Abstract
Background and Aims
Distinguishing an alcohol basis from a nonalcoholic basis for the clinical and histological spectrum of steatohepatitic liver disease is difficult owing to unreliability of alcohol consumption history. Unfortunately, various biomarkers have had limited utility in distinguishing alcoholic liver disease (ALD) from nonalcoholic fatty liver disease (NAFLD). Thus, the aim of our study was to create and validate a model to diagnose ALD in patients with steatohepatitis.
Methods
Cross-sectional cohort study was performed at Mayo Clinic; Rochester, Minnesota to create a model using multivariable logistic regression analysis. This model was validated in three independent data-sets comprising patients of varying severity of steatohepatitis spanning over 10 years.
Results
Logistic regression identified mean corpuscular volume, AST/ALT ratio, body-mass index, and gender as the most important variables that separated patients with ALD from NAFLD. These variables were used to generate the ALD/NAFLD Index (ANI); with ANI of greater than 0 incrementally favoring ALD, and ANI of less than 0 incrementally favoring a diagnosis of NAFLD, thus making ALD unlikely. ANI had a c-statistic of 0.989 in the derivation sample, and 0.974, 0.989, 0.767 in the three validation samples. ANI performance characteristics were significantly better than several conventional and recently proposed biomarkers used to differentiate ALD from NAFLD including the histopathological marker Protein Tyrosine Phosphatase 1b, AST/ALT ratio, gamma-glutamyl transferase and Carbohydrate Deficient Transferrin.
Conclusion
ANI, derived from easily available objective variables, accurately differentiates ALD from NAFLD in hospitalized, ambulatory and pre-transplant patients and compares favorably to other traditional and proposed biomarkers.
Introduction
The clinical, radiographic, and/or histopathologic diagnosis of steatosis/steatohepatitis is common owing to the continued high prevalence of alcoholic liver disease (ALD) in combination with the recent epidemic of nonalcoholic fatty liver disease (NAFLD)1-3. In the clinical arena, it is frequently of paramount importance to know whether steatohepatitic liver injury is related to alcohol or NAFLD, as this distinction may influence patient management and candidacy for liver transplantation4, 5, 6-9. However, distinguishing ALD from NAFLD is often difficult owing to unreliability of alcohol consumption history10. Unfortunately, histology is virtually indistinguishable when comparing patients with similar disease severity and moreover, various standard laboratory parameters and novel biomarkers have had limited discriminatory capacity in distinguishing ALD from NAFLD as well11-13. Thus, the aim of our study was to derive a model based on independent clinical and laboratory markers to distinguish ALD from NAFLD and then to test this model by comparing it to a series of traditional and proposed biomarkers using complimentary validation cohorts.
Patients and Methods
Derivation Cohort
The derivation sample consisted of patients with histological evidence of steatohepatitis who underwent liver biopsy between January 1994 and December 2003 at Mayo Clinic (Rochester, MN). This patient cohort of 241 patients was compiled for a previous study focused on evaluation of the histopathological marker Protein Tyrosine Phosphatase 1b (PTP1b) in patients with steatohepatitis14. This derivation sample was also utilized to compare the c-statistic between the derived model and PTP1b to distinguish ALD from NAFLD. The medical history of the 241 patients with biopsy proven steatohepatitis within this cohort were reviewed; 25 patients were found to have liver disease other than ALD or NAFLD as a cause for their steatohepatitis and were excluded. The remaining 216 patients were coded based on alcohol consumption history; 52 patients were diagnosed to have ALD (greater than 30 g of alcohol intake per day or admitted for alcohol intoxication, withdrawal, or treatment) and 151 patients were diagnosed as NAFLD (less than 20 g of alcohol intake per day). Thirteen other patients with equivocal alcohol intake (20-30 grams per day or alcohol history not available), were excluded. In all patients, including patients in the validation cohorts, other diagnosis of liver disease such as viral and auto-immune hepatitis were excluded.
Validation Cohorts
The first cohort consisted of a case-control combination of patients with ALD or NAFLD (Validation Sample 1). The NAFLD cases consisted of previously published cohorts of patients with persistent elevation of transaminase, daily consumption of alcohol of less than 20g, with biopsy proven steatohepatitis, and exclusion of other liver diseases15, 16. The ALD cases in Validation Sample 1 comprised patients diagnosed at the Mayo Clinic between 1995-2001 with alcoholic hepatitis that was previously characterized and published in a prior study demonstrating the prognostic utility of the Model for End Stage Liver Disease (MELD) in alcoholic hepatitis17, as well as a small number of other patients within the spectrum of ALD that did not have acute alcoholic hepatitis. After exclusion of overlapping patients with the derivation sample, Validation Sample 1 was comprised of 139 NAFLD patients and 88 ALD patients.
Validation Sample 2 was comprised of an ambulatory patient cohort previously used to prospectively evaluate the validity and utility of carbohydrate deficient transferrin (CDT)18 and consisted of 26 patients with NAFLD and 42 patients with ALD collected between 1995 to 1996. CDT refers to the sum of asialo, monosialo, and disialo CDT. Due to the prospective nature of that study, the timing of the last alcohol consumption was well documented, with one-third of the patients with ALD having been abstinent from alcohol for over 2 months.
Validation Sample 3 consisted of 48 ALD and 18 NAFLD patients undergoing evaluation for liver transplantation at Mayo Clinic between 2000-2006. Thus, this cohort consisted entirely of patients with end-stage cirrhotic disease. ALD patients in this cohort were not actively drinking and had undergone psychiatric evaluation and addiction treatment. Validation Sample 3 had no overlap with the other Validation Sample cohorts.
Variables
Laboratory values were recorded from the time closest to the biopsy/initial evaluation/time of hospitalization. To lessen the influence of extreme laboratory values we corrected mean corpuscular volume (MCV) to a range of 92-103 and AST/ALT to be less than, or equal to a ratio of 3. For example, a patient with MCV 90 and AST/ALT 3.5 was analyzed as having MCV of 92 and AST/ALT ratio of 3. This approach was favored over natural logarithm transformation to facilitate calculation and interpretation of the ALD formula by clinicians. Histologic findings of stage III or IV fibrosis were considered as advanced fibrosis. PTP1b positive staining favored a diagnosis of NAFLD as defined as in our previous publication14.
Statistical Analysis
Univariate logistic regression was used to screen variables reported in Table 1. The biopsy fibrosis stage and the MELD Score19 were used to adjust for disease severity as a confounding variable. The United Network for Organ Sharing (UNOS) version of MELD was used which adds 6.4 points to the score independent of the etiology of liver disease. Due to the number of variables considered, only those with p<0.01 were candidates for multivariate logistic regression analysis. Stepwise variable selection was used in the multivariate analysis. The linear combination of independent predictors identified in the multivariate analysis was then applied to the logistic regression formula to predict the probability of ALD based on a formula score. We calculated standard indices of validity including sensitivity, specificity, and area under the Receiver Operating Curve (ROC AUC). The concordance or “c-statistic” between the model and the data was used to assess the utility of the final model; it is represented by the ROC AUC and is the fraction of subject pairs that are correctly ranked by the assigned risk score. A c-statistic value of 0.7 is considered clinically useful, and a c-statistic >0.8 excellent. The c-statistic of different variables was compared using Delong’s Method20. Statistical Software JMP 5.1.2 and SAS 9.1 were used for statistical analyses.
Table 1.
Demographic, Clinical, and Biochemical Features of Derivation and Validation Samples
| Derivation Sample | Validation Sample #1 | Validation Sample #2 | Validation Sample #3 | |||||
|---|---|---|---|---|---|---|---|---|
| ALD
(n=52) |
NAFLD
(n=151) |
ALD
(n=88) |
NAFLD
(n=139) |
ALD
(n=42) |
NAFLD
(n=26) |
ALD
(n=48) |
NAFLD
(n=18) |
|
| Demographic | ||||||||
| Age (yr, mean ± SD) | 50.8±11.3 | 48.8±11.9 | 47±10.6 | 52±12.5 | 54.6±13.6 | 45.9±12.1 | 54.6±11.1 | 56.4±4.6 |
| Sex (% Male) | 38(773%) | 62(41.1%) | 76 (86.3%) | 38(27.3%) | 32(76.2%) | 10(38.5%) | 40(83.3%) | 9(50%) |
| Race | ||||||||
| Caucasian (%) | 34(65.4%) | 117(77.5%) | 57 | 68 | 23(54.8%) | 21(80.8%) | 31(64.6%) | 16(89%) |
| Non Caucasian (%) | 2(3.8%) | 5(3.3%) | 1 | 2 | 3(7.1%) | 1(3.8%) | 3(6.3%) | 0 |
| Unknown | 16(30.8%) | 29(19.2%) | 42 | 30 | 16(38.1%) | 4(15.4%) | 14(29.1%) | 2(11%) |
|
| ||||||||
| Clinical | ||||||||
| Inpatient (%) | 29(55.8%) | 29(19.2%) | 85 | 9 | 0(0%) | 0(0%) | 24(50%) | 6(33%) |
| Alcoholic Hepatitis(%) | 22(42.3%) | N/A | 75(85.2%) | N/A | 5(11.9%) | N/A | 0(0%) | N/A |
| Alcohol consumption within 2 mo(%) | 46(88.5%) | N/A | 85 | N/A | 28(66.7%) | N/A | 0(0%) | N/A |
| Biopsy | ||||||||
| (%) | 52(100%) | 151(100%) | 15 | 100 | 11(26.2%) | 21(80.8%) | 48(100%) | 18(100%) |
| Advanced Fibrosis% | 28(53.8%) | 51(33.8) | 44 | 39 | 7(63.6%) | 3(14.3%) | 48(100%) | 18(100%) |
| DM% | 12(22.6%) | 52(34.4%) | N/A | N/A | N/A | N/A | N/A | N/A |
| BMI(mean + SD) | 27.6±5.7 | 33.2±7.3 | 27.0±5.3 | 33.9±6.7 | 28.2±4.7 | 31.5±6.6 | 28.6±4.7 | 33.1±4.7 |
|
| ||||||||
| Biochemical (mean + SD) | ||||||||
| MELD | 15.4±10.2 | 8.5±4.0 | 17.6±9.0 | 8.2±4.0 | 13.6±6.4 | 7.6±1.1 | 22.0±8.4 | 22.5±9.8 |
| MCV | 101.7±9.3 | 88.7±5.7 | 100.5±8.4 | 89.1±6.8 | 96.5±6.8 | 86.8±6.2 | 98.3±6.3 | 95.7±7.5 |
| AST/ALT | 2.2±1.4 | 0.8±0.4 | 2.9±2.0 | 1.00±1.2 | 2.0±1.5 | 0.7±0.3 | 1.7±0.8 | 1.5±0.8 |
N/A; not applicable or not available
Results
Demographic, Clinical and Biochemical Features of Derivation and Validation Sets
Demographic, clinical and biochemical features of patients from Derivation and Validation patient cohorts are depicted in Table 1.
Discriminatory Variables in Univariate and Multivariate Logistic Regression and ALD Model Derivation
Table 2 depicts the variables that were utilized for univariate logistic regression analysis within the Derivation Sample. After correction by MELD and histologic stage of disease, the variables in bold in Table 2 retained significance (p<0.01) and were candidates for multivariate analysis. These variables comprised the standard risk factors for NAFLD (gender, BMI, diabetes), laboratory abnormalities associated with ALD (AST/ALT ratio, gamma-glutamyl transferase (GGT), MCV), and a number of laboratory and histologic variables related to disease severity. While GGT was significant, there were only 75 patients with an available GGT value and therefore GGT was not entered into multivariate analysis. After correction for histology and MELD, multivariate analysis identified only high MCV, high AST/ALT ratio, low body-mass index (BMI) and male gender as independent predictors of ALD (Table 3; note the change in odds ratio for AST/ALT ratio after correction for MELD). Using the values derived after correction for MELD, we used the four independent predictors in a logistic regression analysis to calculate a nomogram. The derived nomogram, the ALD/NAFLD Index (ANI) was:
-58.5 + 0.637 (MCV) + 3.91 (AST/ALT) – 0.406 (BMI) + 6.35 for male gender.
Table 2.
Univariate Analysis in Derivation Sample
| Without Correction | Correction by MELD/Stage | ||||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | p | c-statistic | Odds ratio (95% CI) | p | |
| MELD | 1.16(1.09-1.23) | <0.0001 | 0.763 | ||
| Stage 3 or 4 | 2.33(1.25-4.33) | 0.007 | 0.646 | ||
| GGT | 1.01(1.01-1.027) | <0.0001 | 0.913 | 1.011(1.01-1.02) | 0.0006 |
| MCV | 1.62 (1.44-1.82) | <0.0001 | 0.902 | 1.61 (1.41-1.83) | <0.0001 |
| AST/ALT | 12.1 (5.7-25.7) | <0.0001 | 0.855 | 9.81(4.32-22.29) | <0.0001 |
| Total Bilirubin | 1.18(1.09-1.26) | <0.0001 | 0.846 | 1.07(0.98-1.18) | 0.14 |
| Alkaline Phosphatase | 1.003 (1.001-1.004) | 0.0003 | 0.833 | 1.001(1.000-1.003) | 0.06 |
| AST | 1.006(1.003-1.010) | 0.0007 | 0.794 | 1.004(0.999-1.008) | 0.08 |
| PTP1b | 0.06(0.03-0.13) | <0.0001 | 0.791 | 0.08(0.04-0.19) | <0.0001 |
| BMI | 0.84(0.79-0.90) | <0.0001 | 0.735 | 0.79( 0.73.0.87) | <0.0001 |
| Albumin | 0.28(0.16-0.47) | <0.0001 | 0.723 | 0.52 (0.28-1.00) | 0.05 |
| Triglyceride | 0.993(0.988-0.998) | 0.005 | 0.669 | 0.996(0.990-1.001) | 0.10 |
| Sex (M=1) | 3.35 (1.72-6.50) | 0.0004 | 0.660 | 4.37(1.96-9.76) | 0.0003 |
| Grade (2 vs 1) | 2.48 (1.24-4.96) | 0.01 | 0.65 | 2.68(1.21-5.89) | 0.01 |
| Grade (3 vs 2) | 6.66 (1.88-23.54) | 0.003 | 0.65 | 3.63(0.78-16.81) | 0.01 |
| INR | 3.07 (1.29-7.29) | 0.01 | 0.634 | 0.35(0.13-0.94) | 0.04 |
| DM | 0.48 (0.23-0.99) | 0.048 | 0.572 | 0.32(0.13-0.79) | 0.01 |
| Fasting Glucose | 0.99(0.98-1.02) | 0.14 | 0.551 | 0.99(0.983-0.999) | 0.03 |
| Age | 1.01(0.99-1.04) | 0.40 | 0.541 | 0.99(0.96-1.02) | 0.55 |
| ALT | 0.998(0.995-1.002) | 0.34 | 0.539 | 0.998( 0.995-1.001) | 0.27 |
Variables are listed in descending order of c-statistic prior to correction by MELD and Stage. The variables remaining significant (p≤0.01) after correction for MELD and fibrosis stage are bolded.
Table 3.
Multivariate Analysis in Validation Sample
| Without Correction | Correction by MELD | Correction by MELD/Stage | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| MCV | 1.49 (1.24-1.79) | <0.0001 | 1.48 (1.23-1.78) | 0.0002 | 1.52 (1.24-1.85) | <0.0001 |
| AST/ALT | 23.2 (5.24-102.8) | <0.0001 | 12.3 (2.58-58.1) | 0.0016 | 13.0 (2.64-64.3) | 0.0016 |
| BMI | 0.78 (0.66-0.91) | 0.0012 | 0.831 (0.716-0.964) | 0.0147 | 0.849 (0.724-0.996) | 0.0441 |
| Sex (M) | 26.5 (3.63-193) | 0.0113 | 24.7 (3.35-181) | 0.0016 | 25.6 (3.33-197) | 0.0018 |
Probability of ALD as compared to NAFLD was calibrated from the logistic regression analysis using the equation as follows:
The resulting value was defined as the ANI, with ANI > 0 incrementally favored a diagnosis of ALD, while ANI < 0 corresponded to a higher likelihood of a diagnosis of NAFLD.
Validation of ANI and Performance Characteristics Compared to Other Biomarkers
In the Derivation Sample, the ANI had a c-statistic of 0.989 which was significantly greater than the c-statistic generated by the proposed histochemical biomarker PTP1b (Table 4) for which this cohort was originally designed to analyze14. We used the history of alcohol consumption as the gold standard for predicting ALD in the Derivation Sample. However, reported alcohol consumption may be unreliable. To partially address this issue, we used the chart diagnosis as an alternative gold standard in the Derivation Sample. The ANI had almost identical c-statistics when the original chart diagnosis was used as gold standard in place of documented alcohol consumption (0.983 vs 0.989, respectively; Table 4).
Table 4.
C-statistic of ANI and comparison to other biomarkers
| Variables | c-statistic | (95% CI) | Cut-point | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Derivation Sample Based on ETOH history | ANI | 0.989 | 0.977-1 | 0 | 87.8 | 97.4 |
| PTP/IR | 0.791 | 0.714-0.867 | 65.4 | 85.9 | ||
|
| ||||||
| Derivation Sample Based on chart diagnosis | ANI | 0.983 | 0.969-0.996 | 0 | 93.5 | 92.0 |
| PTP/IR | 0.795 | 0.722-0.868 | 69.2 | 84.4 | ||
|
| ||||||
| Validation #1 | ANI | 0.974 | 0.955-0.992 | 0 | 95.5 | 92.0 |
| AST/ALT | 0.888 | 0.844-0.933 | 1.39 | 78.4 | 87.8 | |
| GGT | 0.775 | 0.676-0.873 | 109 | 66.7 | 80.0 | |
|
| ||||||
| Validation #2 | ANI | 0.989 | 0.973-1 | 0 | 85.3 | 100 |
| CDT | 0.850 | 0.744-0.955 | 10% | 74.4 | 88.5 | |
|
| ||||||
| Validation #3 | ANI | 0.767 | 0.622-0.913 | 0 | 85.4 | 66.7 |
| AST/ALT | 0.596 | 0.443-0.748* | 1.45 | 56.6 | 72.2 | |
|
| ||||||
| Validation #3 | ANI | 0.925 | 0.780-1 | 0 | 85.0 | 87.5 |
| MELD <20 Subset | AST/ALT | 0.563 | 0.352-0.773 | 1.63 | 40.0 | 100 |
c-statistic of ANI Score > AST/ALT ratio, p=0.02
Next, the ANI was evaluated and compared to other proposed and commonly used biomarkers for ALD, in the validation samples, each of which consisted of a complimentary patient cohort. In Validation Sample 1, which consisted of previously characterized cohorts of patients with NAFLD and ALD12-17, the latter with a high prevalence of acute alcoholic hepatitis, the ANI had a c-statistic of 0.974 (Table 4). The c-statistic for the ANI was significantly higher than AST/ALT ratio or GGT. In Validation Sample 2, which consisted of an ambulatory patient cohort, the c-statistic of the ANI was 0.989. This was significantly higher than the performance characteristics of CDT, the target marker of analysis for which this cohort was derived18. In Validation Sample 3, the pre-transplant sample, the c-statistic of ANI dropped to 0.767. Despite the drop in c-statistic, the ANI was still close to excellent and significantly higher than the traditional AST/ALT ratio marker (c-statistic: 0.596; p=0.02 compared to ANI; Table 4) in Validation Sample 3. Subgroup analysis showed that the c-statistic was 0.925 in patients with MELD score < 20 suggesting that decompensation in cirrhotics may impair the discriminatory capacity of the ANI. The ROC curves in Figure 1 compare ANI (black) to each of the comparator markers that were utilized (AST/ALT ratio, PTP1b staining, CDT) to distinguish ALD from NAFLD in the patient cohorts in aggregate (p<0.05).
Figure 1. ANI compared to other proposed and traditional markers.
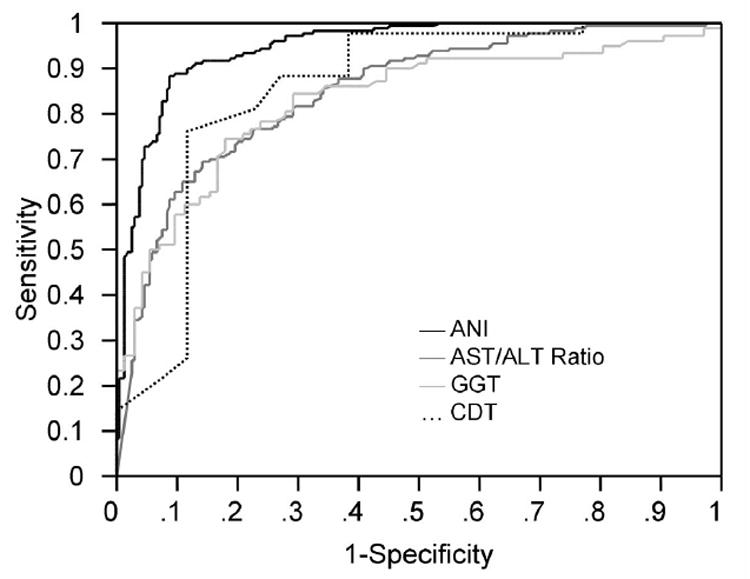
ROC curves and c-statistics were generated to compare ANI (black) to AST/ALT ratio (dark gray), GGT (light gray) and CDT (dotted line) for distinguishing ALD from NAFLD. Data for ANI and AST/ALT ratio are generated from aggregate of the three validation samples. GGT is based on Validation Sample 1 while CDT is based on Validation Sample 2. ANI was significantly more accurate in distinguishing ALD from NAFLD compared to other markers (p<0.05).
In both Validation Samples 2 and 3, which contained ALD patients with prolonged abstinence, sensitivity of ANI was excellent or close to excellent. This led us to examine more closely, if ANI characteristics were affected by abstinence in patients with ALD. ALD patients from the sample cohorts who had been abstinent for at least 2 months had a median ANI of 3.44 Intra Quartile Range (IQR): 1.80 – 7.78), while active drinkers had a median ANI of 6.68 (IQR: 2.49 – 10.30). However, the ANI had a c-statistic of only 0.642 for distinguishing abstinent patients from active drinkers in the ALD patient cohorts. As a comparator, the median ANI in patients with NAFLD was -7.01 (IQR: -2.90 – -9.97). Figure 2 demonstrates the similar distribution of ANI in active drinkers compared to abstinent patients. Thus, the ANI characteristics were not significantly influenced by abstinence.
Figure 2. Distribution of ANI in active drinkers and abstinent patients.
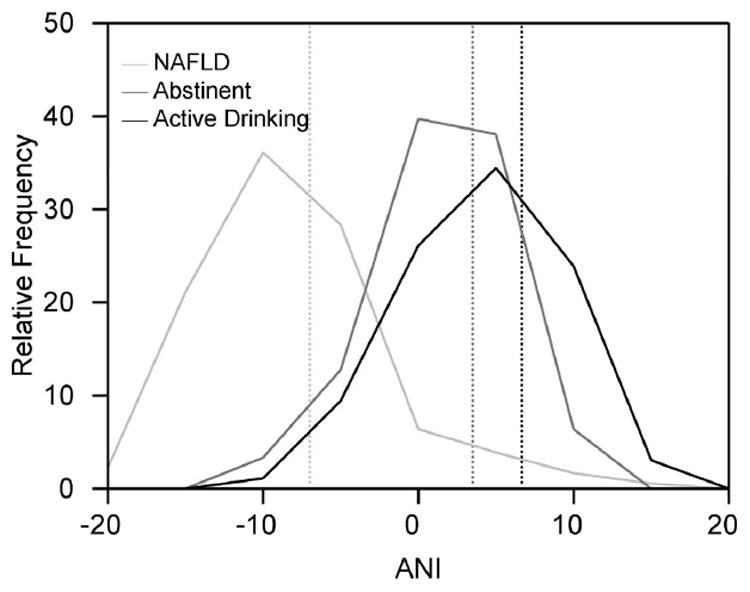
The distribution of ANI in abstinent patients (dark gray line) and actively drinking patients (black line) were similar. ANI in NAFLD patients is shown as a relative comparator (light gray line). The median ANI in NAFLD patients, abstinent ALD patients, and ALD active drinkers were: -7.01 (light gray reference line), 3.44 (dark gray reference line) and 6.68 (black reference line), respectively.
Lastly, we sought to further validate and calibrate the ANI by comparing the predicted probability of ALD based on ANI with the observed fraction of ALD in the combined Validation cohorts. As depicted in Figure 3, the observed fraction of ALD correlated very closely to the predicted probability. ANI of > 2.2 or < -2.2 provided a relatively clear diagnosis, corresponding to a 92.4% or 8.2% probability of ALD, respectively. For example, a male patient with an MCV of 104, AST/ALT ratio of 4, and BMI of 40 would generate an ANI of 8.95 which would correspond with a more than 99 % probability of ALD (e8.951 / (1 + e8.951)), while a female with an MCV of 95, AST/ALT ratio of 1, and BMI of 27, would generate an ANI of -5.04, which would correspond with an ALD probability of less than 1% (e-5.037 / (1 + e-5.037)). Although ANIs between -2.2 and 2.2 were more equivocal in their discriminatory capacity, 83 % of patients had an ANI greater than 2.2 or less than -2.2, indicating that most ANI values fell into a useful and interpretable range, rather than an equivocal range.
Figure 3. Predicted probability of ALD based on ANI.
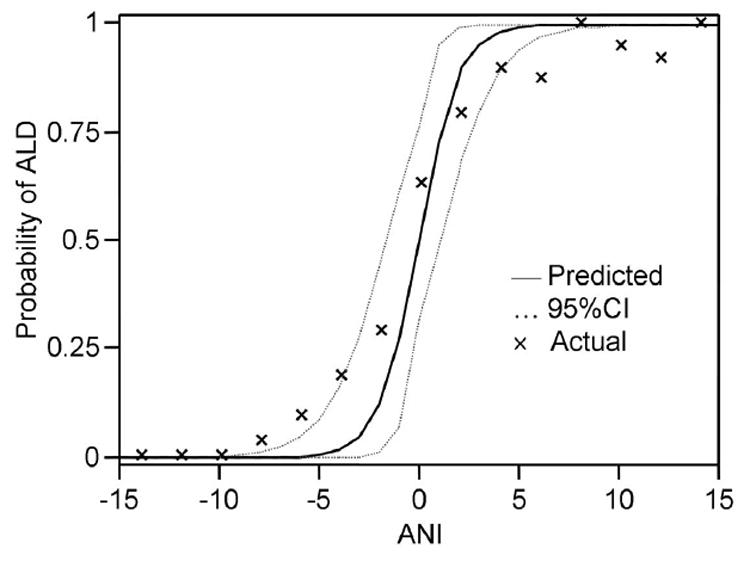
The solid line curve demonstrates the predicted probability of ALD based on a given ANI. Predicted probability is calculated by eANI / (1+ eANI). Actual proportion of ALD is compiled from the three validation samples in aggregate. The dotted line represents the 95% confidence interval of the predicted probability.
Discussion
In the clinical arena, it is frequently important to discern whether steatohepatitic liver injury is related to ALD or NAFLD as this distinction may influence patient management and candidacy for liver transplantation6-9. Despite a number of novel and sometimes expensive biomarkers that have been developed and evaluated, most of these have not been reproducibly demonstrated to perform more effectively than more traditional and inexpensive laboratory values21, 22. Unfortunately, these laboratory values have evidenced limited sensitivity and specificity as well13, 22. In the present studies, we generated a formula derived from a composite set of independent predictors after correction for disease severity, which comprise two commonly ordered and inexpensive laboratory values (AST/ALT ratio, MCV) with two objective clinical parameters (BMI, gender). This model, the ANI was then validated in complimentary patient cohorts including hospitalized, ambulatory and pre-transplant patients and found to compare favorably to several other traditional and proposed biomarkers. The ANI maintains important and unique methodologic aspects that enhance its utility as compared to prior studies designed to predict ALD. First, the ANI is adjusted for disease severity; prior studies have indicated that the validity of clinical predictors of ALD including AST/ALT ratio23, 24, CDT, GGT25, and histology26 are confounded by disease severity. ALD patients frequently present with more severe liver disease as compared to NAFLD patients, which often present as asymptomatic liver enzyme elevations10, 27, and thus adjustment for disease severity in the derivation of the ANI is important. A second methodologic advantage of our study is the use of logistic regression which has facilitated appropriate weighting of the parameters that comprise the ANI. This probably accounts for the improved test characteristics of ANI as compared to prior analyses which analyzed individual parameters that comprise the ANI such as AST/ALT ratio23, 24. Thirdly, the ANI has been derived using multiple complimentary and previously validated patient cohorts14-16, 18 including inpatients, outpatients, variations in disease severity and clinical status, collected at different points in time. Fourthly, the present study compares and validates the proposed ANI to other proposed biomarkers in a head-to-head manner. Thus, the ANI model fulfills many criteria for ideal models.
What caveats should we consider when utilizing the ANI in clinical practice? First, the gold standard of determining ALD from NAFLD remains a thorough interrogation of alcohol consumption history from the patient with corroboration from relatives and friends. However, the ANI may provide an auxiliary tool in the diagnosis, especially in the common scenarios of underreported and surreptitious alcohol consumption. Second, the ANI is a continuous variable. While we used an ANI of 0 as a cut-point to calculate sensitivity and specificity, the magnitude of the ANI must be considered in diagnosis of ALD in individual patients (see Figure 3 for examples). Third, it is well recognized that some patients that consume excess alcohol have features of metabolic syndrome as well, making it difficult to ascribe steatohepatitis to alcohol alone28. A negative ANI makes ALD unlikely and suggests a diagnosis of NAFLD, however a positive ANI, while indicating the presence of ALD, does not exclude co-existing metabolic syndrome. Fourth, the ANI may be less reliable in patients with cirrhosis and MELD Score > 20 as noted in Validation Sample 3. This may be due to the elevation in MCV and AST/ALT ratio that are frequently observed in cirrhosis independent of an alcoholic etiology. Lastly, other liver diseases should be excluded before utilizing the ANI as such patients have not been included in the derivation analysis. In the future, it will be of interest to determine whether our results which focus on distinguishing an alcohol basis to a histologic pattern of steatohepatitis can also be extrapolated to radiographic cases of fatty liver, as clinical evaluations in practice settings often do not progress to biopsy after radiographic ascertainment of fatty liver.
Interestingly, short-term abstinence did not significantly affect the performance characteristics of the ANI, thereby providing utility to this model in clinical scenarios in which patients are abstinent but the etiology of liver damage is still relevant (ie; evaluation for liver transplantation). Whereas the ANI is highly accurate in distinguishing ALD from NAFLD regardless of recent alcohol consumption, it should be noted that the ANI is unlikely to be useful in detecting surreptious alcohol consumption in patients with known ALD. However, this conclusion must be tempered by the understanding that alcohol consumption in our study was not assessed by an objective score to ensure its accuracy. Another limitation of the retrospective nature of the present study related to the GGT. The GGT appeared to be a promising candidate as an independent predictor in our model as it had the highest c-statistic in univariate analysis in which 75 patients had GGT available. Unfortunately this variable had to be excluded from the multivariate analysis because most patients did not have an available GGT value. However, future prospective studies that might be conducted to validate the ANI or evaluate alternative models, may well find that the GGT is a predictive variable.
In conclusion, we have developed a novel scoring system that is highly accurate in distinguishing ALD from NAFLD. The ANI may be a useful tool for the frequent clinical scenarios in which it is useful to ascertain an alcohol basis for steatohepatitic liver injury.
Acknowledgments
Grant Support: R01 AA 013933 (VS).
Abbreviations
- ALD
Alcoholic liver disease
- AUC
area under curve
- BMI
body-mass index
- CDT
carbohydrate deficient transferrin
- GGT
gamma-glutamyl transferase
- IQR
Intra Quartile Range
- MCV
mean corpuscular volume
- MELD
Model for End State Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- PTP
protein tyrosine phosphatase
- ROC
Receiver Operating Curve
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Said A, Williams J, Holden J, Remington P, Musat A, Lucey MR. The prevalence of alcohol-induced liver disease and hepatitis C and their interaction in a tertiary care setting. Clin Gastroenterol Hepatol. 2004;2:928–34. doi: 10.1016/s1542-3565(04)00393-3. [DOI] [PubMed] [Google Scholar]
- 2.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–92. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 3.Kunde SS, Lazenby AJ, Clements RH, Abrams GA. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650–6. doi: 10.1002/hep.20818. [DOI] [PubMed] [Google Scholar]
- 4.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–48. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 5.Carithers RJ, Herlong H, Diehl A, Shaw E, Combes B, Fallon H, Maddrey W. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial [see comments] Annals of Internal Medicine. 1989;110:685–90. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 6.Arteel G, Marsano L, Mendez C, Bentley F, McClain CJ. Advances in alcoholic liver disease. Best Pract Res Clin Gastroenterol. 2003;17:625–47. doi: 10.1016/s1521-6918(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 7.Marsano LS, Mendez C, Hill D, Barve S, McClain CJ. Diagnosis and treatment of alcoholic liver disease and its complications. Alcohol Res Health. 2003;27:247–56. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol. 2005;166:1681–90. doi: 10.1016/S0002-9440(10)62478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan TR, Weiss DG, Nemchausky B, Schiff ER, Anand B, Simon F, Kidao J, Cecil B, Mendenhall CL, Nelson D, Lieber C, Pedrosa M, Jeffers L, Bloor J, Lumeng L, Marsano L, McClain C, Mishra G, Myers B, Leo M, Ponomarenko Y, Taylor D, Chedid A, French S, Kanel G, Murray N, Pinto P, Fong TL, Sather MR. Colchicine treatment of alcoholic cirrhosis: a randomized, placebo-controlled clinical trial of patient survival. Gastroenterology. 2005;128:882–90. doi: 10.1053/j.gastro.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 10.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–33. viii. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Diehl AM, Goodman Z, Ishak KG. Alcohol-like liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95:1056–62. [PubMed] [Google Scholar]
- 12.Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol. 1999;19:261–271. doi: 10.1016/s0741-8329(99)00044-0. [DOI] [PubMed] [Google Scholar]
- 13.Mundle G, Ackermann K, Munkes J, Steinle D, Mann K. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate-deficient transferrin, gamma-glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760–766. doi: 10.1093/alcalc/34.5.760. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson SO, Smyrk TC. The use of protein tyrosine phosphatase 1B and insulin receptor immunostains to differentiate nonalcoholic from alcoholic steatohepatitis in liver biopsy specimens. Am J Clin Pathol. 2005;123:503–9. doi: 10.1309/1PX2-LMPQ-UH1E-E12U. [DOI] [PubMed] [Google Scholar]
- 15.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–8. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 17.Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–8. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 18.Stadheim LM, O’Brien JF, Lindor KD, Gores GJ, McGill DB. Value of determining carbohydrate-deficient transferrin isoforms in the diagnosis of alcoholic liver disease. Mayo Clin Proc. 2003;78:703–7. doi: 10.4065/78.6.703. [DOI] [PubMed] [Google Scholar]
- 19.Kamath P, Wiesner R, Malinchoc M, Kremers W, Therneau T, Kosberg C, D’Amico G, Dickson E, Kim W. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 21.Lumeng L. New diagnostic markers of alcohol abuse. Hepatology. 1986;6:742–745. doi: 10.1002/hep.1840060434. [DOI] [PubMed] [Google Scholar]
- 22.Menon K, Gores G, Shah V. Pathogenesis, diagnosis, and treatment of alcoholic liver disease. Mayo Clin Proc. 2001;76:1021–1029. doi: 10.4065/76.10.1021. [DOI] [PubMed] [Google Scholar]
- 23.Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94:1018–22. doi: 10.1111/j.1572-0241.1999.01006.x. [DOI] [PubMed] [Google Scholar]
- 24.Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004;39:336–9. doi: 10.1093/alcalc/agh074. [DOI] [PubMed] [Google Scholar]
- 25.Nalpas B, Hispard E, Thepot V, Pot S, Dally S, Berthelot P. A comparative study between carbohydrate-deficient transferrin and gamma-glutamyltransferase for the diagnosis of excessive drinking in a liver unit. J Hepatol. 1997;27:1003–8. doi: 10.1016/s0168-8278(97)80143-2. [DOI] [PubMed] [Google Scholar]
- 26.Pinto HC, Baptista A, Camilo ME, Valente A, Saragoca A, de Moura MC. Nonalcoholic steatohepatitis. Clinicopathological comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Dig Dis Sci. 1996;41:172–9. doi: 10.1007/BF02208601. [DOI] [PubMed] [Google Scholar]
- 27.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 28.Balasubramanian S, Kowdley KV. Effect of alcohol on viral hepatitis and other forms of liver dysfunction. Clin Liver Dis. 2005;9:83–101. doi: 10.1016/j.cld.2004.10.004. [DOI] [PubMed] [Google Scholar]


