heart failure was induced in 4-month-old New Zealand white male rabbits with body weight of 3.3±0.06 kg (mean±s.e.mean, n=35) by volume and pressure overload as described previously (Baartscheer et al., 2005). In short, volume overload was produced by rupture of the aortic valve until pulse pressure was increased by about 100%, and after 3 weeks a second surgical procedure pressure overload was created by suprarenal abdominal aortic constriction of 50% (time zero in Figure 1).
Figure 1.
Time table of experimental protocol. †Termination of experiment.
Four groups of rabbits (HF-1, HF-3, HF-car and control (Ctrl)) were studied. The HF-1 (n=10) and HF-3 (n=14) groups received a normal diet until they were killed either after 1 month or after 3 months after aortic constriction. HF-car (n=11) animals received a normal diet for the first month, replaced by a diet supplemented with 0.3% cariporide during the last 2 months until the end of the experiment. Feeding the animals a 0.3% cariporide-supplemented diet gives steady-state cariporide plasma levels of about 5 μM (Baartscheer et al., 2005). This is about five times higher than that needed for complete inhibition of NHE-1 (van Borren et al., 2004). The Ctrl (n=9) group were age-matched animals without surgical procedures on a normal diet. There was no difference in the increase of pulse pressure immediately after aortic valve rupture: 100±3.5, 101±6.2 and 102±3.5% (mean±s.e.mean) for HF-1, HF-3 and HF-car respectively.
In all groups, immediately before termination of the experiment, heart rate and QT and QRS duration were measured and transthoracic two-dimensional, M-mode imaging was performed with a VingMed Sound System Five (General Electric, Fairfeild, Connecticut, USA) using 10 MHz transducer. Interventricular septum thickness, left ventricular posterior wall thickness and left ventricular diameter were measured in diastole and systole from the parasternal long-axis view. In the HF-car group, echocardiographic recordings were also made 1 month after aortic constriction before initiating the cariporide treatment. Left ventricular end diastolic pressure was measured (Millar catheter) immediately before death. Presence of ascites was assessed at autopsy, and heart and lung weights were measured relative to body weight.
Myocytes were isolated from the midmural left ventricular wall as described previously (Baartscheer et al., 1996) and stored at room temperature in vials, each containing about 105 myocytes in 5 mL solution containing (mM) Na+, 156; K+, 4.7; Ca2+, 2.6; Mg2+, 2.0; Cl−, 150.6; HCO3−, 4.3; HPO42−, 1.4; HEPES, 17; glucose, 11 and supplemented with 1% fatty acid-free albumin (pH 7.3).
Measurement of action potential, [Na+]i, [Ca2+]i and SR calcium content
Myocytes were attached to a poly-lysine (0.1 g L−1)-treated coverslip on the stage of a microscope (Nikon Diaphot). A perfusable chamber (height 0.4 mm, diameter 10 mm, volume 30 μL), containing two platinum electrodes for field stimulation (8 mm distance, 40 V cm−1 bipolar square pulses of 0.5-ms duration), was pressed onto the cover slip. The microscope stage and perfusion chamber were maintained at 37 °C. The measuring window was adjusted with a rectangular diaphragm to the cellular surface of one quiescent rod-shaped myocyte. For every experimental condition, fluorescence measurements were taken in at least five myocytes per heart. Action potentials, [Na+]i and [Ca2+]i, were measured in myocytes loaded with the fluorescent probes di-4-Anepps, sodium-binding benzofuran isophathalate and Indo-1 respectively in HEPES solution without albumin as described previously (Baartscheer et al., 2005). Sarcoplasmic reticulum (SR) calcium content was calculated from the response of cytoplasmic [Ca2+]i to rapid cooling. Literature data on cytoplasmic calcium buffer characteristics of normal cardiac myocytes (Hove-Madsen and Bers, 1993) were used to convert the increase of cytoplasmic free [Ca2+]i to total calcium released into the cytoplasm as described previously (Baartscheer et al., 2000). Fractional SR calcium release was estimated from the ratio of the increase of total calcium during a twitch and the increase of total calcium during rapid cooling, ignoring the relatively small influx of calcium across the sarcolemma during a calcium transient. Calcium after-transients were elicited after 10 s of rapid pacing (3 Hz) in the presence of 100 nM noradrenaline (Baartscheer et al., 2003c).
Measurement of sodium channel current
Sodium channel current (INa) was measured at room temperature using the whole-cell patch-clamp technique with pipettes containing (in mM) Na+, 7; Cs+, 133; Mg2+, 2; Cl−, 140; ATP, 2; TEA, 2; HEPES, 5; EGTA, 10 (pH 7.3). The bath solution contained (in mM) Na+, 7; Cs+, 133; Ca2+, 1.8; Mg2+, 1.2; HEPES, 5; glucose, 11; nifedipine, 0.001 (Sigma), pH 7.3. INa was elicited by voltage-clamp steps of 500 ms with 10 mV increments from a holding potential of −120 mV. Current density was calculated by dividing whole-cell current amplitude by cell capacitance (Cm). Cm was estimated by dividing the decay time constant of the capacitive transient in response to 5-mV hyperpolarizing voltage-clamp steps from −40 mV by the series resistance (Rs). Adequate voltage control was achieved by using low-resistance pipettes (1.0–2.0 MΩ) and Rs and Cm compensation >80%.
Statistics
Data are expressed as mean±s.e.mean. Statistical significance (P<0.05) was evaluated with one-way ANOVA with a post hoc test according to Student–Newman–Keuls. Student's t-test or a non-parametric test was used when appropriate.
Reagents
Cariporide and cariporide-supplemented chow were supplied by Aventis Pharma (Frankfurt, Germany). Noradrenaline was purchased from Centrafarm BV (Etten-Leur, The Netherlands). Poly-lysine was purchased from Chemicon (Temecula, California, USA). Indo-1, sodium-binding benzofuran isophathalate and di-4-Anepps were purchased from Invitrogen (Carlsbad, CA, USA). All other chemicals were purchased from Roche BV (Basel, Switzerland). All indicator dyes were first dissolved in dimethyl sulphoxide and then further diluted using buffer solution.
Results
Analysis of hypertrophy and heart failure
Table 1 summarizes morphometric and echocardiographic parameters in all groups of rabbits. One month after the induction of volume and pressure overload (HF-1 group), parameters relevant to development of hypertrophy and heart failure were significantly different from Ctrl. Markers of hypertrophy such as relative heart weight, myocyte dimensions, left ventricular diameter and left ventricular wall and septal thickness, and markers of development of heart failure such as relative lung weight, presence of ascites and left ventricular end diastolic pressure were significantly increased and fractional shortening was significantly decreased. Without treatment with cariporide, hypertrophy and signs of heart failure further deteriorated during the next 2 months (HF-3 group); relative heart weight, myocyte dimensions, left ventricular diameter and left ventricular end diastolic pressure were significantly larger and fractional shortening was significantly less in the HF-3 group compared with the HF-1 group. In contrast, chronic cariporide treatment during these 2 months (HF-car group) significantly reduced hypertrophy as well as signs of heart failure. Hypertrophy was reduced compared with the HF-1 group, but mild hypertrophy remained in comparison with the Ctrl group. Signs of heart failure decreased significantly compared with the HF-1 group but were not different compared with those in the Ctrl group. It is worth noting that echocardiographic data in the HF-car, measured just before initiation of cariporide treatment, were not different from those measured at the same time point in the HF-1 group. Moreover, none of the rabbits in the HF-car group died suddenly after initiation of cariporide treatment, whereas in the HF-3 group, the number of sudden deaths increased to four.
Table 1.
Morphometric and echocardiographic parameters
| Ctrl (9) | HF-1 (10) | HF-3 (14) | HF-Car 1 month (11) | HF-Car 3 months (11) | |
|---|---|---|---|---|---|
| Sudden death | 0 | 1 | 4 | 1 | 1 |
| (9) | (9) | (10) | (10) | ||
| Body weight (kg) | 4.2±0.18 | 4.0±0.06 | 4.2±0.10 | 4.2±0.06 | |
| Relative heart weight (g kg−1) | 2.5±0.08 | 4.7±0.13* | 5.5±0.39*† | 3.7±0.11*†‡ | |
| Relative lung weight (g kg−1) | 3.3±0.08 | 5.5±0.31* | 5.4±0.42* | 3.6±0.10†‡ | |
| Myocyte length (μm) | 147±1.9 | 186±2.4* | 188±3.5* | 163±2.8*†‡ | |
| Myocyte width (μm) | 25±0.3 | 29±0.6* | 32±0.8*† | 26±0.5†‡ | |
| Presence of ascites | 0 | 5* | 7* | 1†‡ | |
| LVEDP (mm Hg) | 4±0.5 | 17±2.6* | 26±3.1*† | 5±1.0†‡ | |
| (9) | (9) | (10) | (10) | (10) | |
| IVS dia (mm) | 2.5±0.08 | 3.2±0.17* | 3.5±0.15* | 3.3±0.18* | 2.8±0.09†‡ |
| IVS sys (mm) | 4.6±0.31 | 4.8±0.30 | 4.5±0.22 | 4.6±0.24 | 4.7±0.22 |
| LVWP dia (mm) | 2.8±0.11 | 3.9±0.14* | 4.0±0.21* | 3.7±0.16* | 3.1±0.11†‡ |
| LVWP sys (mm) | 3.9±0.15 | 4.4±0.16* | 4.4±0.17* | 4.2±0.22 | 4.0±0.20 |
| LV diameter dia (mm) | 15.4±0.34 | 20.8±0.54* | 23.4±0.73*† | 20.9±0.51*‡ | 17.7±0.17*†‡ |
| LV diameter sys (mm) | 8.3±0.44 | 14.2±0.59* | 18.2±0.86*† | 14.5±0.54*‡ | 10.3±0.23*†‡ |
| Fractional shortening (%) | 46±2.0 | 32±1.8* | 22±2.0*† | 30±1.8*‡ | 40±1.9*†‡ |
Abbreviations: dia, diastolic; sys, systolic; IVS, interventricular septum; LVEDP, left ventricular end diastolic pressure; LVWP, left ventricular wall posterior.
Mean±s.e.mean; P<0.05, *versus Ctrl, †versus HF-1, ‡versus HF-3; the number of rabbits is given in parentheses.
Electrophysiology
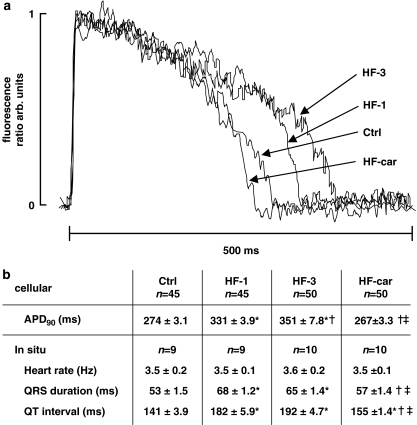
Figure 2a shows representative examples of action potentials of myocytes stimulated at 2 Hz. Figure 2b summarizes data on action potential duration (APD90) in isolated myocytes and in situ body-surface ECG parameters of all groups. APD90 was significantly prolonged in both the HF-1 and HF-3 groups relative to the Ctrl group. In the HF-car group, APD90 returned to control values after 2 months of chronic cariporide treatment. Heart rate was the same in all groups. QRS duration and QT interval were significantly prolonged in the HF-1 and HF-3 groups relative to those in the Ctrl group. QRS duration and the QT interval in the HF-car group remained significantly prolonged compared with Ctrl, but were significantly shortened compared with HF-1 and HF-3. The in situ data on duration of the QT interval reflect prolongation of the action potential data measured in the isolated myocytes.
Figure 2.
Electrophysiological data. (a) Raw data of action potentials in 2 Hz stimulated myocytes from Ctrl, HF-1, HF-3 and HF-car groups of animals, measured using di-4-Anepps fluorescence ratio in dual-emission mode. (b) Mean±s.e.mean; P<0.05, *versus Ctrl, †versus HF-1, ‡versus HF-3. APD90, action potential duration at 90% repolarization in 2 Hz stimulated myocytes.
[Na+]i and activity of NHE-1
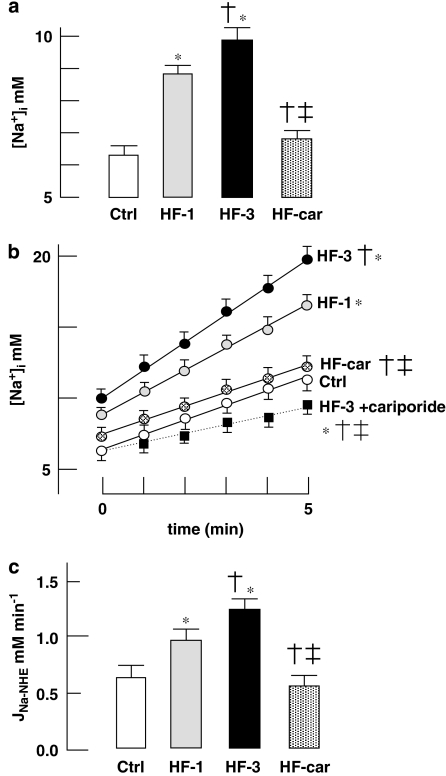
Figure 3a shows steady-state cytoplasmic [Na+]i in isolated myocytes stimulated at 2 Hz. [Na+]i progressively increased after induction of volume and pressure overload in myocytes of HF-1 and HF-3 animals. After 2 months of cariporide treatment, [Na+]i in the HF-car group was the same as that in the Ctrl group. Figure 3b shows the increase of [Na+]i in stimulated myocytes following inhibition of the sodium pump with 100 μM ouabain, which allows estimation of the steady-state rate of influx of sodium. In the HF-1 and HF-3 groups, the rate of sodium influx was increased more than twofold over that in the Ctrl group. In the HF-car group, the steady-state rate of sodium influx was not different from that in myocytes from Ctrl rabbits. In all HF groups, acute application of cariporide in addition to ouabain caused reduction of the steady-state rate of sodium influx to the same value as measured in HF-3 (only data in the HF-3 group in these conditions are shown). The difference between steady-state rates of sodium influx in the presence and absence of cariporide reflects the activity of NHE-1 (JNa-NHE). Figure 3c shows the cariporide-sensitive sodium influx, JNa-NHE, in all groups. JNa-NHE was significantly increased in HF-1 and progressively increased further in HF-3. In the HF-car group, JNa-NHE measured after 2 months of treatment had returned to a value not different from that measured in myocytes from Ctrl rabbits.
Figure 3.
Activity of the Na+/H+ exchanger and cytoplasmic [Na+]i. (a) Cytoplasmic [Na+]i in 2 Hz stimulated myocytes of Ctrl (nine rabbits, 45 cells), HF-1 (nine rabbits, 45 cells), HF-3 (10 rabbits, 45 cells) and HF-car (10 rabbits, 50 cells). (b) Time course of increase of [Na+]i in 2 Hz stimulated myocytes after inhibition of Na+/K+-ATPase with 100 μM ouabain in the absence of cariporide (solid lines) of Ctrl (nine rabbits, 45 cells), HF-1 (nine rabbits, 27 cells), HF-3 (10 rabbits, 45 cells) and HF-car (10 rabbits, 50 cells). Myocytes from all four groups were also pre-incubated for 5 min with 5 μM cariporide before assay. Only one set of data is shown, for HF-3 myocytes (nine rabbits, 45 cells: dotted line), as the increase of [Na+]i was the same in all of the myocyte groups pre-incubated with cariporide. (c) The activity of NHE-1 was measured as the rate of cariporide-sensitive sodium influx (JNa-NHE). JNa-NHE was obtained by subtracting the initial rates of sodium influx immediately following sodium pump inhibition with 100 μM ouabain in the absence and presence of 5 μM cariporide (see also Baartscheer et al., 2003c). Data: mean±s.e.mean; P<0.05, *versus Ctrl, †versus HF-1 and ‡versus HF-3. NHE-1, Na+/H+ exchanger-1.
Sodium current
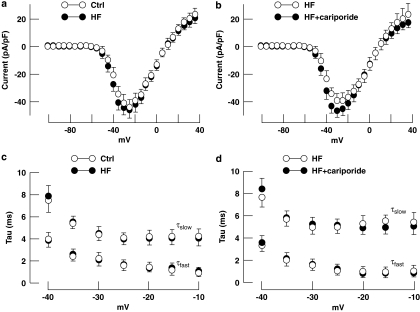
It has been suggested that time of inactivation of INa, the so-called late sodium current, is increased in heart failure (Maltsev et al., 2007) and that several NHE-1 inhibitors reduced this slowing of inactivation (Chattou et al., 2000). Sodium current was studied to investigate whether changes in the kinetics of the Na channel during development of heart failure could be responsible for the increase of [Na+]i in isolated HF myocytes. Figure 4a shows that the peak current density and current–voltage relationship of INa were not different in myocytes from HF groups compared with Ctrl myocytes. Double-exponential fits of inactivation of INa revealed that the time constants of fast and slow inactivation (τfast and τslow, respectively) were not different (Figure 4b). Figures 4c and d show that 5 μM cariporide did not affect the peak current density, the current–voltage relationship or the time constants of fast and slow inactivation of INa in failing myocytes. In addition, the voltage dependence of activation and inactivation was the same in HF and Ctrl myocytes and were not altered by 5 μM cariporide (data not shown). These results suggest that the so-called late sodium current in cardiac myocytes from our model of heart failure is not different compared to that from the Ctrl group and that the characteristics of INa are not altered by 5 μM cariporide, applied ex vivo.
Figure 4.
Sodium current (INa) in Ctrl and failing myocytes. (a) Peak current density and current–voltage relation of INa in HF-3 (n=9) and Ctrl myocytes (n=9). (b) Peak current density and current–voltage relation of INa in HF-3 myocytes in the presence (n=8) and absence (n=8) of 5 μM cariporide. (c and d) The corresponding time constants (τfast and τslow) of a double-exponential fit of inactivation of INa. Data: mean±s.e.mean.
Calcium handling
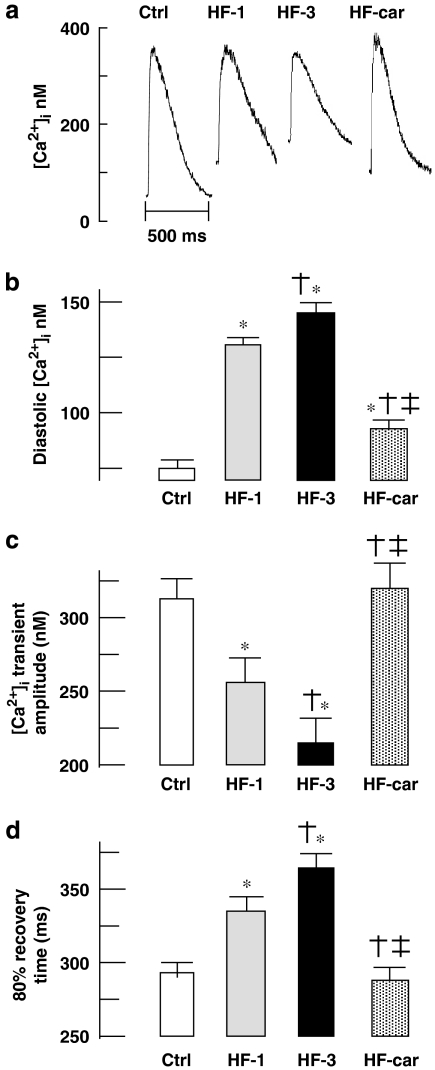
Figure 5a shows representative examples of calcium transients in stimulated myocytes of all groups. Myocytes from the HF-1 and HF-3 groups showed increased diastolic [Ca2+]i, decreased calcium transient amplitude and slowing of relaxation compared with those from the Ctrl group. In the HF-car group, the calcium transient measured after 2 months of cariporide treatment was almost normalized compared to the transient in Ctrl myocytes, although some small, but significant, increase of diastolic calcium remained. These data are summarized in Figures 5b–d.
Figure 5.
Calcium transient characteristics. (a) Typical example of steady-state calcium transients in stimulated myocytes (2 Hz; average of five successive calcium transients). (b) Average data of diastolic [Ca2+]i, (c) average data of calcium transient amplitude and (d) calcium transient duration at 80% recovery. Ctrl (nine rabbits, 45 cells), HF-1 (nine rabbits, 45 cells), HF-3 (10 rabbits, 50 cells) and HF-car (10 rabbits, 50 cells). Data: mean±s.e.mean; P<0.05, *versus Ctrl, †versus HF-1 and ‡versus HF-3.
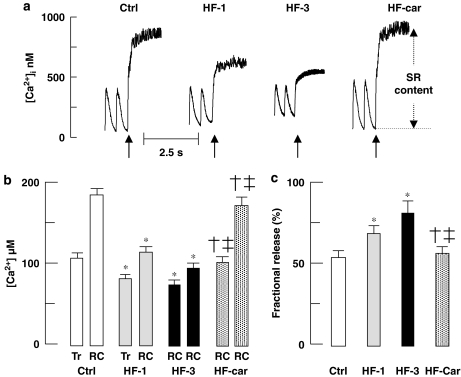
Figure 6a shows representative examples of the increase of [Ca2+]i upon rapid cooling in stimulated (2 Hz) myocytes of all groups. The increase of [Ca2+]i in myocytes of the HF-1 and HF-3 groups was less compared with Ctrl. In the HF-car group, the response of [Ca2+]i to rapid cooling was significantly larger than in HF-1 and HF-3 and was not different from that in the Ctrl group. These data indicate that the SR calcium content is reduced in the HF-1 and HF-3 groups compared with the Ctrl group, but the SR calcium content in the HF-car group is not different from that in the Ctrl group.
Figure 6.
SR calcium content and fractional release. (a) Typical examples of response to rapid cooling in stimulated (2 Hz) myocytes. Rapid cooling (indicated by arrows) was used to release all calcium from SR. (b) The increase of total calcium during an electrically stimulated calcium transient (Tr) and the increase of total calcium during rapid cooling (RC). (c) Fractional SR calcium release during a calcium transient (ratio of Tr/RC). Ctrl (nine rabbits, 45 cells), HF-1 (nine rabbits, 45 cells), HF-3 (10 rabbits, 50 cells) and HF-car (10 rabbits, 50 cells). Data: mean±s.e.mean; P<0.05, *versus Ctrl, †versus HF-1 and ‡versus HF-3. SR, sarcoplasmic reticulum.
At any value of cytoplasmic free [Ca2+]i, the associated total cytoplasmic calcium can be calculated using proper cytoplasmic calcium buffering data (see Methods). The SR calcium content can be estimated from the difference between total cytoplasmic calcium during diastole and that after rapid cooling (RC). The amount of calcium released from SR during a stimulated calcium transient can be estimated from the difference between total cytoplasmic calcium during diastole and that at the peak of the calcium transient (Tr), assuming no change in calcium entry. These data are summarized in Figure 6b. In the HF-1 and HF-3 groups, SR calcium content was significantly lower, but in the HF-car group after 2 months of treatment, SR calcium content was not different from that in the Ctrl group.
Figure 6b also showed the increase in total calcium during a stimulated calcium transient (Tr). From these data, it can be appreciated that the fractional calcium release from SR during stimulation is higher in myocytes from the HF-1 and HF-3 groups, compared with Ctrl and HF-car groups. Figure 6c shows the associated fractional SR depletion during stimulation; 70 and 80% of the SR calcium content is released in the HF-1 and HF-3 groups compared with 52 and 54% in Ctrl and HF-car group respectively.
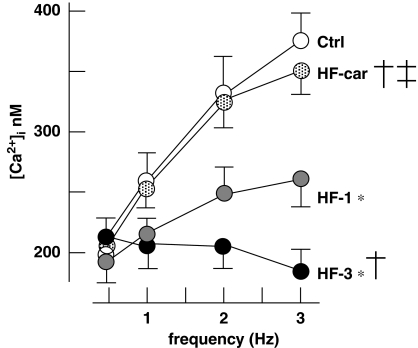
In healthy humans and rabbits, the force–frequency relationship is positive, but becomes negative with development of heart failure. This is reflected in the relationship between calcium transient amplitude and stimulation frequency in isolated myocytes (Baartscheer et al., 2003c). Figure 7 shows the calcium transient amplitudes measured in myocytes stimulated from 0.5 to 3 Hz in all animal groups. In Ctrl animals, the relation between transient amplitude and stimulation is positive. In the HF-1 group, the relation becomes significantly less positive and even negative in the HF-3 group. In the HF-car group, 2 months of treatment with cariporide restores the slope of the relationship to a positive value that was not statistically different from Ctrl.
Figure 7.
The stimulation-rate dependence of the calcium transient amplitude. The calcium transient amplitudes measured in myocytes stimulated from 0.5 to 3 Hz. Ctrl (nine rabbits, 45 cells), HF-1 (nine rabbits, 45 cells), HF-3 (10 rabbits, 50 cells), HF-car (10 rabbits, 50 cells). Data: mean±s.e.mean; P<0.05, *versus Ctrl, †versus HF-1 and ‡versus HF-3.
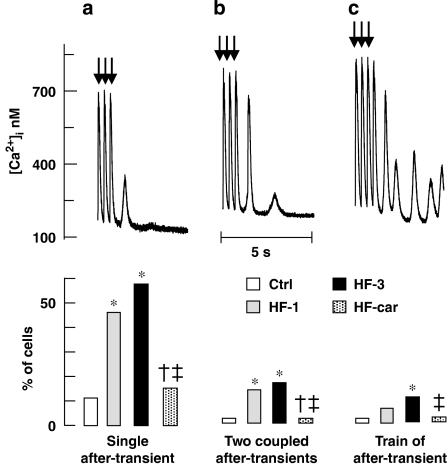
In heart failure, altered calcium handling is associated with an increased propensity to generate delayed after-depolarizations, related to calcium after-transients. The after-transients can be elicited in myocytes by cessation of stimulation after rapid pacing (3 Hz, 10 s) with β-adrenoceptor stimulation (100 nM noradrenaline) (Baartscheer et al., 2003c). Figure 8 shows representative examples of [Ca2+]i during this protocol (top panels) and the corresponding incidence of calcium after-transients (lower panels). Calcium after-transients were divided into three groups according to arrhythmogenic severity: one single after-transient (A), two coupled after-transients (B) and a train of after-transients (C). The propensity to develop after-transients and the arrhythmogenic severity significantly increased in the HF-1 and HF-3 groups. In the HF-car group, the incidence of calcium after-transients in myocytes after 2 months of cariporide treatment was not different from that measured in the Ctrl group.
Figure 8.
Incidence of calcium after-transients. Upper panels: representative examples of calcium after-transients evoked by cessation of 10 s of rapid stimulation (3 Hz) in the presence of 100 nM noradrenaline, arrows mark the last three stimulated beats. (a) Single calcium after-transient, (b) double calcium after-transient and (c) train of calcium after-transient. Lower panels: corresponding incidence of calcium after-transients in myocytes of Ctrl (nine rabbits, 45 cells), HF-1 (nine rabbits, 45 cells), HF-3 (10 rabbits, 50 cells) and HF-car (10 rabbits, 50 cells). P<0.05, *versus Ctrl, †versus HF-1 and ‡versus HF-3.
Discussion
In this study, we demonstrate for the first time that chronic inhibition of NHE-1 by treatment with dietary cariporide, initiated at a time when heart failure has already developed, reduced the electrophysiological and ionic remodelling to almost normal (control) values. As a consequence, the propensity to develop calcium after-transients, which underlie the development of triggered arrhythmias, was reduced to normal in the treated group. Inhibition of NHE-1 also restored the relation between stimulation rate and calcium transient amplitude. The functional improvement induced by NHE-1 inhibition was paralleled by almost complete normalization of echocardiographic parameters and reduction of the morphological signs of hypertrophy and ventricular dilatation in the group treated with cariporide.
Experimental studies show that the detrimental effect of increased NHE-1 activity is the result of increased [Na+]i, which leads to calcium overload through the Na+/Ca2+ exchanger (Cingolani and Ennis, 2007). This explains why inhibition of NHE-1 prevents disturbances of calcium handling and the occurrence of triggered arrhythmias. In experimental ischaemia/reperfusion studies, NHE-1 inhibition reduces calcium overload and prevented lethal cell injury and activation of intracellular pathways leading to hypertrophy and heart failure. Regarding ischaemia/reperfusion injury, the beneficial effect of NHE-1 inhibition obtained in experimental studies was not confirmed in two large clinical trials (Guard During Ischemia Against Necrosis (Guardian) and Evaluation of the Safety and Cardioprotective Effects of Eniporide in Acute Myocardial Infarction (ESCAMI)) (Theroux et al., 2000; Zeymer et al., 2001). However, the most recent clinical trial, the EXPEDITION study (Sodium–Hydrogen Exchange Inhibition to Prevent Coronary Events in Acute Cardiac condition), showed benefits of cariporide treatment in patients with ischaemic heart disease. Unfortunately, the treatment was associated with an increased (although small) incidence of stroke (Mentzer, 2003). However, it must be stressed that the adverse effect of cariporide was found in a subgroup of patients following coronary artery bypass graft surgery, but not in patients with heart failure. Nevertheless, our present and previous results with cariporide in experimental studies clearly suggest a high potential efficacy of specific NHE-1 inhibition for clinical treatment of heart failure, even after development of hypertrophy and signs of heart failure. Therefore, a search for other specific NHE-1 inhibitors, lacking the adverse effects of cariporide, seems indicated.
Inhibition of NHE-1 has been demonstrated to lead to reduction of cardiac hypertrophy when the agent was administered not only before but also after induction of hypertrophy (Camillión de Hurtado et al., 2002; Chen et al., 2004; Baartscheer et al., 2005; Baartscheer, 2006). In rats with myocardial infarction, NHE-1 inhibition was more successful than ACE inhibition. Combined treatments tended to provide the best protection (Ruetten et al., 2005). Our study corroborates these results: chronic administration of cariporide started at the time when development of heart failure had reached its maximum led to complete normalization of most of the echocardiographic and other parameters used to quantify heart failure-related changes within 2 months.
The novelty of the present study resides in the cariporide-induced functional changes that accompany the structural alterations, further strengthening the causal relation between the two. Heart failure is associated with an increase in [Na+]i caused by increased NHE-1-dependent sodium influx leading to Na+/Ca2+ exchanger-mediated increase of [Ca2+]i (Baartscheer et al., 2003b; Aiello et al., 2005; Chahine et al., 2005). Inhibition of this NHE-1-dependent increase in [Na+]i by chronic cariporide administration initiated before the induction of heart failure effectively prevents heart failure development (Baartscheer et al., 2005). Although it may be argued that, in these experiments, the absence of the development of heart failure (both structural and functional changes) may have been the result of the absence of the trigger for heart failure; the present study provides strong evidence for a causal relation between the morphological and functional changes. Both normalized in concert when specific NHE-1 inhibition was started once the changes had developed. In addition, our study demonstrates that the incidence of spontaneous release of calcium from the SR, responsible for calcium after-transients and triggered activity-dependent arrhythmias, returns to normal following 2 months of cariporide treatment. These cellular arrhythmias facilitate life-threatening cardiac arrhythmias in patients with heart failure (Janse, 2004).
Although recovery occurred in most parameters, there were some exceptions: the recovery of myocyte dimensions was more pronounced than the relative heart weight. This could suggest that inhibition of NHE-1 mainly affects cardiomyocyte structure and function but with less effects on intercellular matrix. As a consequence, the recovery of both conduction and contractile function should be less compared with the recovery of myocyte function. Indeed, calcium handling almost totally recovered to control values, where recovery of fractional shortening (contractility) and QRS duration (conduction) was not complete. Regarding contractility, this variable indicates that reversal of functional cellular alterations at this stage of heart failure did not lead to full recovery of structural changes. In this respect, it has been shown that NHE-1 inhibition had some effect on contractility (van Borren et al., 2006).
In most studies in which chronic inhibition of NHE-1 has been applied experimentally, treatment started immediately following the intervention use to induce hypertrophy and failure (such as myocardial infarction, pressure and/or volume overload, rapid pacing and β-adrenoceptor stimulation (Baartscheer, 2006)). Two studies report on reversibility of hypertrophy and improvement of cardiac function by chronic inhibition of NHE-1 (Camillión de Hurtado et al., 2002; Chen et al., 2004). In a pressure overload model of heart failure in mice, release of the pressure overload reversed the development of hypertrophy and heart failure (Gao et al., 2005) and inhibition of ACE by lisinopril in rats after myocardial infarction reversed severity of heart failure (Mulder et al., 1997). However, these studies did not evaluate reversibility of ionic and electrical remodelling.
Prolonged hypertrophy is associated with increased sudden cardiac death and progression to heart failure, mostly caused by ventricular arrhythmias (Janse, 2004). The major stimulus to initiate pathological hypertrophy is sustained increased workload, which activates cellular pathways resulting in altered protein expression and cellular remodelling. Although most of the altered protein expression is proportional to the change in cell size (Siehl et al., 1985), a small but important number of genes (<2%) is either unproportionally up- or downregulated or re-expressed (Hwang, 2001). Among them are genes involved in sodium handling, calcium handling, excitation–contraction coupling, contractility, energy metabolism, cytoskeleton and extracellular matrix.
Enhanced activity of the cardiac sarcolemmal NHE-1 is associated with and/or contributes to cellular growth, hypertrophy, electrical and ionic remodelling and development of heart failure (Takewaki et al., 1995; Chen et al., 2001; Engelhardt et al., 2002; Baartscheer et al., 2003b; Chahine et al., 2005). The mechanisms involved are not fully understood, but it has been suggested that sodium handling plays a key role (Cingolani and Ennis, 2007). Both increased [Na+]i and [Ca2+]i is recognized as a cell growth signal (Marban and Koretsune, 1990; Eguchi et al., 1996; Zou et al., 1996) and, therefore, may play a role in the hypertrophic response, cellular remodelling and the development of heart failure. Several lines of evidence support this contention: (1) in animal models of hypertrophy and heart failure, chronic inhibition of NHE-1 prevents not only the increase of [Na+]i and [Ca2+]i, but also hypertrophy, development of heart failure and ionic and electrical cellular remodelling (Baartscheer, 2006); (2) reduction of sodium efflux associated with Na+/K+ pump inhibition causes elevation of [Na+]i and promotes cardiac hypertrophy (Peng et al., 1996; Gu et al., 1998) and (3) rapid pacing enhances Na channel-related sodium influx and causes hypertrophy and development of heart failure (Yao et al., 1998). Our study supports this contention, because normalization of [Na]i was directly associated with normalization of structural changes.
It has been suggested (Bers and Despa, 2006) that NHE-1 inhibitors might also inhibit the late sodium current, slowly inactivating Na+ channels (Chattou et al., 2000), which could provide an alternative explanation for elevated [Na]i in heart failure (Maltsev et al., 2007). Inhibition of the late sodium current can shorten AP duration and decrease the incidence of early after-depolarization-related arrhythmias (Maltsev et al., 2007) and improve mechanical efficiency (Chandler et al., 2002). Yet, no data are available showing that inhibition of the late sodium current can either prevent/reduce an increase of [Na+]i or prevent/reduce the development of hypertrophy. Our experiments demonstrating slowing of Na+ channel inactivation in myocytes from HF rabbits were performed at room temperature at which inactivation is much slower compared with more physiological temperatures, which complicates estimation of a possible contribution of the late sodium current to the influx of Na+.
We measured INa in Ctrl and HF myocytes before and after application of cariporide ex vivo (Figure 4). We did not find any difference in inactivation of INa between Ctrl and HF myocytes. Neither we could demonstrate any effect of 5 μM cariporide on INa in Ctrl and HF myocytes. Therefore, we feel confident that our results were due to an action of cariporide, other than inhibition of NHE-1. In another study, Chattou et al. (2000) have shown that NHE-1 inhibitors can inhibit the veratrine-induced late sodium current (INa). However, the inhibitory effect of cariporide on the late sodium current (INa) was low compared with other NHE-1 inhibitors.
In conclusion, increased activity of NHE-1 in heart failure caused elevation of cytoplasmic [Na]i and consequently disrupted cellular calcium handling and was instrumental in electrophysiological and functional remodelling. This study demonstrated for the first time that ionic and electrical cellular remodelling could be reversed by chronic inhibition of NHE-1, when treatment was initiated at a time when substantial hypertrophy and signs of heart failure were already present. This treatment also markedly reduced hypertrophy, induced contractile recovery and prevented sudden arrhythmic death. Such pharmacological treatment may be beneficial and life preserving in patients with hypertrophy and developing heart failure not only by preservation of contractile function but also by its antiarrhythmic potential.
Acknowledgments
We gratefully acknowledge support of this study by Sanofi-Aventis Pharma (Frankfurt, Germany) who kindly supplied the cariporide-supplemented chow.
Abbreviations
- NHE-1
Na+/H+ exchanger-1
Conflict of interest
The authors state no conflict of interest.
References
- Aiello EA, Villa-Abrille MC, Dulce RA, Cingolani HE, Perz NG. Endothelin-1 stimulates the Na+/Ca2+-exchanger reverse mode through intracellular Na+ (Na+i)-dependent pathways. Hypertension. 2005;45:288–243. doi: 10.1161/01.HYP.0000152700.58940.b2. [DOI] [PubMed] [Google Scholar]
- Baartscheer A. Chronic inhibition of the Na+/H+-exchanger in the heart. Curr Vasc Pharmacol. 2006;4:23–29. doi: 10.2174/157016106775203117. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Borren v MMC, Schumacher CA, Belterman CNW, Coronel R, Fiolet JWT. Increased Na+/H+-exchange activity is the cause of increased [Na+]i in heart failure and underlies disturbed calcium handling. Cardiovasc Res. 2003b;57:1015–1024. doi: 10.1016/s0008-6363(02)00809-x. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, Belterman CNW, Coronel R, Fiolet JWT. [Na+]i and the driving force of the Na+/Ca2+-exchanger in heart failure. Cardiovasc Res. 2003a;57:986–995. doi: 10.1016/s0008-6363(02)00848-9. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, Belterman CNW, Coronel R, Fiolet JWT. SR calcium handling and calcium after-transients in a rabbit model of heart failure. Cardiovasc Res. 2003c;58:99–108. doi: 10.1016/s0008-6363(02)00854-4. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, Belterman CNW, Coronel R, Fiolet JWT. Chronic inhibition of Na+/H+-exchanger attenuates cardiac hypertrophy and prevents cellular remodelling in heart failure. Cardiovasc Res. 2005;65:83–92. doi: 10.1016/j.cardiores.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, Fiolet JWT. SR calcium depletion following reversal of the Na+/Ca2+-exchanger in rat ventricular myocytes. J Mol Cell Cardiol. 2000;32:1025–1037. doi: 10.1006/jmcc.2000.1145. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, Opthof T, Fiolet JWT. The origin of increased cytoplasmic calcium upon reversal of the Na+/Ca2+-exchanger in isolated rat ventricular myocytes. J Mol Cell Cardiol. 1996;28:1963–1973. doi: 10.1006/jmcc.1996.0189. [DOI] [PubMed] [Google Scholar]
- Bers DM, Despa S. Cardiac myocytes Ca2+ and Na+ regulation in failing and normal hearts. J Pharmacol Sci. 2006;100:315–322. doi: 10.1254/jphs.cpj06001x. [DOI] [PubMed] [Google Scholar]
- Camillión de Hurtado MC, Portiansky EL, Pérez NG, Rebolledo OR, Cingolani HE. Regression of cardiomyocyte hypertrophy in SHR following chronic inhibition of the Na+/H+-exchanger. Cardiovasc Res. 2002;53:862–868. doi: 10.1016/s0008-6363(01)00544-2. [DOI] [PubMed] [Google Scholar]
- Chahine M, Bkaily G, Nader M, Al-Khoury J, Jacques D, Beier N, et al. NHE-1-dependent intracellular sodium overload in hypertrophic hereditary cardiomyopathy: prevention by NHE-1 inhibitor. J Mol Cell Cardiol. 2005;38:571–582. doi: 10.1016/j.yjmcc.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Chandler MP, Stanley WC, Morita H, Suzuki G, Roth BA, Blackburn B, et al. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res. 2002;91:278–280. doi: 10.1161/01.res.0000031151.21145.59. [DOI] [PubMed] [Google Scholar]
- Chattou S, Coulombe A, Diacono J, Le Grand B, John G, Feuvray D. Slowly inactivating component of sodium current in ventricular myocytes is decreased by diabetes and partially inhibited by known Na+-H+ exchange blockers. J Mol Cell Cardiol. 2000;32:1181–1192. doi: 10.1006/jmcc.2000.1151. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen CX, Can XT, Beier N, Scolz W, Karmazyn M. Inhibition and reversal of myocardial infarction-induced hypertrophy and heart failure by NHE-1 inhibition. Am J Physiol. 2004;286:H381–H387. doi: 10.1152/ajpheart.00602.2003. [DOI] [PubMed] [Google Scholar]
- Chen L, Gan XT, Haist JV. Attenuation of compensatory right ventricular hypertrophy and heart failure following monocrotaline-induced pulmonary vascular injury by the Na+/H+-exchange inhibitor cariporide. J Pharmacol Exp Ther. 2001;298:469–476. [PubMed] [Google Scholar]
- Cingolani HE, Ennis IL. Sodium-hydrogen exchanger, cardiac overload and myocardial hypertrophy. Circulation. 2007;115:1090–1100. doi: 10.1161/CIRCULATIONAHA.106.626929. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Matsumoto T, Motley ED, Utsunomiya H, Inagami T. Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells. J Biol Chem. 1996;271:14169–14175. doi: 10.1074/jbc.271.24.14169. [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Hein L, Keller U, Klämbt K, Lohse MJ. Inhibition of Na+-H+ exchange prevents hypertrophy, fibrosis, and heart failure in β1-adrenergic receptor transgenic mice. Circ Res. 2002;90:814–819. doi: 10.1161/01.res.0000014966.97486.c0. [DOI] [PubMed] [Google Scholar]
- Gao X, Kiriazis H, Moore X, Feng X, Sheppard K, Dart A, et al. Regression of pressure overload-induced left ventricular hypertrophy in mice. Am J Physiol. 2005;288:H2702–H2707. doi: 10.1152/ajpheart.00836.2004. [DOI] [PubMed] [Google Scholar]
- Gu JW, Anand V, Shek EW, Moore MC, Brady AL, Kelly EC. Sodium induces hypertrophy of cultured myocardial myoblasts and vascular smooth muscle cells. Hypertension. 1998;31:1083–1087. doi: 10.1161/01.hyp.31.5.1083. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L, Bers DM. Passsive Ca buffering and SR Ca uptake in permeabilized rabbit ventricular myocytes. Am J Physiol. 1993;33:C677–C686. doi: 10.1152/ajpcell.1993.264.3.C677. [DOI] [PubMed] [Google Scholar]
- Hwang JJ. Genomics and the pathophysiology of heart failure. J Am Coll Curr Cardiol Rep. 2001;3:198–207. doi: 10.1007/s11886-001-0023-z. [DOI] [PubMed] [Google Scholar]
- Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res. 2004;61:208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail. 2007;9:219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E, Koretsune Y. Cell calcium, oncognese and hypertrophy. Hypertension. 1990;15:652–658. doi: 10.1161/01.hyp.15.6.652. [DOI] [PubMed] [Google Scholar]
- Mentzer RM.The EXPEDITION study Investigators. Effect of Na+/H+-exchange inhibition by cariporide on death and nonfatal myocardial infarction in patient undergoing coronary artery bypass graft surgery: the EXPEDITION study Circulation 20031083M(abstract) [Google Scholar]
- Mulder P, Devaux B, Richard V. Early versus delayed angiotensin-converting enzyme inhibition in experimental chronic heart failure. Effects on survival, hemodynamics and cardiovascular remodelling. Circulation. 1997;95:1314–1319. doi: 10.1161/01.cir.95.5.1314. [DOI] [PubMed] [Google Scholar]
- Peng M, Huang L, Xie Z, Huang WH, Askari A. Partial inhibition of Na+/K+-ATPase by oubain induces Ca2+-dependent expression of early response genes in cardiac myocytes. J Biol Chem. 1996;271:10372–10378. doi: 10.1074/jbc.271.17.10372. [DOI] [PubMed] [Google Scholar]
- Ruetten H, Gehring D, Hiss K, Schindler U, Gerl M, Busch AE, et al. Effects of combined inhibition of the Na+-H+-exchanger and angiotension-converting enzyme in rats with congestive heart failure after myocardial infarction. Br J Pharmacol. 2005;146:723–731. doi: 10.1038/sj.bjp.0706381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehl D, Chua BH, Lautensack-Belser N, Morgan HE. Faster protein and ribosome synthesis in thyroxine-induced hypertrophy of rat heart. Am J Physiol. 1985;248:C309–C319. doi: 10.1152/ajpcell.1985.248.3.C309. [DOI] [PubMed] [Google Scholar]
- Takewaki S, Kuro-o M, Hiroi Y. Activation of Na(+)-H+ antiporter (NHE-1) gene expression during growth, hypertrophy and proliferation of the rabbit cardiovascular system. J Moll Cell Cardiol. 1995;25:729–742. doi: 10.1016/s0022-2828(08)80063-6. [DOI] [PubMed] [Google Scholar]
- Theroux P, Chaitman BR, Danchin N, Erhardt L, Meinertz T, Schroeder JS, et al. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardaial infarction in high-risk ischemic situations. Main results of the GUARDIAN Trial Guard During Ischemic Against Necrosis (GUARDIAN) Investigators. Circulation. 2000;102:3032–3038. doi: 10.1161/01.cir.102.25.3032. [DOI] [PubMed] [Google Scholar]
- van Borren MMGJ, Baartscheer A, Wilders R, Ravesloot JH. NHE-1 and NBC during pseudo-ischemia/reperfusion in rabbit ventricular myocytes. J Mol Cell Cardiol. 2004;37:567–577. doi: 10.1016/j.yjmcc.2004.05.017. [DOI] [PubMed] [Google Scholar]
- van Borren MM, Zeegers JG, Baartscheer A, Ravesloot JH. Contribution of NHE-1 to cell length shortening of normal and failing rabbit cardiac myocytes. J Mol Cell Cardiol. 2006;41:706–715. doi: 10.1016/j.yjmcc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Yao A, Su Z, Nonaka A, Zubair I, Spitzer KW, Bridge JH, et al. Abnormal myocyte Ca2+ homeostasis in rabbits with pacing-induced heart failure. Am J Physiol. 1998;275:H1441–H1448. doi: 10.1152/ajpheart.1998.275.4.H1441. [DOI] [PubMed] [Google Scholar]
- Zeymer U, Suryapranata H, Monassier JP, Opolski G, Davies J, Rasmanis G, et al. The Na+-H+-exchange inhibitor eniporide as an adjunct reperfusion therapy for acute myocardial infarction. Results of the Evaluation of the Safety and Cardioprotective Effects of Eniporide in Acute Myocardial Infarction (ESCAMI) Trial. J Am Coll Cardiol. 2001;38:1644–1650. doi: 10.1016/s0735-1097(01)01608-4. [DOI] [PubMed] [Google Scholar]
- Zou Y, Komuro I, Yamazaki T. Protein kinase C, but not tyrosine kinase of Ras, plays a critical role in angiotensin II-induced activation of Raf-I kinase and extracellular signal regulated protein kinase in cardiac myocytes. J Biol Chem. 1996;271:33592–33597. doi: 10.1074/jbc.271.52.33592. [DOI] [PubMed] [Google Scholar]