Abstract
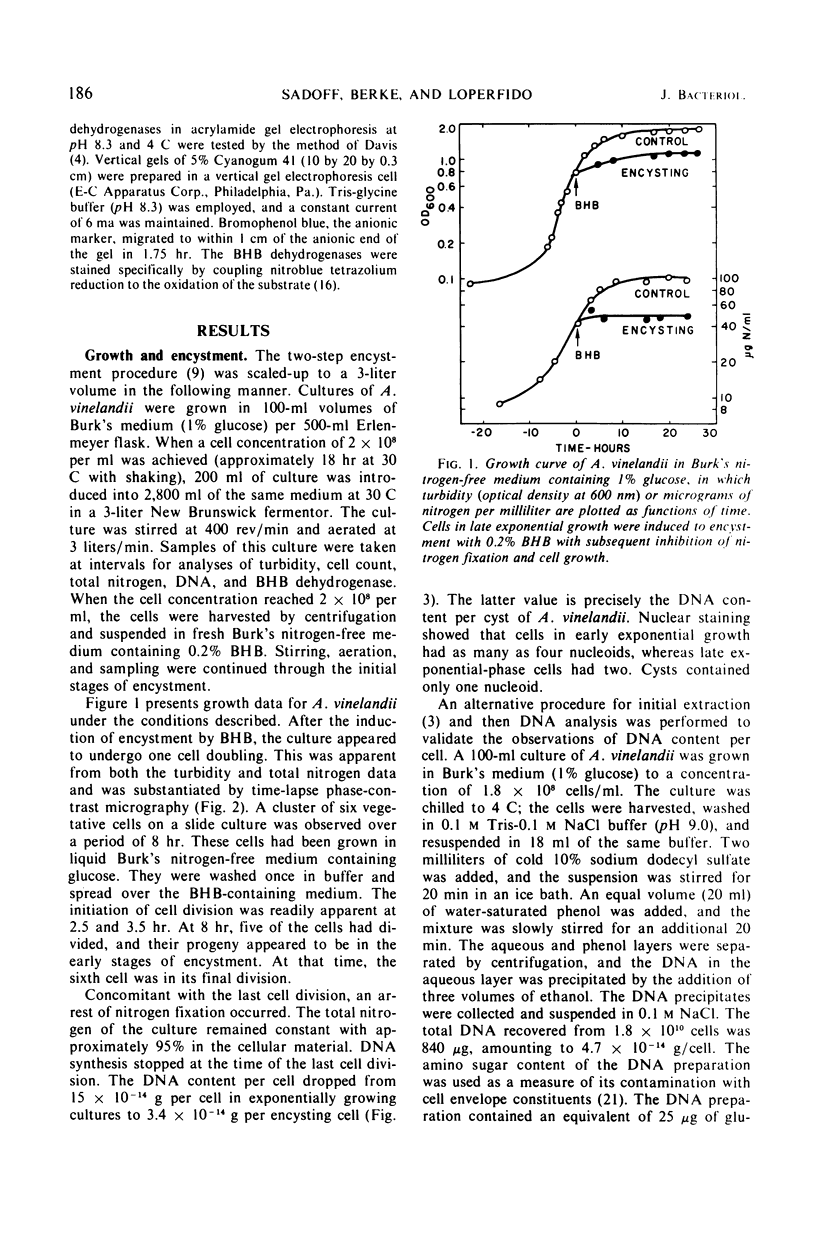
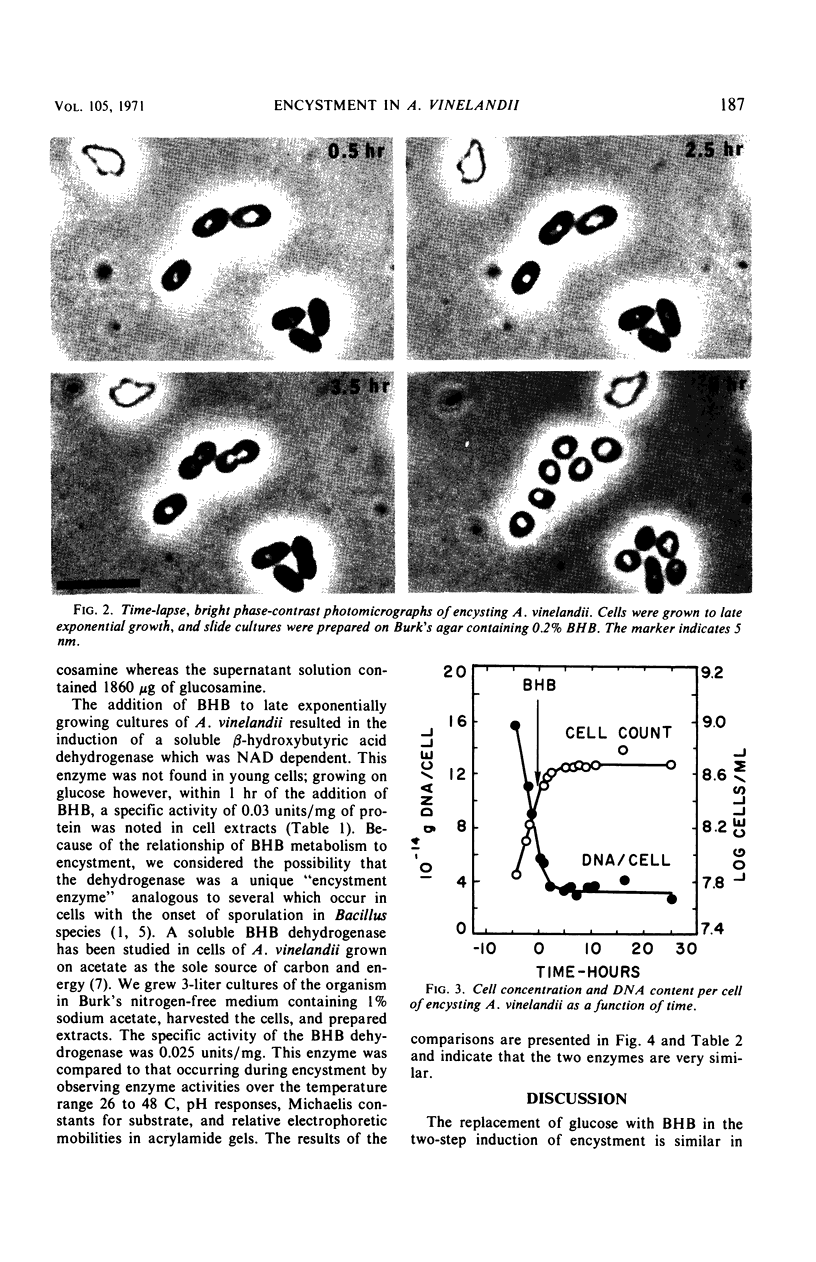
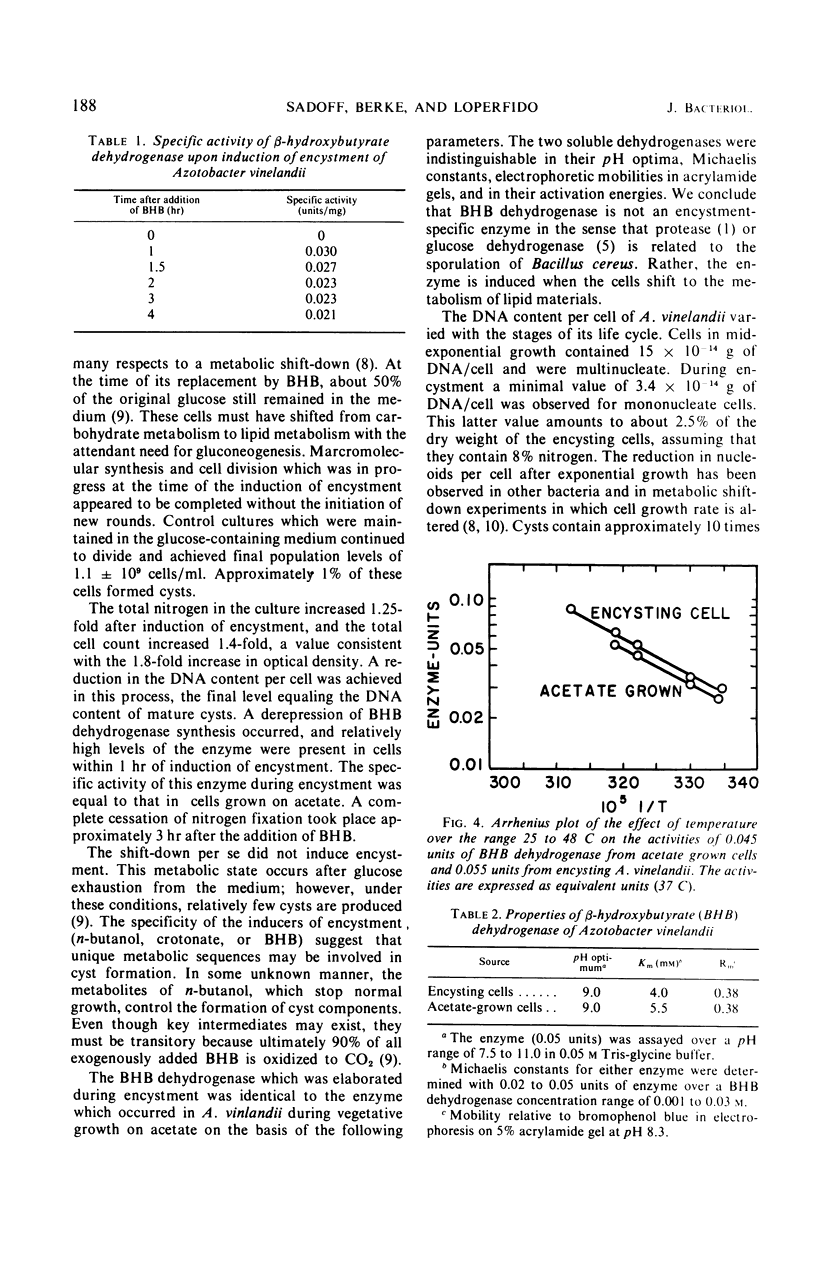
Azotobacter vinelandii, in late exponential growth phase, encysts when the glucose in the medium is replaced with β-hydroxybutyrate. A final cell division then occurs without apparent deoxyribonucleic acid (DNA) synthesis, resulting in a reduction from two to one nucleoids per cell and a final DNA content of 3.2 × 10−14 g per cell. This is also the DNA content per cyst. A β-hydroxybutyrate dehydrogenase is derepressed by the addition of the inducer and is identical to the enzyme in acetate-grown cells in its pH optimum, Michaelis constant for substrate, temperature-activity response, and mobility during electrophoresis in acrylamide gel.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DOI R., HALVORSON H., CHURCH B. Intermediate metabolism of aerobic spores. III. The mechanism of glucose and hexose phosphate oxidation in extracts of Bacillus cereus spores. J Bacteriol. 1959 Jan;77(1):43–54. doi: 10.1128/jb.77.1.43-54.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
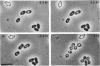
- Hitchins V. M., Sadoff H. L. Morphogenesis of cysts in Azotobacter vinelandii. J Bacteriol. 1970 Oct;104(1):492–498. doi: 10.1128/jb.104.1.492-498.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Manning S., Barrera C. R. Isolation and purification of the D(-)beta-hydroxybutyric dehydrogenase of Azotobacter vinelandii. Can J Microbiol. 1968 Jul;14(7):775–783. doi: 10.1139/m68-129. [DOI] [PubMed] [Google Scholar]
- KJELDGAARD N. O., MAALOE O., SCHAECHTER M. The transition between different physiological states during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):607–616. doi: 10.1099/00221287-19-3-607. [DOI] [PubMed] [Google Scholar]
- Lin L. P., Sadoff H. L. Encystment and polymer production by Azotobacter vinelandii in the presence of beta-hydroxybutyrate. J Bacteriol. 1968 Jun;95(6):2336–2343. doi: 10.1128/jb.95.6.2336-2343.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A. K., Wyss O. An adenine-requiring mutant of Azotobacter vinelandii blocked in inosinic acid synthesis. Experientia. 1969 Jan 15;25(1):85–85. doi: 10.1007/BF01903913. [DOI] [PubMed] [Google Scholar]
- Müller H. P., Kern H. Strahlenresistenz, Gehalt und Basenzusammensetzung der DNA einiger strahleninduzierter Mutanten von Azotobacter chroococcum. Z Naturforsch B. 1967 Dec;22(12):1330–1336. [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOCOLOFSKY M. D., WYSS O. Resistance of the Azotobacter cyst. J Bacteriol. 1962 Jul;84:119–124. doi: 10.1128/jb.84.1.119-124.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L., Hitchins A. D., Celikkol E. Properties of fructose 1,6-diphosphate aldolases from spores and vegetative cells of Bacillus cereus. J Bacteriol. 1969 Jun;98(3):1208–1218. doi: 10.1128/jb.98.3.1208-1218.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCHAN Y. T., BIRCH-ANDERSEN A., JENSEN H. L. The ultrastructure of vegetative cells and cysts of Azotobacter chroococcum. Arch Mikrobiol. 1962;43:50–66. doi: 10.1007/BF00408395. [DOI] [PubMed] [Google Scholar]
- WHEAT R. W., ROLLINS E. L., LEATHERWOOD J. M., BARNES R. L. Studies on the cell wall of Chromobacterium violaceum: the separation of lipopolysaccharide and mucopeptide by phenol extraction of whole cells. J Biol Chem. 1963 Jan;238:26–29. [PubMed] [Google Scholar]
- WYSS O., NEUMNN M. G., SOCOLOFSKY M. D. Development and germination of the Azotobacter cyst. J Biophys Biochem Cytol. 1961 Aug;10:555–565. doi: 10.1083/jcb.10.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]