Abstract
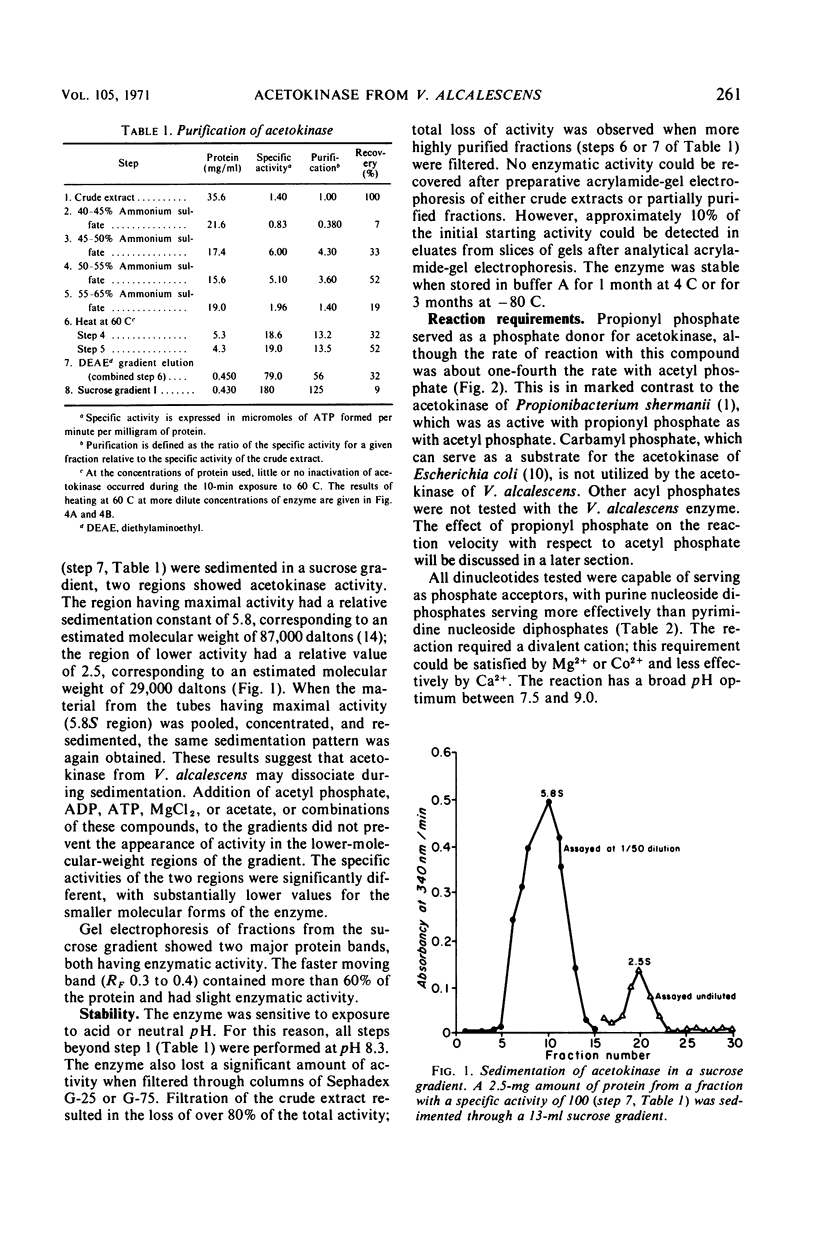
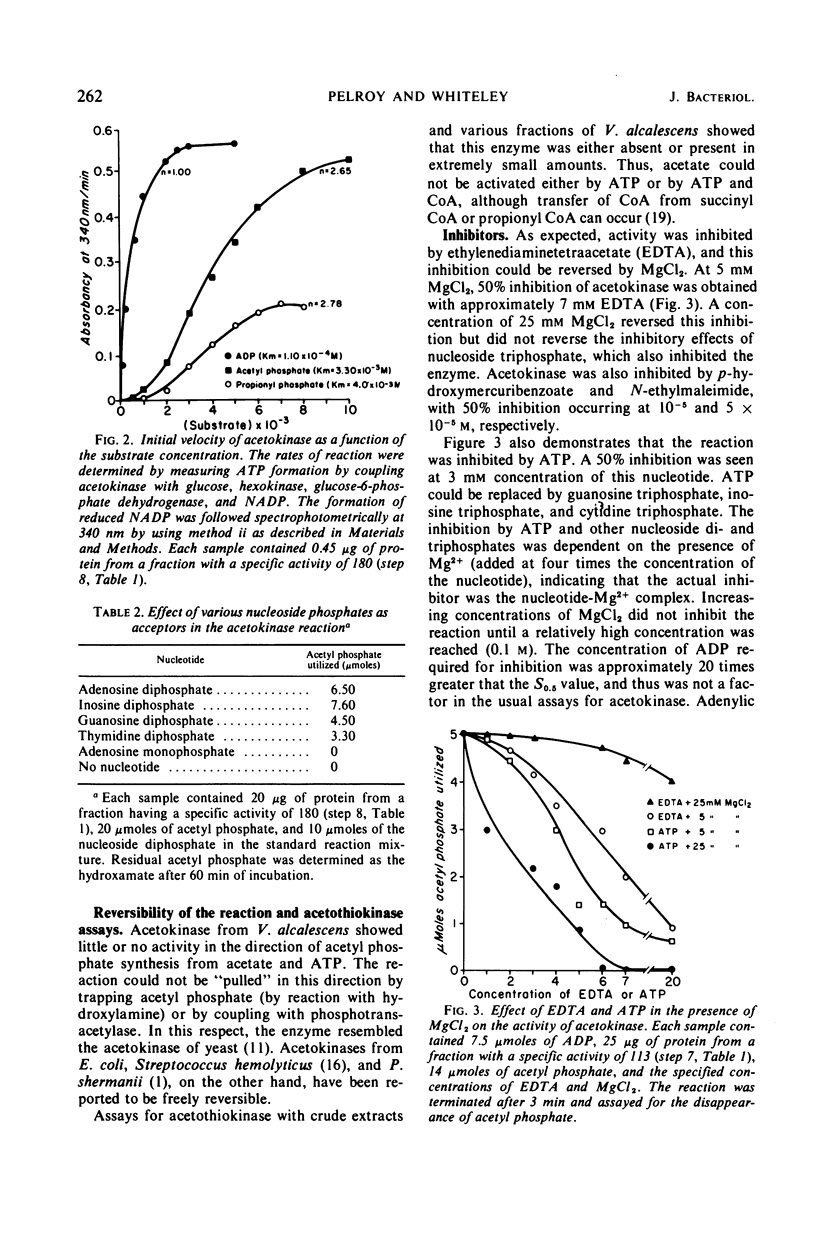
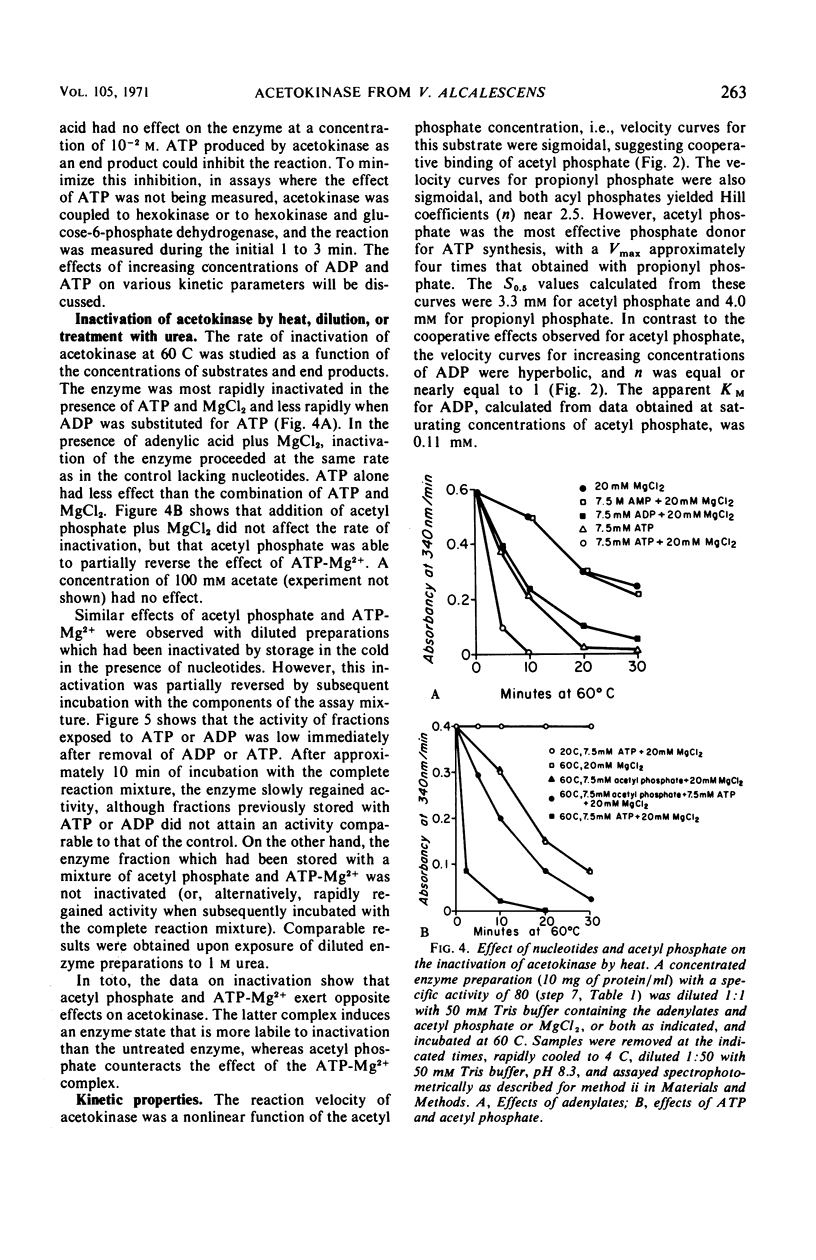
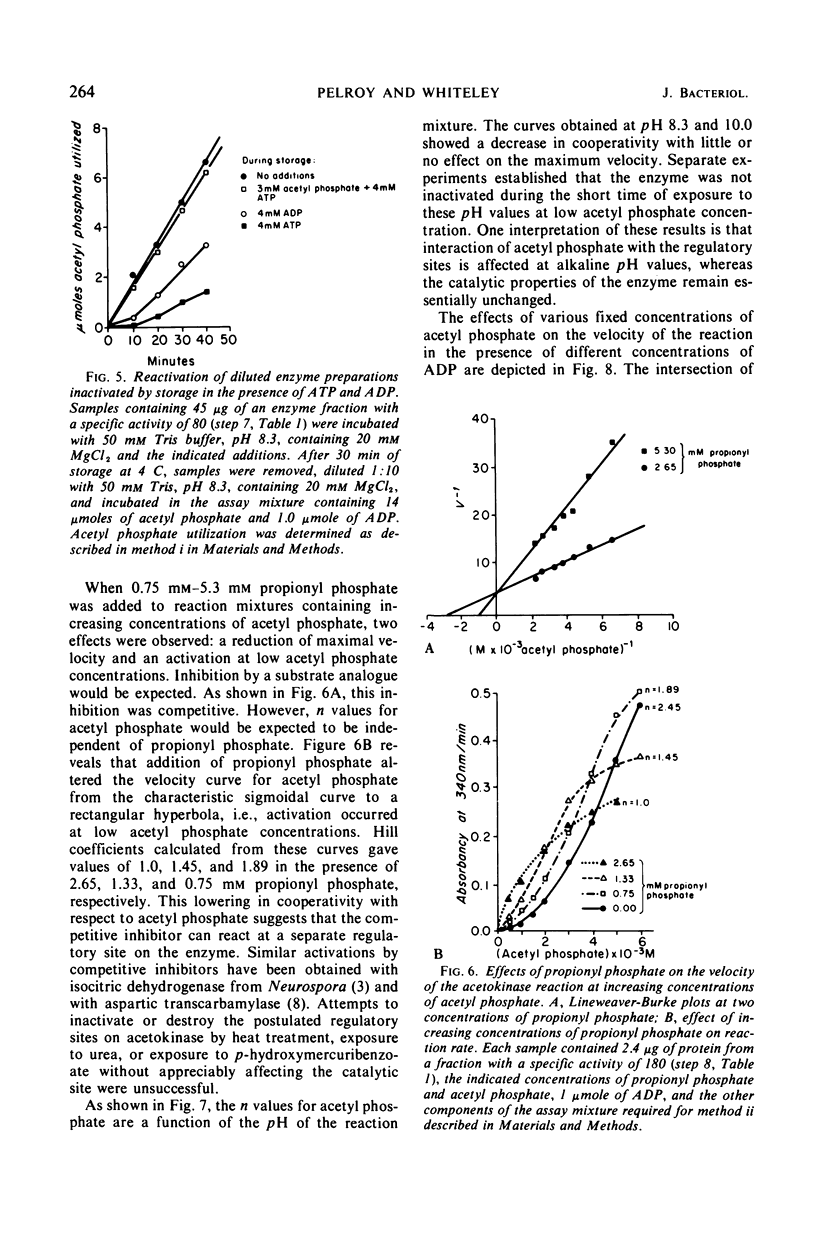
Acetokinase from Veillonella alcalescens catalyzes the virtually irreversible synthesis of adenosine triphosphate from acetyl phosphate and adenosine diphosphate. Kinetic analysis revealed that the enzyme was activated by acetyl phosphate and inhibited by adenosine triphosphate. Velocity curves obtained with increasing amounts of adenosine diphosphate were of the Michaelis-Menten type (rectangular hyperbolas) under all conditions employed. However, velocity curves generated by varying the level of acetyl phosphate were sigmoidal, suggesting homotropic interactions for this substrate. Alteration of the pH of the reaction mixture from 7.4 to 10 or addition of a substrate analogue, propionyl phosphate, resulted in the loss of cooperativity and changed the values of the Hill coefficient from 3 to 1. Data obtained in experiments with propionyl phosphate suggested the existence of separate effector sites on the enzyme. The inhibition curves for adenosine triphosphate were also sigmoidal with Hill coefficients ranging between 1 and 3, depending on the concentration of acetyl phosphate. Sedimentation studies with the partially purified enzyme indicated a polymeric structure with a maximum molecular weight of about 90,000 daltons; a subunit of approximately 29,000 daltons was also detected.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN S. H., KELLERMEYER R. W., STJERNHOLM R. L., WOOD H. G. PURIFICATION AND PROPERTIES OF ENZYMES INVOLVED IN THE PROPIONIC ACID FERMENTATION. J Bacteriol. 1964 Jan;87:171–187. doi: 10.1128/jb.87.1.171-187.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- Allen S. H. The isolation and characterization of malate-lactate transhydrogenase from Micrococcus lactilyticus. J Biol Chem. 1966 Nov 25;241(22):5266–5275. [PubMed] [Google Scholar]
- Biggins D. R., Dilworth M. J. Control of pyruvate phosphoroclastic activity in extracts of Clostridium pasteurianum by ADP and acetyl phosphate. Biochim Biophys Acta. 1968 Mar 11;156(2):285–296. doi: 10.1016/0304-4165(68)90257-2. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GRISOLIA S., HARMON P., RAIJMAN L. Studies on the mechanism of action of carbamate kinase. Biochim Biophys Acta. 1962 Aug 13;62:293–299. doi: 10.1016/0006-3002(62)90042-2. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Joseph R., Mortlock R. P. Requirement for coenzyme A in the phosphoroclastic reaction of anaerobic bacteria. J Bacteriol. 1969 Dec;100(3):1328–1334. doi: 10.1128/jb.100.3.1328-1334.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREIL-KISS G., HOFFMANN-OSTENHOF O. Enzymic formation of adenosine triphosphate with acetyl phosphate as donor in a yeast extract. Biochim Biophys Acta. 1963 Jan 8;67:168–170. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Pearce J., Carr N. G. The metabolism of acetate by the blue-green algae, Anabaena variabilis and Anacystis nidulans. J Gen Microbiol. 1967 Nov;49(2):301–313. doi: 10.1099/00221287-49-2-301. [DOI] [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- STADTMAN E. R. The purification and properties of phosphotransacetylase. J Biol Chem. 1952 May;196(2):527–534. [PubMed] [Google Scholar]
- WHITELEY H. R., McCORMICK N. G. Degradation of pyruvate by Micrococcus lactilyticus. III. Properties and cofactor requirements of the carbon dioxide-exchange reaction. J Bacteriol. 1963 Feb;85:382–393. doi: 10.1128/jb.85.2.382-393.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley H. R. The Mechanism of Propionic Acid Formation by Succinate Decarboxylation: II. The Formation and Decarboxylation of Succinyl-CoA. Proc Natl Acad Sci U S A. 1953 Aug;39(8):779–785. doi: 10.1073/pnas.39.8.779. [DOI] [PMC free article] [PubMed] [Google Scholar]



