Abstract
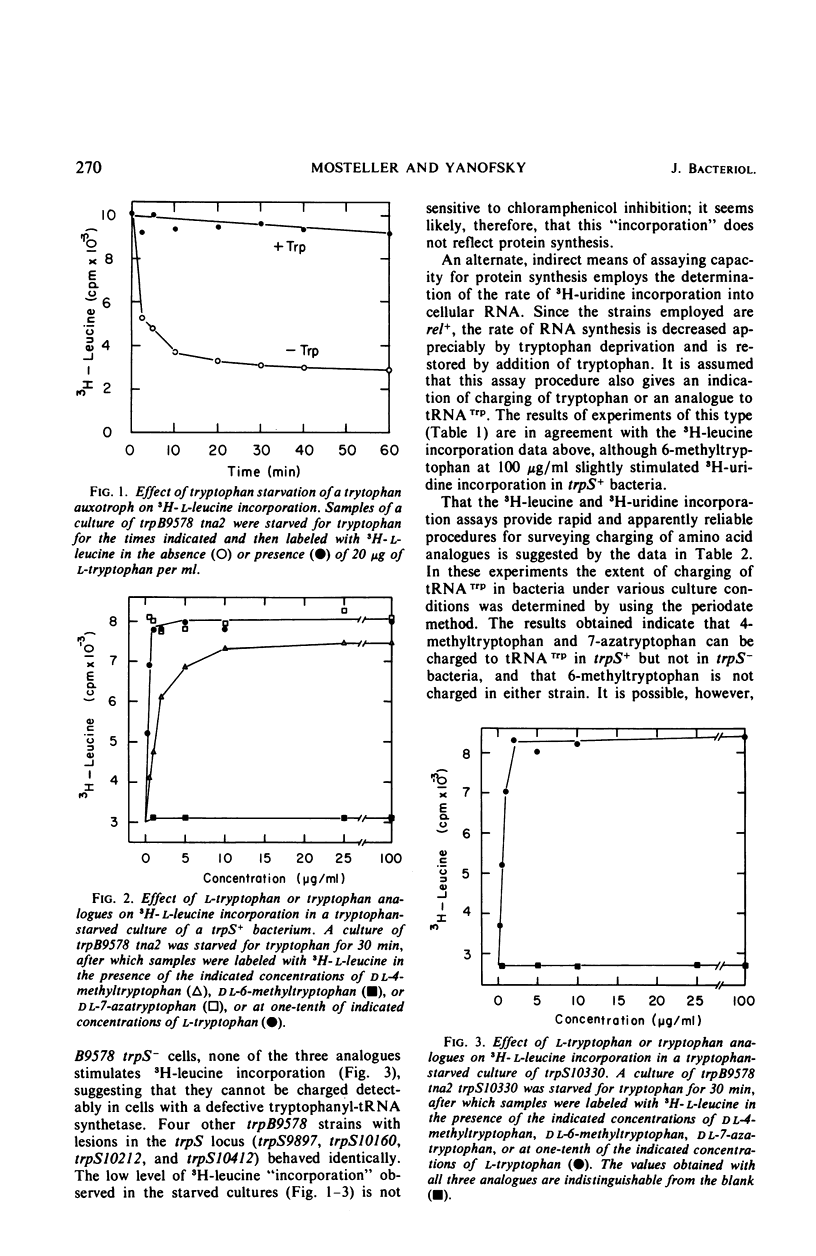
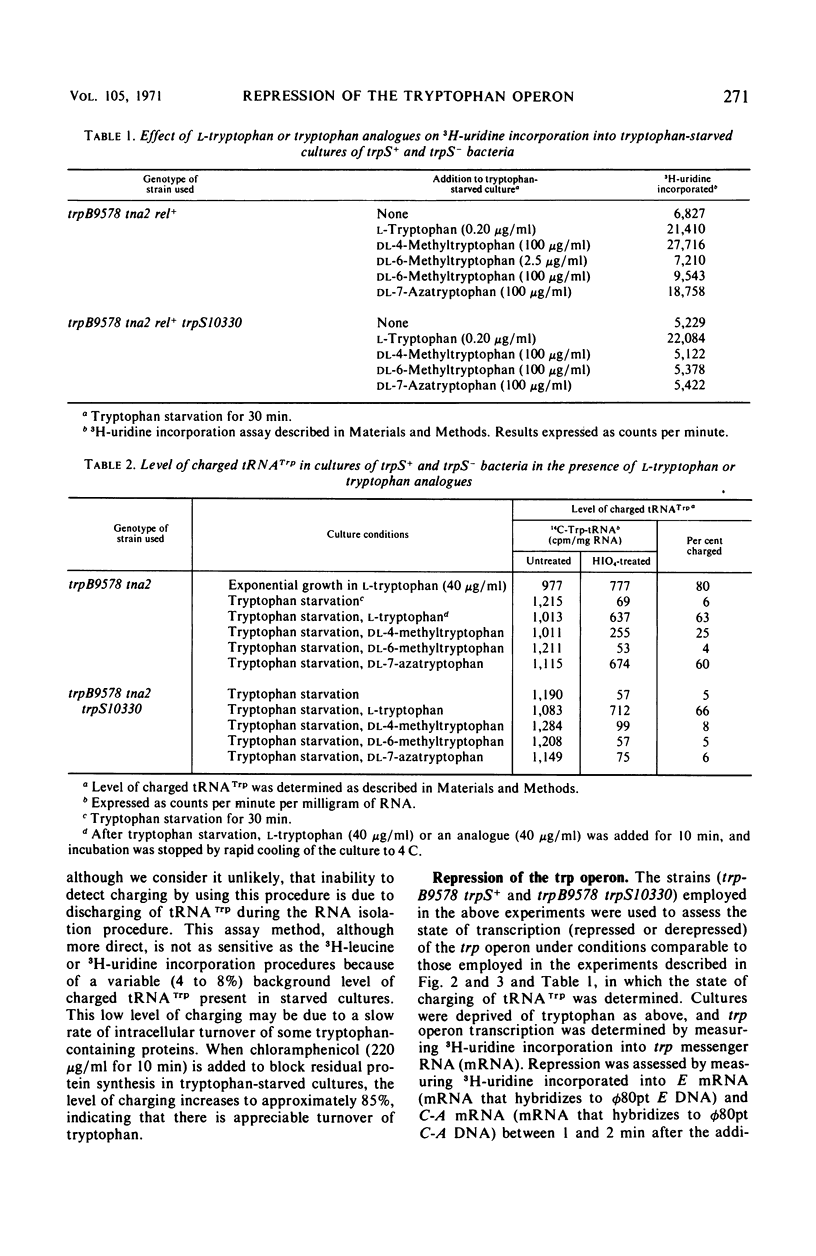
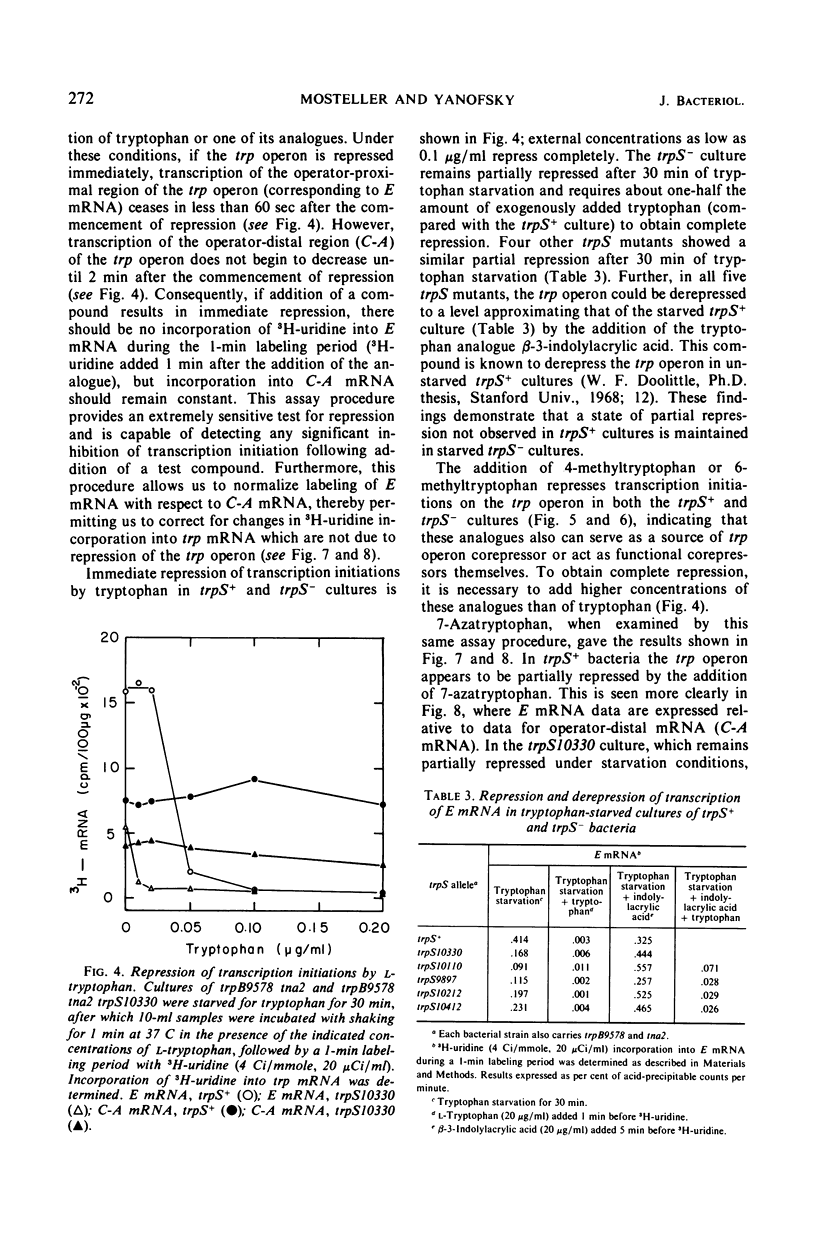
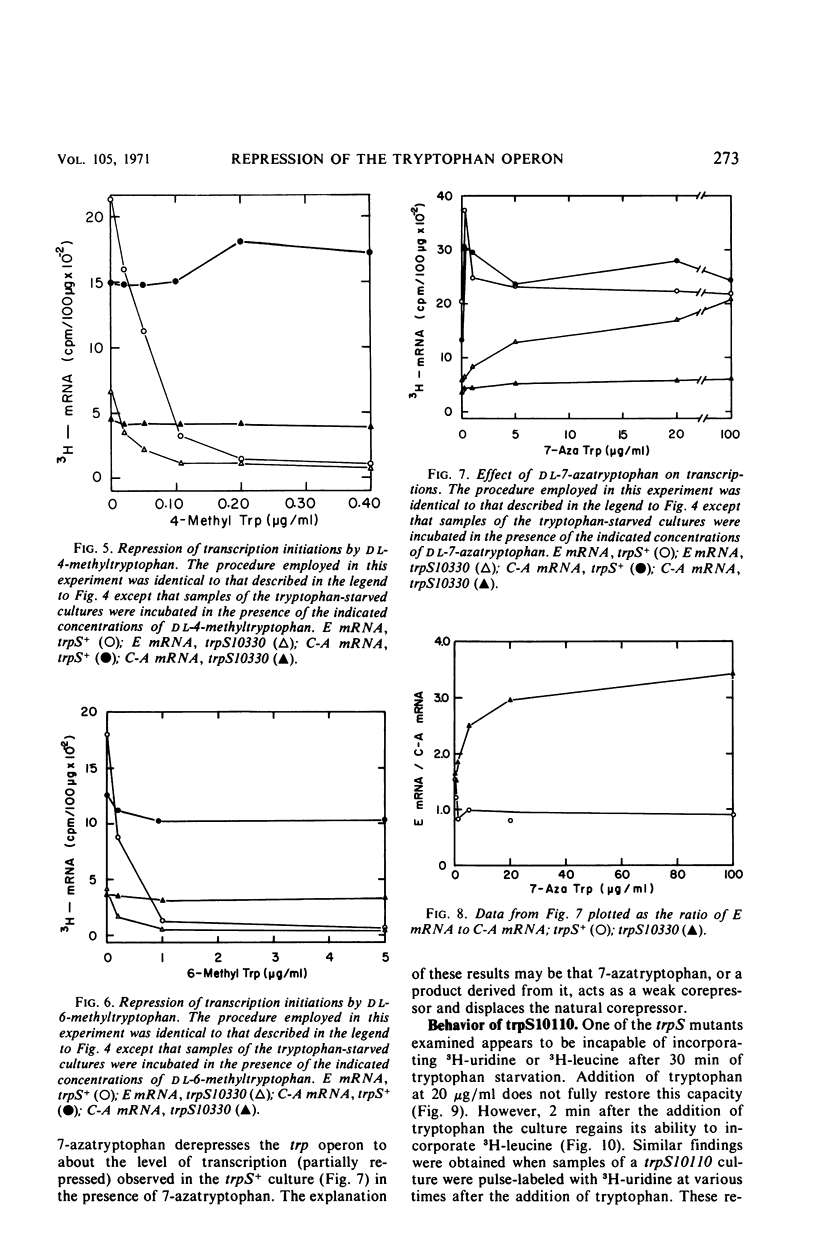
When a growing culture of a tryptophan auxotroph of Escherichia coli is transferred to a tryptophan-free medium, the bacteria exhaust their supply of Trp-transfer ribonucleic acid (tRNA). Under these conditions transcription of the trp operon is derepressed. When l-tryptophan, dl-4-methyltrytophan, dl-6-methyltryptophan, or dl-7-azatryptophan is added to a tryptophan-starved culture, charging to tRNATrp can be detected with each of these compounds except 6-methyltryptophan. Under these conditions, transcription initiations on the trp operon are repressed completely by tryptophan, 4-methyltryptophan, and 6-methyltryptophan, but only slightly by 7-azatryptophan. If a culture of a bacterium containing an altered tryptophanyl-tRNA synthetase (trpS−) is starved for tryptophan in the same manner, tRNATrp is uncharged, but transcription of the operon is derepressed to only about one-third the level observed in trpS+ cultures. When tryptophan, 4-methyltryptophan, 6-methyltryptophan, or 7-azatryptophan is added to a tryptophan-starved trpS− culture, tRNATrp is charged with tryptophan but apparently not with the analogues. However, tryptophan, 4-methyltryptophan, and 6-methyltryptophan completely repress transcription initiations, whereas 7-azatryptophan derepresses transcription initiations to approximately the level of partial repression observed in trpS+ cultures in the presence of 7-azatryptophan. When tryptophan is added to a tryptophan-starved trpS10110 culture, there is a 2-min delay before tRNATrp appears to be charged. However, under these conditions, transcription initiations on the trp operon are repressed immediately by the addition of tryptophan. We interpret these results as indicating that Trp-tRNA is not the corepressor of the trp operon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour S. D., Pardee A. B. The establishment of beta-galactosidase repression in the mating system of Escherichia coli K12. J Mol Biol. 1966 Oct;20(3):505–515. doi: 10.1016/0022-2836(66)90006-4. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Characterization of altered forms of glycyl transfer ribonucleic acid synthetase and the effects of such alterations on aminoacyl transfer ribonucleic acid synthesis in vivo. J Bacteriol. 1970 Apr;102(1):204–212. doi: 10.1128/jb.102.1.204-212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Ito K., Hamada K., Yura T. A new regulatory gene for the tryptophan operon of Escherichia coli. Biochem Biophys Res Commun. 1967 Mar 9;26(5):522–527. doi: 10.1016/0006-291x(67)90095-2. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Yanofsky C. Transcription of the tryptophan operon in polarity mutants of Escherichia coli. I. Characterization of the tryptophan messenger RNA of polar mutants. J Mol Biol. 1967 Aug 28;28(1):1–23. doi: 10.1016/s0022-2836(67)80073-1. [DOI] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Temperature-sensitive repression of the tryptophan operon in Escherichia coli. J Bacteriol. 1969 Jul;99(1):279–286. doi: 10.1128/jb.99.1.279-286.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Tryptophanyl transfer RNA synthetase and expression of the tryptophan operon in the trpS mutants of Escherichia coli. Genetics. 1969 Mar;61(3):521–538. doi: 10.1093/genetics/61.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y., Matsushiro A., Shimura Y. Isolation of the novel regulatory mutants of the tryptophan biosynthetic system in Escherichia coli. Mol Gen Genet. 1968;102(1):15–26. doi: 10.1007/BF00341866. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Incorporation of 5-methyltryptophan into the protein of Escherichia coli 15T- (555-7). J Bacteriol. 1969 Feb;97(2):980–982. doi: 10.1128/jb.97.2.980-982.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Mosteller R., Baker R. F., Yanofsky C. Direction of in vivo degradation of tryptophan messenger RNA--a correction. Nature. 1969 Jul 5;223(5201):40–43. doi: 10.1038/223040a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. A transcription-initiating mutation within a structural gene of the tryptophan operon. J Mol Biol. 1969 May 14;41(3):317–328. doi: 10.1016/0022-2836(69)90278-2. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Amber mutants of the trpR regulatory gene. J Mol Biol. 1969 Aug 28;44(1):185–193. doi: 10.1016/0022-2836(69)90413-6. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Effects of azatryptophan on bacterial enzymes and bacteriophage. Biochim Biophys Acta. 1958 Feb;27(2):330–344. doi: 10.1016/0006-3002(58)90340-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]


