Abstract
A surprising proportion of patients with inflammatory bowel disease (IBD) remain refractory to all classes of drugs presently in clinical use. Kinins are inflammatory mediators of potential relevance in IBD, because at least the kinin B1 receptor subtype is upregulated in human or animal intestinal inflammation and also both B1 and B2 receptors for kinins support inflammation and epithelial electrogenic ion transport that leads to secretory diarrhoea. In this issue of the BJP, Hara et al. report the therapeutic effect of a modern and selective nonpeptide kinin B1 receptor antagonist, SSR240612 ((2R)-2-(((3R)-3-(1,3-benzodioxol-5-yl)-3-(((6-methoxy-2-naphthyl)sulphonyl)amino)propanoyl)amino)-3-(4-((2R,6S)-2,6-dimethylpiperidinyl)methyl)phenyl)-N-isopropyl-N-methylpropanamide hydrochloride), with benefits such as decreased neutrophil influx and improved macroscopic tissue scoring. The results were corroborated using kinin B1 receptor gene-knockout mice. Further, kinin B1 receptor upregulation in this inflammatory model is partially dependent on TNF-α, a recognized target for IBD pharmacotherapy. More work is warranted to evaluate the value of the kinin B1 receptor antagonists as a novel anti-inflammatory therapeutic option for IBD.
Keywords: kinin B1 receptor, bradykinin B2 receptor, inflammatory bowel disease
Approximately 1.4 million persons in the United States of America and 2.2 million individuals in Europe suffer from inflammatory bowel disease (IBD, ulcerative proctitis/colitis, Crohn's disease; Loftus, 2004). IBD remains a therapeutic challenge because a surprisingly large proportion of patients do not benefit from available treatments, including corticosteroids, immunosuppressive drugs, 5-aminosalicylate and its derivatives, and recent biotechnological proteins (Katz, 2007). The objectives of IBD pharmacotherapy may be more or less comprehensive and include symptomatic relief, induction of clinical remission, inhibition of nutritional deficit and growth retardation in affected children, healing and prevention of fistulas and the prevention of long-term complications such as colon carcinoma.
The kallikrein–kinin system consists of circulating kininogens, the proteolytic enzymes kallikreins, kinins (bradykinin-related peptides, which are produced through the cleavage of kininogens by kallikreins) and two G-protein-coupled receptors termed kinin B1 and bradykinin B2 receptors, which mediate the biological effects of kinins (Leeb-Lundberg et al., 2005; Alexander et al., 2008). Endogenous kinins exhibit a double pharmacological personality: they are vasodilators that may be recruited in stressful situations, such as renal ischemia (Kakoki et al., 2007), and, usually via bradykinin B2 receptors constitutively expressed in endothelial cells, release nitric oxide and other negative regulators of vascular smooth muscle tone and platelet function. ACE is a major kinin-destroying enzyme and protection of endogenous kinins may account for some part of the beneficial effects of ACE inhibitors (Leeb-Lundberg et al., 2005). However, kinins also reproduce all four cardinal signs of inflammation when injected into tissues and thus qualify as mediators of inflammation. In that context, the kinin B1 receptor is usually absent from healthy tissues, but is inducible following tissue injury under the action of cytokines and other agents, such as bacterial lipopolysaccharide (Leeb-Lundberg et al., 2005).
Despite the intensive work to develop effective peptide and non-peptide kinin receptor antagonists in the last decades, the clinical impact of these agents is still minimal. Icatibant, a peptide antagonist of bradykinin B2 receptors, is now a recognized therapeutic option in a rare congenital disease, hereditary angioedema, where endogenous bradykinin is clearly inflammatory (Bernstein, 2008). Medicinal chemistry efforts have been particularly targeted at producing analgesic drugs, both B1 and B2 kinin receptor antagonists showing activity in animals, but a clinically useful drug from these classes is yet to emerge. Kinins are potentially significant mediators of IBD: both B1 and B2 receptors for kinins have been immunolocalized in the epithelial cells of affected human intestinal tissue; the kinin B1 receptor was constitutively expressed in normal colonic epithelium, possibly because the normal bacterial flora activates the innate immune system, but was upregulated by inflammation (at least at the level of the protein; Stadnicki et al., 2005). Further, kinin B1 receptor expression was extended to macrophages present in granulomas (Stadnicki et al., 2005; Figure 1). In relation to a prominent symptom of IBD, both inducible B1 and constitutive B2 basolateral kinin receptors support the electrogenic ion transport in intestinal epithelium, the luminal secretion of Cl− being accompanied with that of Na+ and water, thus leading to secretory diarrhoea (Cuthbert, 2001). The effects of kinins were largely prostaglandin-independent in this system. As the kinin B1 receptor is more resistant to functional desensitization than the bradykinin B2 receptor, the participation of the former was felt to be more likely in the genesis of clinically significant diarrhoeal states (Cuthbert, 2001).
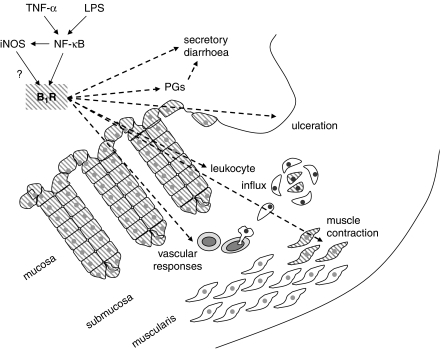
Figure 1.
Schematic representation of the mechanisms of kinin B1 receptor induction (solid arrows) and its functions (dashed arrows) in colonic inflammation, according to Hara et al. (2008) and other sources. The hatched surface is the symbol for kinin B1 receptor presence in cells. B1R, kinin B1 receptor; iNOS, inducible NOS; LPS, lipopolysaccharide; NF-κB, nuclear factor κ-B; PGs, prostaglandins.
In this issue of the BJP, Hara et al. (2008) report the therapeutic action of a non-peptide and orally bio-available antagonist of the kinin B1 receptors, SSR240612, in a mouse model of colitis. The drug treatment reduced polymorphonuclear leukocyte influx, improved the macroscopic tissue damage score and had favourable effects, both in preventing colitis induced by 2,4,6-trinitrobenzene sulphonic acid and alleviating the inflammation in established colitis. Importantly, the results were corroborated by exploiting kinin B1 receptor gene-knockout mice that are partially refractory to this model of colitis. Hara et al. (2008) also studied kinin B1 receptor upregulation in the tissue; interestingly, the radioligand-binding assay applied to colonic membranes showed the inflammation-induced upregulation of kinin B1 receptors, but from a significant control baseline population. On the other hand, the freshly isolated control colonic smooth muscle did not contract in response to the kinin B1 receptor agonist des-Arg9-bradykinin, whereas the inflamed tissues were responsive. The binding assay may reveal the constitutive kinin B1 receptors in the colonic epithelium, as in human tissues (Stadnicki et al., 2005), but transmural inflammation may be needed to induce the expression of these receptors in the underlying smooth muscle cells (Figure 1). Additional findings include a significant role for tumour necrosis factor-α (TNF-α) and its receptor in the expression of the kinin B1 receptor in murine colon, as TNF-α is a recognized target for therapy in IBD (Katz, 2007). The documented therapeutic effects of anti-TNF-α agents, such as infliximab, in IBD may derive in part from the repression of kinin B1 receptor expression. Thus, the experimental system shows robust anti-inflammatory effects of a modern and selective kinin B1 receptor antagonist in a model of IBD (Hara et al., 2008). Among unresolved issues, the therapeutic place of bradykinin B2 receptor antagonists has not been addressed, but it may be significant, as this receptor type is also upregulated in the same murine model (Hara et al., 2007).
In this post-Vioxx era, who will dare to develop kinin receptor antagonists as anti-inflammatory drugs in IBD? The potentially salutary effect of kinins in the peripheral circulation may discourage systemic treatments with kinin receptor antagonists (although predictable safety issues are less clear for kinin B1 receptor antagonists). On the other hand, drug delivery to the intestine may exploit topical or galenic forms that minimize systemic distribution, as for 5-aminosalicylate and its derivatives. Further, despite the fact that almost all kinin receptor antagonists developed so far exhibit a strong selectivity for one of the two receptor subtypes, there are prototypes of drugs that block both. An example is the non-peptide Compound 1 from the studies of Ritchie et al. (2004), a balanced B1 and B2 kinin receptor antagonist. Therefore, more work is warranted to evaluate the value of the novel anti-inflammatory therapeutic option for IBD offered by the kinin receptor antagonists.
Acknowledgments
FM is supported by the Canadian Institutes of Health Research.
Abbreviations
- IBD
inflammatory bowel disease
- SSR240612
(2R)-2-(((3R)-3-(1,3-benzodioxol-5-yl)-3-(((6-methoxy-2-naphthyl)sulphonyl)amino)propanoyl)amino)-3-(4-((2R,6S)-2,6-dimethylpiperidinyl)methyl)phenyl)-N-isopropyl-N-methylpropanamide hydrochloride
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA.Guide to Receptors and Channels (GRAC) Br J Pharmacol 2008153Suppl 2S1–S209.3rd edn. (2008 revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JA. Hereditary angioedema: a current state-of-the-art review, VIII: current status of emerging therapies. Ann Allergy Asthma Immunol. 2008;100:S41–S46. doi: 10.1016/s1081-1206(10)60585-6. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW. Kinins and epithelial ion transport in the alimentary tract. Biol Chem. 2001;382:57–60. doi: 10.1515/BC.2001.009. [DOI] [PubMed] [Google Scholar]
- Hara DB, Fernandes ES, Campos MM, Calixto JB. Pharmacological and biochemical characterization of bradykinin B2 receptors in the mouse colon: influence of the TNBS-induced colitis. Regul Pept. 2007;141:25–34. doi: 10.1016/j.regpep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Hara DB, Leite DFP, Fernandes ES, Passos GF, Guimarães AO, Pesquero JB, et al. The relevance of kinin B1 receptor up-regulation in a mouse model of colitis Br J Pharmacol 20081541276–1286.this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoki M, McGarrah RW, Kim HS, Smithies O. Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2007;104:7576–7581. doi: 10.1073/pnas.0701617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. Mind the gap' an unmet need for new therapy in IBD. J Clin Gastroenterol. 2007;41:799–809. doi: 10.1097/MCG.0b013e318033d71d. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg LMF, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. International Union of Pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Ritchie TJ, Dziadulewicz EK, Culshaw AJ, Muller W, Burgess GM, Bloomfield GC, et al. Potent and orally bioavailable non-peptide antagonists at the human bradykinin B1 receptor based on a 2-alkylamino-5-sulfamoylbenzamide core. J Med Chem. 2004;47:4642–4644. doi: 10.1021/jm049747g. [DOI] [PubMed] [Google Scholar]
- Stadnicki A, Pastucha E, Nowaczyk G, Mazurek U, Plewka D, Machnik G, et al. Immunolocalization and expression of kinin B1R and B2R receptors in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2005;289:G361–G366. doi: 10.1152/ajpgi.00369.2004. [DOI] [PubMed] [Google Scholar]