Abstract
Background:
Macrophage elastase (MMP-12) is involved in the inflammatory process of chronic obstructive pulmonary disease (COPD). The aim of this study was to investigate in mice the effect of MMP-12 inhibition on the inflammatory process induced by cigarette smoke (CS) or by lipopolysaccharide (LPS) exposure of the airways.
Experimental approach:
C57BL/6 mice were given, orally, either the selective MMP-12 inhibitor AS111793 (3, 10, 30 and 100 mg kg−1), the PDE-4 inhibitor roflumilast (3 mg kg−1) or vehicle, then exposed to CS (for 3 days) or to LPS (100 μg mL−1, 30 min). Subsequent to the last smoke or LPS exposure, bronchoalveolar lavages (BAL) were performed and lungs were removed and homogenized to analyze various markers of inflammation at appropriate times.
Key results:
Inhibition of MMP-12 by AS111793 (10 and 30 mg kg−1) was associated with a reduction of the increase in neutrophil number in BAL fluids after 4 days and of macrophages after 11 days. On day 4, AS111793 also significantly reduced all the inflammation markers that had increased after CS exposure, including soluble TNF receptors I and II, MIP-1γ, IL-6 and pro-MMP-9 activity in BAL fluids, and KC/CXCL1, fractalkine/CX3CL1, TIMP-1 and I-TAC/CXCL11 in lung parenchyma. In contrast, inhibition of MMP-12 did not reduce neutrophil influx, pro-MMP-9 activity or KC/CXCL1 release in BAL fluids of mice exposed to LPS.
Conclusion:
Inhibition of MMP-12 with AS111793, reduced the inflammatory process associated with exposure of mice to CS, strongly suggesting a specific involvement of MMP-12 in lung inflammation following CS exposure.
Keywords: MMP-12, cigarette smoke, lung, inflammation, COPD, LPS
Introduction
Chronic obstructive pulmonary disease (COPD) is a rapidly increasing global health problem, predicted to be the third leading causes of death in developed countries by 2020. COPD is characterized by slowly progressive and largely irreversible, airflow limitation due to chronic bronchitis, emphysema or both, associated with an abnormal inflammatory response of the lungs. This inflammatory process in lung parenchyma is characterized, among other changes, by an accumulation of inflammatory cells such as macrophages and neutrophils in the alveoli, bronchioli and small airways. The chronic airflow obstruction is also, in part, due to a loss of lung elasticity due to enzymatic destruction of the lung parenchyma by proteases.
Cigarette smoke (CS) has been identified as the most important risk factor for the development of COPD. Hence, the protease-antiprotease imbalance is thought to play a key role in CS-induced chronic lung disease. Most studies have focused on the role of serine protease neutrophil elastase (Stockley, 2000; Ohbayashi, 2002), but there is increasing evidence that matrix metalloproteinases (MMPs) may have a pivotal role in cigarette smoking-related COPD (Barnes et al., 2003; Belvisi and Bottomley, 2003).
Macrophage metalloelastase (MMP-12; EC 3.4.24.65) is mainly produced by macrophages and seems to be involved in acute and chronic pulmonary inflammatory diseases associated with intense airway remodelling (Nénan et al., 2005a). MMP-12 is able to degrade different substrates, among which is elastin, the major constituent of alveolar walls. Recently, a study has shown MMP-12 overexpression in cells recovered from bronchoalveolar lavage (BAL) and bronchial biopsies of COPD patients, suggesting this overexpression as a critical step in the pathogenesis of COPD and emphysema (Molet et al., 2005). Moreover, MMP-12 is found in greater amounts in sputum from smokers and correlates with the decline of lung function (Demedts et al., 2006). Also, there is evidence in preclinical rodent models that MMP-12 participates in the development of lung inflammation and emphysema. It is well established that mice deficient in the gene encoding MMP-12 (MMP-12−/−) show a diminished inflammatory response induced by long-term exposure to CS and are resistant to the development of emphysema (Hautamaki et al., 1997). Moreover, in an acute model of CS exposure in mice, MMP-12 gene deletion was shown to protect against the neutrophil influx induced by CS (Leclerc et al., 2006).
The development of selective MMP-12 inhibitors would provide insight into these processes and may be useful in the development of new therapies. It was recently reported that a dual MMP-9/MMP-12 inhibitor, AZ11557272, protected against smoke-mediated increases in small airway wall thickness, in guinea pigs exposed daily to CS for up to 6 months (Churg et al., 2007). The aim of this study was to further investigate the role of MMP-12 in the early stages of lung inflammation induced by CS exposure, using a new selective MMP-12 inhibitor, AS111793 (2-hydroxy-3-[1-(thiophenyl-oxadiazolyl)-2,2-dimethyl-propylcarbamoyl]-methyl hexanohydroxamic acid) (Ayscough et al., 2003). In contrast to the lung inflammation elicited by a lipopolysaccharide (LPS) challenge, our study shows that inflammation induced by exposure to CS can be prevented by the MMP-12 inhibitor AS111793.
Materials and methods
Materials
AS111793 and roflumilast were provided by Serono Pharmaceutical Research Institute (Geneva, Switzerland).
Lipopolysaccharide (from Escherichia coli, serotype 055:B5), PEG 400, Triton X-100 and gelatin were purchased from Sigma Chemicals (St Louis, MO, USA). Kentucky 1R3 cigarettes (Tobacco Health Research) were provided by University of Kentucky, Lexington, KY, USA. Sodium pentobarbital was from Sanofi Santé Animal (Libourne, France). May-Grünwald and Giemsa stains were from RAL (Paris, France). Acrylamide, sodium dodecyl sulphate and BSA were from Eurobio (Les Ulis, France). Coomassie Brilliant Blue was from Biorad (Munich, Germany). The ELISA kits for murine IL-6 and KC/CXCL1 detection and recombinant MMP-2 and MMP-9 were purchased from R&D Systems (Abingdon, UK). Mouse Cytokine Antibody Arrays were from Ray Biotech, (Norcross, GE, USA).
Animals and experimental protocols
CS and LPS exposure
All animal procedures were approved by the local Ethical Committee for experiments using animals and these complied with the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Marketing and Education, New York Academy of Sciences' Ad Hoc Committee on Animal Research.
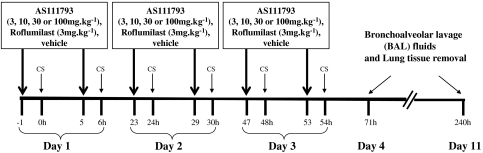
Ten-week-old C57/BL6 male mice (CERJ, Le Genest Saint Isle, France) were orally treated with either the MMP-12 inhibitor AS111793 (3, 10, 30, 100 mg kg−1) or the PDE-4 inhibitor roflumilast (3 mg kg−1) or with vehicle (poly-ethyleneglycol (PEG) 400/distilled water (1:1)) in a volume of 300 μL, 1 h before each CS exposure. Mice were exposed to the smoke of two cigarettes, twice a day for 3 days (Figure 1) as described previously (Leclerc et al., 2006).
Figure 1.
Experimental protocol of exposure to cigarette smoke (CS). Mice were orally treated with either AS111793 (3, 10, 30,100 mg kg−1) or roflumilast (3 mg kg−1) or vehicle (poly-ethyleneglycol (PEG) 400/distilled water (1:1)), 1 h before each CS exposure. Mice were exposed to the whole smoke of two cigarettes twice a day for 3 days. Bronchoalveolar lavage (BAL) fluids and lung tissues were collected 4 (day 4) or 11 days (day 11) after the first CS exposure.
Ten-week-old Balb/c mice (CERJ) were orally treated with either AS111793 (10, 30 or 100 mg kg−1) or vehicle (PEG 400/distilled water (1:1)) in a volume of 300 μL, 1 h before the exposure to an aerosol of LPS (100 μg mL−1) for 30 min as described previously (Corbel et al., 2002).
Positive controls (mice treated with vehicle and exposed either to CS or to LPS) were systematically performed in each experiment to assess that the level of airway inflammatory response is comparable in each experiment.
Bronchoalveolar lavage and tissue collection
Bronchoalveolar lavage was performed, 4 (Day 4) or 11 (Day 11) days after the first CS exposure (Figure 1), or 24 h after LPS exposure. Total and differential cell counts were determined in BAL fluids as described previously (Corbel et al., 2002).
After lavage of the airspaces, lungs were removed, snap frozen in liquid nitrogen and then stored at −80 °C until tissue homogenization using Lysing Matrix D tubes (Q-biogene Inc., Illkirch, France) and Fast-Prep FP 120 cell disrupter (Q-biogene Inc.).
Measurement of MMPs, cytokines and other markers of inflammation
Metalloproteinases were detected in the supernatants of BAL fluids and lung homogenates using zymography and ELISA method (R&D system, Abingdon, UK) as described previously (Corbel et al., 2002). The amounts of interleukin-6 (IL-6) and keratinocyte-derived chemokine (KC/CXCL1; the IL-8/CXCL8 counterpart in mice) in BAL fluids and in lung homogenate supernatants were quantified by ELISA method. A commercial antibody-based protein array designed to detect 40 inflammatory mediators was used according to the manufacturer's instructions (Ray Bio Mouse Cytokine Antibody Array I and 1.1 (40), Ray Biotech Inc., Norcross, GE, USA).
Analysis and expression of results
The results are expressed as means±s.e.m. Analysis of effects between treatments was performed with the non-parametric Mann–Whitney test. For each analysis, P-values less than 5% were considered statistically significant.
Results
Selectivity of AS111973 as MMP inhibitor
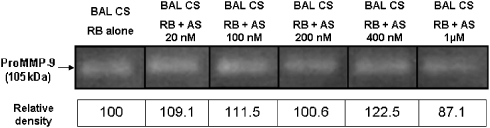
AS111793 inhibits recombinant human MMP-12 in vitro with an IC50 value of 20 nM and displays selectivity ratios for MMP-1 (interstitial collagenase), MMP-2 (gelatinase A) and MMP-9 (gelatinase B) of 1:30, 1:30 and 1:40, respectively. To rule out possible inhibition by AS111793 of MMP-9 activity in our ex vivo system, we used BAL fluids sampled from the lungs of mice exposed to CS (see methods). Aliquots of BAL fluids were subjected to electrophoresis in the presence of sodium dodecyl sulphate in acrylamide gel containing 1 mg mL−1 gelatin under non-reducing conditions. After electrophoresis, gels were washed with 2.5% Triton X-100, rinsed with water and incubated at 37 °C overnight in reaction buffer containing various concentrations of AS111793 (up to 1 μM). The results are presented in Figure 2 and show that AS111793 did not inhibit MMP-9 activity present in the BAL fluids at concentrations up to 400 nM and that only a very limited inhibition was observed in the presence of 1 μM AS111793 (12.9% inhibition vs control).
Figure 2.
Effect of AS111793 on matrix metalloproteinase (MMP)-9 activity ex vivo. Zymography was performed with aliquots of bronchoalveolar lavage (BAL) fluids recovered in the airways of mice exposed to cigarette smoke (BAL CS). After electrophoresis, gels were incubated at 37 °C overnight in reaction buffer (RB) containing various concentrations of AS111793 (AS 0, 20, 100, 200, 400 nM and 1 μM). Quantification of the 105 kDa MMP-9 gelatinase activity was performed by image analysis and densitometry on zymograms.
Effects of AS111793 on cell composition in BAL fluid of mice exposed to CS
Mice exposed to the smoke of two cigarettes, twice daily for 3 days, elicited a marked and significant influx of inflammatory cells in BAL fluids at day 4, as compared with control conditions, that is exposure to air (Table 1). These inflammatory cells were mainly neutrophils, whereas the number of macrophages did not change significantly. Interestingly, these two cell types represented the majority of the cells recovered from BAL fluids, as lymphocytes and eosinophils accounted for a maximum of 0.5% of the total cells. Treatment of the mice with the selective MMP-12 inhibitor, AS111793, significantly reduced the increase in total number of inflammatory cells in BAL fluids (P<0.01 at 3, 10 and 30 mg kg−1 vs vehicle-treated controls), as well as in the number of neutrophils at 10 and 30 mg kg−1 (P<0.01). The PDE-4 inhibitor roflumilast (3 mg kg−1) also significantly reduced the number of total cells and of neutrophils in the same conditions, compared with control mice (P<0.01 and 0.001, respectively).
Table 1.
Effects of AS111793 on CS-induced changes in BAL cell composition at day 4
| Treatment | n | Total cells | Macrophages | Neutrophils | Eosinophils | Lymphocytes | |
|---|---|---|---|---|---|---|---|
| Air | 5 | 280±38 | 279±38 | 1±0.5 | 0 | 0 | |
| Vehicle | CS | 15 | 474±19## | 290±18a | 182±14### | 1±0.8 | 0.4±0.4 |
| AS111793 | Air | 6 | 236±14 | 235±14 | 1±1 | 0 | 0 |
| 3 mg kg−1 | CS | 6 | 357±19** | 202±20* | 154±18a | 0.3±0.3 | 0 |
| AS111793 | Air | 6 | 281±29 | 280±29 | 1±0.3 | 0 | 0.1±0.1 |
| 10 mg kg−1 | CS | 7 | 311±42** | 220±34a | 91±20** | 0 | 0.1±0.1 |
| AS111793 | Air | 6 | 192±36 | 192±36 | 0 | 0 | 0.3±0.3 |
| 30 mg kg−1 | CS | 6 | 273±48** | 181±29* | 91±28** | 0.2±0.2 | 0.1±0.1 |
| Roflumilast | Air | 6 | 275±38 | 275±38 | 0.5±0.3 | 0 | 0 |
| 3 mg kg−1 | CS | 7 | 279±31** | 210±31* | 69±16*** | 0.2±0.2 | 0 |
Abbreviations: BAL, bronchoalveolar lavage; CS, cigarette smoke; n, number of mice.
Total and differential cell counts in BAL fluids collected at day 4 in mice exposed to CS or air and treated orally with either vehicle (v:v), AS111793 (3, 10, 30 mg kg−1) or roflumilast (3 mg kg−1), 1 h before CS exposure. Results are presented as means ( × 103 cells)±s.e.m.
Nonsignificant.
##P<0.01, ###P<0.001 for mice exposed to CS compared with control mice exposed to air. *P<0.05, **P<0.01, ***P<0.001 compared with mice exposed to CS and treated with vehicle.
Using the same protocol of CS exposure (two cigarettes twice a day for 3 days) the total number of cells, as well as the cell composition in BAL fluids were also examined 11 days after the first cigarette (Table 2). At that time, a significant increase in the total number of cells was also observed but mainly due to an increase in the number of macrophages. The number of neutrophils, eosinophils and lymphocytes were not statistically different from those in the control group (Table 2). Also at day 11, AS111793 prevented the increase in macrophages, in a statistically significant manner at 30 and 100 mg kg−1 (P<0.05 vs controls). In contrast, 3 mg kg−1 roflumilast was ineffective, and did not significantly modify the macrophage number in mice exposed to CS.
Table 2.
Effects of AS111793 on CS-induced changes in BAL cell composition at day 11
| Treatment | n | Total cells | Macrophages | Neutrophils | Eosinophils | Lymphocytes | |
|---|---|---|---|---|---|---|---|
| Air | 6 | 210±37 | 210±37 | 0 | 0.2±0.2 | 0 | |
| Vehicle | CS | 12 | 415±37## | 414±37## | 1±0.8 | 0 | 0 |
| AS11193 | Air | 6 | 274±42 | 273±42 | 0.3±0.3 | 0 | 0 |
| 10 mg kg−1 | CS | 7 | 378±21a | 378±21a | 0.2±0.2 | 0 | 0 |
| AS111793 | Air | 6 | 206±30 | 206±30 | 0.2±0.2 | 0 | 0 |
| 30 mg kg−1 | CS | 7 | 315±33* | 313±32* | 1±0.6 | 1±1 | 0 |
| AS111793 | Air | 6 | 229±16 | 229±16 | 0.2±0.2 | 0 | 0 |
| 100 mg kg−1 | CS | 7 | 306±15* | 304±15* | 2±0.6 | 0 | 0 |
| Roflumilast | Air | 6 | 239±31 | 237±31 | 1.5±0.6 | 0 | 0 |
| 3 mg kg−1 | CS | 4 | 379±23a | 378±23a | 0.5±0.4 | 0.5±0.4 | 0.5±0.4 |
Abbreviations: BAL, bronchoalveolar lavage; CS, cigarette smoke; n, number of mice.
Total and differential cell counts in BAL fluids collected at day 11 in mice exposed to CS or air, and treated orally with either vehicle (v:v), AS111793 (10, 30, 100 mg kg−1) or roflumilast (3 mg kg−1), 1 h before CS exposure. Results are presented as means ( × 103 cells)±s.e.m.
Nonsignificant.
##P<0.01 for mice exposed to CS compared with control mice exposed to air.
*P<0.05 compared with mice exposed to CS and treated with vehicle.
Effects of AS111793 on gelatinase activity in BAL fluids and in lung homogenate supernatants of mice exposed to CS
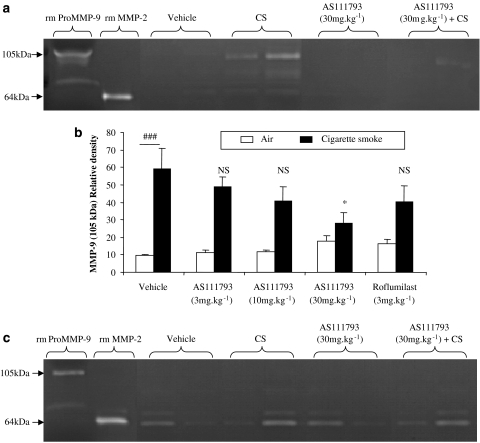
By zymography, the following gelatinolytic bands were identified as metalloproteinase activities: pro-MMP-9 (105 kDa), pro-MMP-2 (72 kDa) and MMP-2 (64 kDa). At day 4, exposure to CS induced a significant increase of pro-MMP-9 (105 kDa) activity in BAL fluids, in comparison with mice only exposed to air (Figure 3a). AS111793 partially inhibited the MMP-9 increase in mice exposed to CS, which was statistically significant at 30 mg kg−1 (P<0.05), whereas the treatment with roflumilast did not significantly modify MMP-9 activity (Figure 3b). Also, no significant increase of MMP-2 (64 kDa) was observed after CS exposure in the airways (Figure 3a). In lung homogenate supernatants, no difference in both MMP-9 and MMP-2 activities could be observed between controls and CS exposed mice (data not shown).
Figure 3.
Zymographic analysis of the bronchoalveolar lavage (BAL) fluids recovered in the airways of mice exposed to two cigarettes twice daily for 3 days smoke exposure, after 4 (a) or after 11 days (c) following the first smoke exposure. Mice were orally treated with either vehicle (poly-ethyleneglycol (PEG) 400/distilled water) or AS111793 (3, 10, 30, 100 mg kg−1) or roflumilast (3 mg kg−1), 1 h before each CS exposure. (a and c) Representative gelatin zymograms performed with samples of BAL fluids from mice exposed to air and orally treated with vehicle (lanes 3–4) or with AS111793, 30 mg kg−1 (lanes 7–8), or from mice exposed to CS and treated with vehicle (lanes 5–6) or with AS111793, 30 mg kg−1 (lanes 9–10). Lane 1, recombinant mouse pro-matrix metalloproteinase (MMP)-9 (rm pro-MMP-9); lane 2, recombinant mouse MMP-2 (rm MMP-2). (b) Quantification of the 105 kDa MMP-9 gelatinase activity by image analysis and densitometry on zymograms of BAL fluids performed at day 4, in mice exposed to CS or to air. ###P<0.001 for mice exposed to CS compared with control mice exposed to air. *P<0.05 compared with mice orally treated with vehicle and exposed to CS. NS, nonsignificant. N=6–7 per group.
At day 11 (Figure 3c), no significant differences in both MMP-9 and MMP-2 activities between control and CS exposed mice were observed in BAL fluids or supernatants of lung homogenates.
Quantification of IL-6 and KC/CXCL1
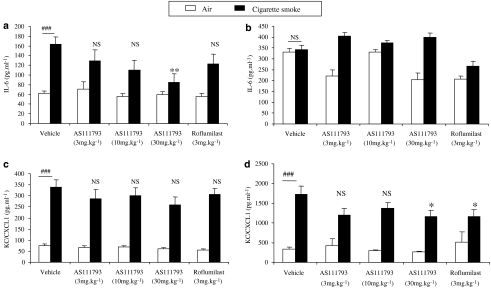
At day 4, IL-6 concentration significantly increased in BAL fluids of mice exposed to CS in comparison with controls (P<0.001; Figure 4a). AS111793 elicited an inhibition of IL-6 release in BAL fluids of CS-exposed mice, which was statistically significant at 30 mg kg−1 (P<0.01 vs vehicle-treated mice). Treatment with roflumilast did not reduce IL-6 levels in BAL fluids of mice exposed to CS (Figure 4a). In contrast, despite higher levels of IL-6, there was no significant change after CS exposure and after treatment with either AS111793 or roflumilast (Figure 4b) in lung homogenates.
Figure 4.
Levels of interleukin (IL)-6 (a and b) and KC/CXCL-1 (c and d) in bronchoalveolar lavage (BAL) fluids (a and c) and in lung homogenate supernatants (b and d) from mice exposed to cigarette smoke (CS) or to air, orally treated with either vehicle ((poly-ethyleneglycol (PEG) 400/distilled water) or AS111793 (3, 10, 30 mg kg−1) or roflumilast (3 mg kg−1). BAL fluids and lung tissues were collected 24 h after the last CS exposure (on day 4). Results are expressed in pg mL−1 (means±s.e.m). ###P<0.001 for mice exposed to CS compared with mice exposed to air. **P<0.01, *P<0.05 compared with mice orally treated with vehicle and exposed to CS. NS, nonsignificant. N=6–7 per group.
Exposure of mice to CS also induced a significant increase in KC/CXCL1 levels in BAL fluids after 4 days in comparison with control mice and neither AS111793 nor roflumilast significantly prevented this increase (Figure 4c). Treatment with AS111793 (30 mg kg−1) and with roflumilast (3 mg kg−1) moderately, but significantly (P<0.05), decreased KC/CXCL1 levels in lung homogenate supernatants (Figure 4d).
Eleven days after the first CS exposure, no significant increase in IL-6 and KC/CXCL1 could be observed in BAL fluids or in lung homogenates, when compared with control mice (data not shown).
Characterization of several cytokines and chemokines secreted at day 4 in BAL fluids and lung homogenate supernatants
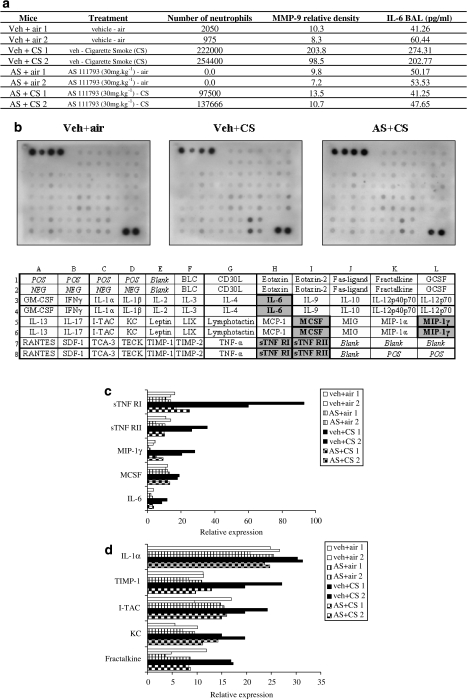
Bronchoalveolar lavage fluids and lung homogenate supernatants were further analysed at day 4 using protein arrays to examine whether other major inflammatory mediators were affected by CS exposure or by the treatment with AS111793 (30 mg kg−1). Two animals of each condition (vehicle+air, vehicle+CS, AS111793+air, AS111793+CS) were tested using these arrays (Figure 5a). Forty mediators were semiquantitatively analysed (Figure 5b) and only those mostly affected by CS exposure are presented in Figures 5c and d. Exposure to CS elicited a rise in soluble tumour necrosis factor-α receptors (sTNFR)-I and to a lesser degree of sTNFR-II, macrophage inflammatory protein-1γ (MIP-1γ), IL-6 and macrophage colony-stimulating factor in BAL fluids (Figure 5c). In lung homogenates, CS exposure induced an increase in tissue inhibitor of metalloproteinase-1 (TIMP-1), interferon-inducible T-cell chemoattractant (I-TAC/CXCL11), fractalkine/CX3CL1, KC/CXCL1 and IL-1α (Figure 5d). All these mediators were strongly reduced by treatment with AS111793 (30 mg kg−1) (Figures 5c and d).
Figure 5.
Determination of several inflammatory mediators at day 4, using protein microarrays. (a) Characteristics of the representative mice chosen to perform the arrays. (b) Pictures of three arrays performed on bronchoalveolar lavage (BAL) fluids of controls (vehicle+air), mice treated with vehicle and exposed to cigarette smoke (veh+CS) and mice treated with AS111793 and exposed to CS (AS+CS); and design of the array (POS, positive control; NEG, negative control). Mediators in BAL fluids (c) and in lung homogenate supernatants (d) of mice orally treated with either vehicle ((poly-ethyleneglycol (PEG) 400/distilled water) or AS111793 (30 mg kg−1) and exposed to air or CS. Density of each spot on the array was corrected for adjacent background intensity and normalized to the membrane's positive controls. Results are presented as relative expression.
Effects of AS111793 on cell composition in BAL fluids recovered in mice exposed to LPS
Total and differential cell counts were determined after a single exposure of mice for 30 min to an aerosol of LPS (100 μg mL−1). Similar to the experiments using CS, exposure to LPS elicited a marked and significant influx of neutrophils in BAL fluids 24 h after exposure, in comparison with control mice exposed to an aerosol of saline (Table 3). There were no changes in the number of macrophages. However, in this model, treatment of mice with AS111793, in doses of up to 100 mg kg−1, did not induce any significant change in the increased number of neutrophils in BAL fluids.
Table 3.
Effects of AS111793 on LPS-induced changes in BAL cell composition
| Treatment | n | Total cells | Macrophages | Neutrophils | Eosinophils | Lymphocytes | |
|---|---|---|---|---|---|---|---|
| Saline | 6 | 276±33 | 257±32 | 18±2 | 0 | 0 | |
| Vehicle | LPS | 16 | 808±83### | 213±23a | 595±71### | 0 | 0 |
| AS111793 | Saline | 6 | 229±44 | 215±44 | 14±5 | 0 | 0 |
| 10 mg kg−1 | LPS | 7 | 637±57a | 161±22a | 475±42a | 0 | 0 |
| AS111793 | Saline | 6 | 234±36 | 221±33 | 12±7 | 0 | 0 |
| 30 mg kg−1 | LPS | 6 | 676±49a | 184±25a | 492±64a | 0 | 0 |
| AS111793 | Saline | 6 | 189±19 | 182±19 | 7±3 | 0 | 0 |
| 100 mg kg−1 | LPS | 8 | 953±32a | 263±46a | 690±37a | 0 | 0 |
Abbreviations: BAL, bronchoalveolar lavage; CS, cigarette smoke; LPS, lipopolysaccharide; n, number of mice.
Total and differential cell counts in BAL fluids collected 24 h in mice exposed to an aerosol of LPS (30 min, 100 μg mL−1) or of saline (controls). Mice were orally treated with either vehicle (v:v) or AS111793 (10, 30, 100 mg kg−1) or roflumilast (3 mg kg−1), 1 h before LPS exposure. Results are presented as means ( × 103 cells)±s.e.m.
Nonsignificant compared with mice exposed to LPS and treated with vehicle.
###P<0.001 for mice exposed to LPS compared with mice exposed to NaCl.
Effects of AS111793 on pro-MMP-9 activity in BAL fluid of mice exposed to LPS
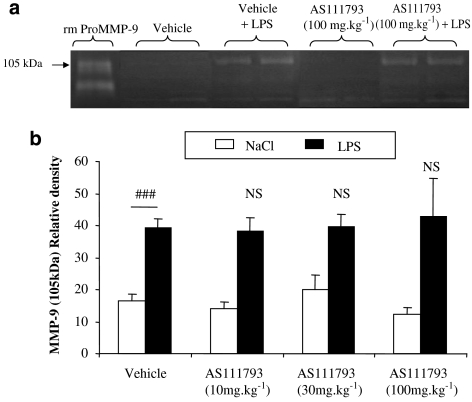
Exposure of mice to an aerosol of LPS (100 μg mL−1) induced a significant increase in pro-MMP-9 (105 kDa) activity in BAL fluids in comparison with control mice exposed to saline (Figure 6a). The treatment of mice with AS111793 at doses up to 100 mg kg−1 did not affect the increase in MMP-9 activity (Figure 6b).
Figure 6.
Zymographic analysis of the bronchoalveolar lavage (BAL) fluids recovered in the airways of mice exposed to an aerosol of lipopolysaccharide (LPS) or saline. Mice were orally treated with either vehicle ((poly-ethyleneglycol (PEG) 400/distilled water) or AS111793 (10, 30, 100 mg kg−1), 1 h before LPS or saline exposure. (a) Representative gelatin zymograms performed with samples of BAL fluids from mice exposed to NaCl and orally treated with either vehicle (lanes 2–3) or AS111793, 100 mg kg−1 (lanes 6–7), or from mice exposed to LPS and treated with vehicle (lanes 4–5) or with AS111793, 100 mg kg−1 (lanes 8–9). Lane 1, recombinant mouse pro-matrix metalloproteinase (MMP)-9 (rm pro-MMP9). (b) Quantification of the 105 kDa MMP-9 gelatinase activity by image analysis and densitometry on zymograms of BAL fluids performed in mice exposed to LPS or to NaCl. ###P<0.001 for mice exposed to LPS compared with control mice exposed to NaCl. NS, nonsignificant. N=6–7 per group.
Discussion
This study reports that the new MMP-12 inhibitor, AS111793, reduced the lung inflammation associated with CS exposure in mice.
A marked influx of neutrophils was observed in airways on day 4 after 3 days of exposure to CS. This influx was associated with the release of cytokines and chemokines and with increased gelatinase, mainly MMP-9, activity. Then, 7 days after the last exposure to CS (day 11), the number of macrophages in the airways increased whereas neutrophil number returned to control values and levels of MMP-9 activity and cytokines were not statistically different, in comparison with controls. Both increases in neutrophil number at day 4 and macrophage number at day 11 were significantly smaller after treatment with the MMP-12 inhibitor AS111793. In contrast, treatment of mice with AS111793 at dose levels up to 100 mg kg−1 did not significantly modify the neutrophil number in the airways of mice exposed to an aerosol of LPS.
The pharmacokinetic profile following a single oral dose of 5 mg kg−1 AS111793 in mice was investigated and Cmax and area under the curve were found to be 1771 ng mL−1 and 989 ng h mL−1, respectively. Other doses were not tested in mice, but a more comprehensive study in rats suggests that area under the curve could be extrapolated to 1980 ng h mL−1and 5940 ng h mL−1 after 10 and 30 mg kg−1, respectively. The compound has a short half-life in mice; it is entirely cleared 6 h after administration of 10 mg kg−1 and 8 h after 30 mg kg−1, respectively. Therefore, Caveg can be extrapolated in the present experiment to 330 or 743 ng mL−1 at these two doses when using a b.i.d. regimen. Also, the compound is 95% bound to plasma proteins in rodents, which would result in a free fraction of only 15 or 37 ng mL−1 of blood, equivalent to 35 or 87 nM. Furthermore, our in vitro studies using zymography on BAL fluids of mice exposed to CS (Figure 2) showed that AS111793 did not significantly affect MMP-9 activity up to a concentration of 1 μM, when added to the reaction medium. Therefore, we consider that, at the plasma concentrations of AS111793 likely to be attained in our experiments, selectivity of inhibition of MMP-12 over MMP-1, -2 and -9 would be maintained.
The hypothesis for a role of MMP-12 in the inflammatory process associated with CS exposure was first suggested by Hautamaki et al. (1997). These authors reported that mice lacking the MMP-12 gene were protected against the influx of macrophages and also resistant to emphysema induced by long-term exposure to smoke. They hypothesized that the proinflammatory effect of MMP-12 in response to CS could be linked to its elastolytic properties. A more recent study confirmed that hypothesis, showing that MMP-12 released after cigarette exposure, was able to degrade extracellular matrix components, including elastin and that these elastin fragments had chemotactic properties towards monocytes (Houghton et al., 2006). Moreover, after a limited exposure to tobacco smoke, MMP-12 gene deletion also protected mice from neutrophil influx to the airways, suggesting an early role for MMP-12 in lung inflammation associated with CS exposure (Churg et al., 2002).
It has been, however, difficult to study the role of the MMP-12 because of the lack of selective inhibitors. Therefore, the positive results obtained with different compounds, especially broad spectrum inhibitors of MMPs in smoke-exposed guinea-pigs or mice (Martin et al., 2001; Selman et al., 2003), are not easily attributable solely to MMP-12 inhibition. It was recently reported that a dual MMP-9/MMP-12 inhibitor, AZ11557272, protected against smoke-mediated increases in small airway wall thickness, in guinea-pigs exposed to daily CS for up to 6 months (Churg et al., 2007). In this study, we showed that the MMP-12 inhibitor AS111793 prevented the development of lung inflammation in an acute model of CS-induced inflammation.
Treatment with AS111793 induced an inhibition of neutrophil influx and concomitantly of increased MMP-9 levels in the airways of mice exposed to CS, although to a lesser extent than that reported with mice lacking the MMP-12 gene (Leclerc et al., 2006). Although, in COPD patients, MMP-9 has been reported to be secreted by macrophages (Finlay et al., 1997; Russell et al., 2002), our data suggest that the high level of MMP-9 in the mouse model 4 days after the first exposure to CS should rather be linked to the neutrophil influx. Consistent with this hypothesis is the absence of a significant increase in MMP-9 activity at day 11, at a time when macrophage number was significantly higher in the airways. This hypothesis is also consistent with several reports from our group in different preclinical models (Corbel et al., 2001; Manoury et al., 2007; Nénan et al., 2005b), as well as from others (Opdenakker et al., 2001; Atkinson and Senior, 2003). Thus, a closely linked reduction of MMP-9 level and of neutrophil number in BAL fluids was observed after depletion of neutrophils using rabbit anti-mouse polymorphonuclear in mice treated with bleomycin (Manoury et al., 2007) or with rhMMP-12 (Nénan et al., 2005b). This would indicate that MMP-9 is an appropriate marker of neutrophil number. In addition, our finding that in vitro MMP-9 activity in BAL fluids was not significantly decreased by up to 1 μM AS111793 suggests that the decrease in MMP-9 activity after AS111793 treatment, 4 days after the first CS exposure is unlikely to be due to a direct effect of the drug on the enzyme present in the airways. In contrast to BAL fluids, we were not able to detect an increase of MMP-9 activity in lung homogenates of mice exposed to CS (data not shown), but in this experiment, we did not evaluate the presence of neutrophils.
Consistent with this hypothesis and with the role of this chemokine in the chemotaxis and activation of neutrophils (Baggiolini et al., 1994), a significant reduction in KC/CXCL1 by AS111793 treatment was observed in lung parenchyma. IL-8/CXCL8, the human counterpart of KC/CXCL1, was also reported to be increased in induced sputum from patients with COPD (Keatings et al., 1996) and to stimulate neutrophils to release stored MMP-9 (Van den Steen et al., 2000). Interestingly, we observed decreased levels of KC/CXCL1 only in lung parenchyma but not in BAL fluids after AS111793 administration, when compared with controls. The reason for this difference is unknown and would deserve further investigations.
In contrast to KC/CXCL1, IL-6 was significantly elevated in BAL fluids at day 4, but not in lung parenchyma of mice exposed to CS and was also reduced after inhibition of MMP-12 by AS111793. As IL-6 is mainly produced by macrophages, these results suggest that MMP-12 inhibition could diminish the activation of these cells, even though no change in their number was noted 4 days after smoke exposure. This would suggest an early role for macrophage activation in the inflammatory process associated with CS exposure.
To further investigate the potential mechanisms underlying the anti-inflammatory effects linked to the reduction of MMP-12 activity, some BAL fluid and lung tissue samples were analysed using an inflammatory protein microarray. These samples were collected at day 4 in mice treated with either AS111793 or the vehicle and their counterpart controls exposed to air. Despite the limited number of animals per group, results in BAL fluids clearly show an increase in sTNFR-I and to a lesser extent in sTNFR-II, MIP-1γ/CCL-15, macrophage colony-stimulating factor and IL-6. Some other markers also increased slightly in comparison with the air-exposed controls, including LIX, eotaxin-2 and IL-12p40p70. The arrays also showed a dramatic increase in tissue inhibitor of metalloproteinase-1 levels in pulmonary tissues, but not in BAL fluids, supporting the hypothesis that pulmonary cells produce greater amounts of natural MMP inhibitors in response to the MMP increase induced by CS. Fractalkine/CX3CL1 and I-TAC/CXCL11, two chemokines involved in inflammatory cell recruitment in airway inflammatory diseases (Imai et al., 1997; Sauty et al., 1999; Sukkar et al., 2004), were also upregulated in lung tissues of mice exposed to CS. Interestingly, the arrays confirmed the increase in KC/CXCL1 level in lung homogenate supernatants of mice exposed to CS that was also observed by ELISA. The arrays also showed that inhibition of MMP-12 by AS111793 induced a decrease of both sTNFR-I and sTNFR-II, IL-6 and MIP-1γ/CCL-9 in BAL fluids, with levels equivalent to that observed in control mice. These data are in agreement with the reduction of IL-6 observed in BAL fluids using ELISA quantification and are consistent with the finding (Churg et al., 2003) that MMP-12 mediates smoke-induced inflammation by releasing TNF-α from macrophages. As MIP-1γ/CCL-15 is also mainly produced by macrophages (Maurer and von Stebut, 2004), this suggests that macrophage-derived cytokines play a central role in producing airway inflammation and also supports the hypothesis that reduction of their production or activity may be an interesting therapeutic approach (Antoniu, 2006). Levels of tissue inhibitor of metalloproteinase-1, fractalkine/CX3CL1, I-TAC/CXCL11 and KC/CXCL1 in lung tissue from AS111793-treated mice were similar to those in control mice. These preliminary data would deserve further validation with a greater number of animals and also using ELISA measurements.
Interestingly, the early influx of neutrophils in the airways at day 4 was followed by an increase in the number of macrophages in the BAL fluids at day 11. It is likely that this was due to the migration of monocytes/macrophages from blood. Indeed, production of chemotactic mediators for monocytes may be a consequence of the elastin fragments resulting from the degradation of elastin by MMP-12 (Houghton et al., 2006). Therefore, it is conceivable that the reduction of the macrophage influx observed at day 11 after treatment with AS111793 is a consequence of MMP-12 inhibition. Unexpectedly, there was no simultaneous change in the levels of either IL-6, or KC/CXCL1 or MMP-9 at day 11. The lack of increase of KC/CXCL-1 and MMP-9 is consistent with the very low level of neutrophils in BAL fluids. The lack of IL-6 release may reflect the absence of activated macrophages. Previous studies have demonstrated an increased number of macrophages after smoke exposure (Hautamaki et al., 1997; Churg et al., 2002), but without evidence of activation of these cells. Following airway instillation of rhMMP-12 in mice (Nénan et al., 2005b), a delayed (Days 4 to 15) macrophage influx was observed and, again, it was not possible to demonstrate a macrophage activation associated with IL-6 and MIP-1α release (Nénan et al., 2005a). The fact that AS111793 significantly reduced the CS-induced influx of both neutrophils and macrophages strongly suggests mechanisms that are similar to those involved after instillation of rhMMP-12.
We also report here that pulmonary inflammation induced by LPS was not significantly affected by treatment with AS111793, even at the high dose of 100 mg kg−1. These results contrast with those observed in the CS-induced lung inflammation model. A possible explanation of this could be that a single administration of AS111793 might not be sufficient to prevent the LPS-induced lung inflammation. However, we previously reported that airway inflammation induced by LPS was sensitive to both the PDE-4 inhibitor cilomilast and to dexamethasone, and that mice lacking the MMP-12 gene had the same degree of inflammation as the wild-type controls (Leclerc et al., 2006). These results suggest that the events that trigger inflammation might be different after CS or after LPS exposure, even if they both result in a rise in neutrophils and of MMP-9 in the airways.
Among molecules developed for the treatment of COPD, the most advanced drugs are based on PDE-4 inhibition. We found that roflumilast significantly reduced the neutrophil influx in the airways at day 4, confirming previous data with cilomilast (Leclerc et al., 2006). However, roflumilast was ineffective in reducing the increase in macrophage number at day 11. In a previous study, we found that rolipram was also ineffective in decreasing macrophage recruitment, whereas it was able to elicit a significant decrease in neutrophil numbers, in cytokine levels and pro-MMP-9 release in BAL fluids after instillation of rhMMP-12 in mice (Nénan et al., 2007). This suggest the resistance of certain components of the lung inflammatory response to inhibition of PDE-4, as previously reported (Germain et al., 1998). In contrast, the broad spectrum MMP inhibitor, marimastat, was able to reduce both the increase in the number of neutrophils and that of macrophages, after human MMP-12 instillation (Nénan et al., 2007). We report here that the MMP-12 inhibitor, AS111793, was also able to hamper the development of lung inflammation associated with CS exposure, suggesting that MMP-12 inhibitors may be an interesting therapeutic approach to the treatment of the inflammation associated with COPD.
Acknowledgments
We thank Hélène Pasche for the advice regarding the statistical analysis.
Abbreviations
- BAL
bronchoalveolar lavage
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- IL-6
interleukin-6
- I-TAC
interferon-inducible T-cell chemoattractant
- KC
keratinocyte-derived chemokine
- LPS
lipopolysaccharide
- MIP
macrophage inflammatory protein
- MMP
matrix metalloproteinase
- PDE-4
phosphodiesterase type 4
- PEG
poly-ethyleneglycol
- TNFR
tumour necrosis factor receptor
Conflict of interest
Jean-Yves Gillon and Valérie Cayron-Elizondo were employed by Merck-Serono. Vincent Lagente received research grants from Merck-Serono.
References
- Antoniu SA. Infliximab for chronic obstructive pulmonary disease: towards a more specific inflammation targeting. Expert Opin Investig Drugs. 2006;15:181–184. doi: 10.1517/13543784.15.2.181. [DOI] [PubMed] [Google Scholar]
- Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- Ayscough A, Davies S, Pain G, Gillon J-Y. Oxa- and thiadiazoles and their use as metalloproteinase inhibitors. Patent WO 03070711. 2003.
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Bottomley KM. The role of matrix metalloproteinases (MMPs) in the pathophysiology of chronic obstructive pulmonary disease (COPD): a therapeutic role for inhibitors of MMPs. Inflamm Res. 2003;52:95–100. doi: 10.1007/s000110300020. [DOI] [PubMed] [Google Scholar]
- Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL. An MMP-9/-12 inhibitor prevents smoke-induced emphysema and small airway remodeling in guinea pigs. Thorax. 2007;62:706–713. doi: 10.1136/thx.2006.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, et al. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med. 2003;167:1083–1089. doi: 10.1164/rccm.200212-1396OC. [DOI] [PubMed] [Google Scholar]
- Churg A, Zay K, Shay S, Xie C, Shapiro SD, Hendricks R, et al. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol. 2002;27:368–374. doi: 10.1165/rcmb.4791. [DOI] [PubMed] [Google Scholar]
- Corbel M, Germain N, Lanchou J, Molet S, R e Silva PM, Martins MA, et al. The selective phosphodiesterase 4 inhibitor RP 73-401 reduced matrix metalloproteinase 9 activity and transforming growth factor-beta release during acute lung injury in mice: the role of the balance between Tumor necrosis factor-alpha and interleukin-10. J Pharmacol Exp Ther. 2002;301:258–265. doi: 10.1124/jpet.301.1.258. [DOI] [PubMed] [Google Scholar]
- Corbel M, Theret N, Caulet-Maugendre S, Germain N, Lagente V, Clement B, et al. Repeated endotoxin exposure induces interstitial fibrosis associated with enhanced gelatinase (MMP-2 and MMP-9) activity. Inflamm Res. 2001;50:129–135. doi: 10.1007/s000110050736. [DOI] [PubMed] [Google Scholar]
- Demedts IK, Morel-Montero A, Lebecque S, Pacheco Y, Cataldo D, Joos GF, et al. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax. 2006;61:196–201. doi: 10.1136/thx.2005.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay GA, O'Driscoll LR, Russell KJ, D'Arcy EM, Masterson JB, FitzGerald MX, et al. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med. 1997;156:240–247. doi: 10.1164/ajrccm.156.1.9612018. [DOI] [PubMed] [Google Scholar]
- Germain N, Bertin B, Legendre A, Martin B, Lagente V, Payne A, et al. Selective phosphodiesterase inhibitors modulate the activity of alveolar macrophages from sensitized guinea-pigs. Eur Respir J. 1998;12:1334–1339. doi: 10.1183/09031936.98.12061334. [DOI] [PubMed] [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- Leclerc O, Lagente V, Planquois JM, Berthelier C, Artola M, Eichholtz T, et al. Involvement of MMP-12 and phosphodiesterase type 4 in cigarette smoke-induced inflammation in mice. Eur Respir J. 2006;27:1102–1109. doi: 10.1183/09031936.06.00076905. [DOI] [PubMed] [Google Scholar]
- Manoury B, Nenan S, Guenon I, Lagente V, Boichot E. Influence of early neutrophil depletion on MMPs/TIMP-1 balance in bleomycin-induced lung fibrosis. Int Immunopharmacol. 2007;7:900–911. doi: 10.1016/j.intimp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Martin RL, Shapiro SD, Tong SE, Van Wart HE. Macrophage metalloelastase inhibitors. Prog Respir Res. 2001;31:177–180. [Google Scholar]
- Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Molet S, Belleguic C, Lena H, Germain N, Bertrand CP, Shapiro SD, et al. Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary disease. Inflamm Res. 2005;54:31–36. doi: 10.1007/s00011-004-1319-4. [DOI] [PubMed] [Google Scholar]
- Nénan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12): a pro-inflammatory mediator. Mem Inst Oswaldo Cruz. 2005a;100 Suppl 1:167–172. doi: 10.1590/s0074-02762005000900028. [DOI] [PubMed] [Google Scholar]
- Nénan S, Lagente V, Planquois JM, Hitier S, Berna P, Bertrand CP, et al. Metalloelastase (MMP-12) induced inflammatory response in mice airways: effects of dexamethasone, rolipram and marimastat. Eur J Pharmacol. 2007;559:75–81. doi: 10.1016/j.ejphar.2006.11.070. [DOI] [PubMed] [Google Scholar]
- Nénan S, Planquois JM, Berna P, De Mendez I, Hitier S, Shapiro SD, et al. Analysis of the inflammatory response induced by rhMMP-12 catalytic domain instilled in mouse airways. Int Immunopharmacol. 2005b;5:511–524. doi: 10.1016/j.intimp.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Ohbayashi H. Neutrophil elastase inhibitors as treatment for COPD. Expert Opin Investig Drugs. 2002;11:965–980. doi: 10.1517/13543784.11.7.965. [DOI] [PubMed] [Google Scholar]
- Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- Russell RE, Culpitt SV, DeMatos C, Donnelly L, Smith M, Wiggins J, et al. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2002;26:602–609. doi: 10.1165/ajrcmb.26.5.4685. [DOI] [PubMed] [Google Scholar]
- Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, et al. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- Selman M, Cisneros-Lira J, Gaxiola M, Ramirez R, Kudlacz EM, Mitchell PG, et al. Matrix metalloproteinases inhibition attenuates tobacco smoke-induced emphysema in guinea pigs. Chest. 2003;123:1633–1641. doi: 10.1378/chest.123.5.1633. [DOI] [PubMed] [Google Scholar]
- Stockley RA. New approaches to the management of COPD. Chest. 2000;117:58S–62S. doi: 10.1378/chest.117.2_suppl.58s. [DOI] [PubMed] [Google Scholar]
- Sukkar MB, Issa R, Xie S, Oltmanns U, Newton R, Chung KF. Fractalkine/CX3CL1 production by human airway smooth muscle cells: induction by IFN-gamma and TNF-alpha and regulation by TGF-beta and corticosteroids. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1230–L1240. doi: 10.1152/ajplung.00014.2004. [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]