Abstract
We report robust HPLC/UV methods for quantifying retinyl esters (RE), retinol (ROL) and retinal (RAL) applicable to diverse biological samples, with lower limits of detection of 0.7 pmol, 0.2 pmol, and 0.2 pmol, respectively, and linear ranges >3 orders of magnitude. These assays function well with small, complex biological samples (10–20 mg tissue). Coefficients of variation range from: intra-day, 5.9–10.0%; inter-day, 5.9–11.0%. Quantification of endogenous RE, ROL, and RAL in mouse serum and tissues (liver, kidney, adipose, muscle, spleen, testis, skin, brain, and brain regions) reveals utility. Ability to discriminate spatial concentrations of ROL and RE is illustrated with C57BL/6 mouse brain loci (hippocampus, cortex, olfactory bulb, thalamus, cerebellum, and striatum.) We also developed a method to distinguish isomeric forms of ROL to investigate precursors of retinoic acid. The ROL isomer assay has limits of detection between 3.5–4.5 pmol and a similar linear range and % CV as the ROL/RE and RAL assays. The assays described here provide for sensitive and rigorous quantification of endogenous RE, ROL, and RAL to elucidate retinoid homeostasis in disease states, such as Alzheimer’s disease, type 2 diabetes, obesity, and cancer.
Keywords: HPLC/UV, retinol, retinyl ester, retinal, retinoids, vitamin A, quantification, mouse, tissue
Introduction
Retinoid homeostasis involves balance among multiple retinoids in multiple tissues effected through dietary intake, storage, mobilization, transport, and metabolism (1–5). Specific binding proteins, enzymes, and receptors control flux through the central pathways of retinoid homeostasis, ultimately to produce retinoic acid, an active form of vitamin A (Figure 1) (6–7). RA effects a wide range of physiological processes, including development, nervous system function, immune response, cell proliferation, cell differentiation, and reproduction through activation of type II nuclear receptors (RAR, RXR, PPARβ/δ) (8–15). RA exerts additional biological effects through dimerization of RXR with an array of other type II nuclear receptors (13). Various approaches have been used to determine effectors of retinoid metabolism, such as genetic alteration of retinoid-binding proteins (16–21), enzymes (22–25), and receptors (12,13,26), dietary manipulation of vitamin A intake (28), and exposure to xenobiotics (29–32). Quantifying how manipulation of retinoid metabolism effects the flux of retinoids through metabolic paths and/or effects availability of substrate for RA production will provide insight into retinoid homeostasis and metabolism, and thereby function. Dysfunctions in retinoid homeostasis have been linked to dyslipidemia, diabetes, obesity, cancer, and Alzheimer’s Disease (33–40).
Figure 1.
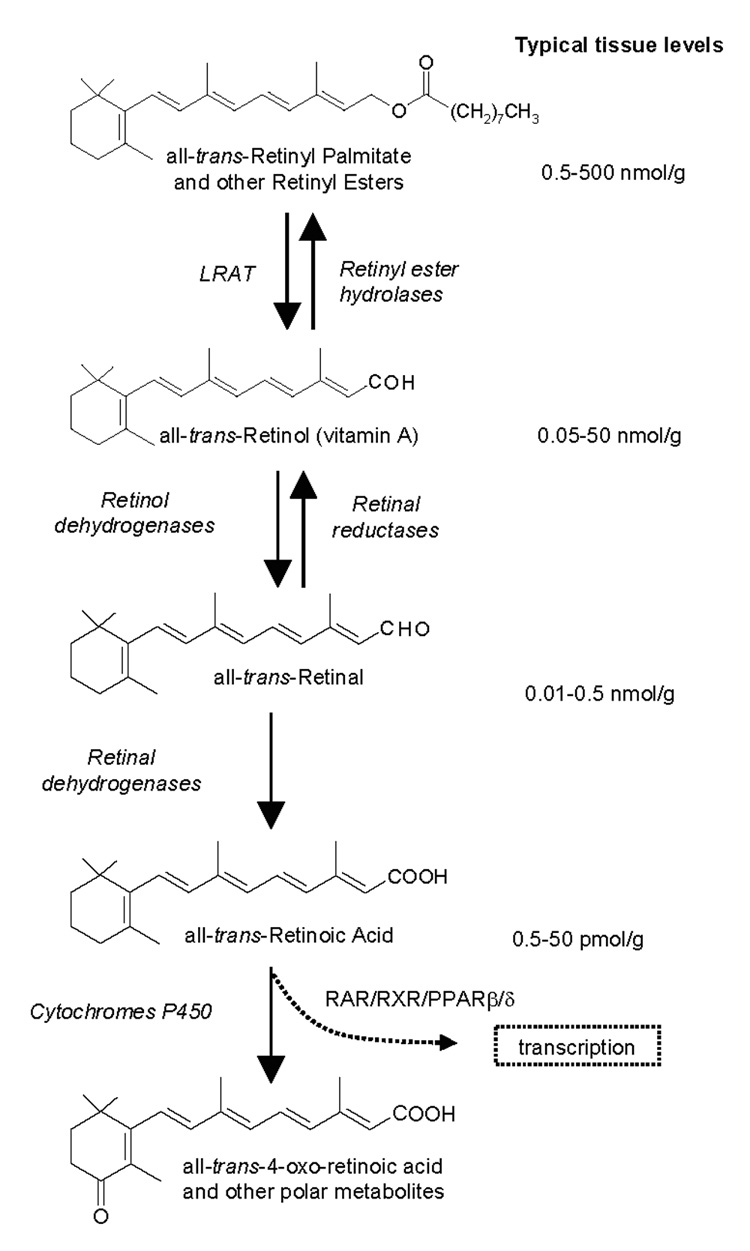
Structures of analytes in the central pathway of retinoid metabolism. Typical in vivo levels of each analyte are listed. Ranges reflect variation among tissues.
To date, many reports of retinoid quantification by HPLC/UV have not included essential supportive data, such as % recovery, % CV, standard curves defining linear ranges, illustration of effectiveness, or verification for use with multiple tissues/matrices. We have developed improved, simple, versatile HPLC/UV methods to measure endogenous levels of RE, ROL, RAL and isomers of ROL. These methods coupled with RA quantification (41,42) will provide opportunities for more complete and rigorous characterization of retinoid metabolism and homeostasis.
Experimental
Materials
Solvents were purchased from Fisher Scientific. O-Ethylhydroxylamine and retinoids, except 9cROL, were purchased from Sigma-Aldrich. Retinoid standards were prepared the day of use. Concentrations were verified spectrophotometrically using ε values (43).
9cROL preparation
9cROL was prepared by reducing 9cRAL with excess NaBH4 in 95%tetrahydrofuran/methanol. Acetone was added to destroy excess NaBH4. The reaction mixture was evaporated to dryness and resuspended in 50% hexane/water. The hexane layer containing 9cROL was removed and washed several times with water. Identity of 9cROL was confirmed spectrophotometrically and chromatographically.
Animals and tissues
Male SV129 mice (Charles River) were fed an AIN93G diet with 4 IU vitamin A/g as retinyl palmitate from weaning and were bred from dams fed the same diet. Male C57BL/6 mice (Charles River) were bred from dams fed a stock diet and were fed either a stock diet or an AIN93G diet with 4 IU vitamin A/g from weaning. The stock diet (Harlan Teklad Global, 18% protein rodent diet) contained 30.9 IU vitamin A/g (15.4 IU/g retinyl acetate and 15.5 IU/g retinol). Mice were fasted 16 hr prior to euthanasia. Tissues were dissected under yellow light. Brain dissections were performed with a Nikon SMZ-10A dissection microscope equipped with a Volpi (Auburn, NY) NCL 150 light source with a yellow filter. Tissues were frozen in liquid nitrogen immediately after harvest and kept frozen at −80 °C until assay, within three days of harvest. Tissues were homogenized on ice using ground glass homogenizers (Kontes, Duall size 21), either manually or with a Heidolph motorized homogenizer (280 RPM) in cold 0.9% saline to produce 10–25% homogenates. Serum was recovered by centrifuging clotted blood at 10,000 g for 10 min at 4 °C.
Extraction
Total ROL/RE and ROL isomer
Samples were extracted with a two-step acid-base method that recovers multiple retinoids. Here we describe only ROL and RE quantification, but the protocol also extracts RA and RA metabolites. Approximately 100 pmol of internal standard (5 µL of ~20 µM retinyl acetate in ethanol) was added to each sample. One to 3 ml of 0.025 M KOH in ethanol was added to tissue homogenates (up to 500 µL) or serum (100–200 µL). Ten ml of hexane were added to the aqueous ethanol phase. The samples were vortexed and centrifuged for 1 to 3 min at 60 rpm in a Dunac centrifuge (Becton Dickinson) to facilitate phase separation and to pellet precipitated protein. The hexane (top) phase containing nonpolar retinoids (ROL and RE) was removed. If RA and RA metabolites were assayed from the same samples, 4 M HCl (60– 180 µL) was added to the aqueous ethanol phase, and polar retinoids (RA and metabolites) were removed by extraction with a second 10 ml aliquot of hexane. Organic phases were removed under nitrogen while heating at ~25–30 °C in a water bath (Organomation Associates Inc. model N-EVAP 112, Berlin, MA). ROL/RE extracts were resuspended in 120 µL acetonitrile for serum and tissues except liver. Liver ROL/RE extracts were dissolved in 500 µL acetonitrile. Only glass containers, pipettes, and syringes were used to handle retinoids. Measurements from 10–20 mg tissue produce rigorous data from limited samples (e.g., embryo, brain regions), but routine assays from adult mice used 40–115 mg of tissue.
RAL-oxime method
RAL contains a reactive aldehyde group optimally converted to a stable product for accurate quantification. O-ethylhydroxylamine was used to convert RAL into an O-ethyl oxime derivative. One to 2 mL of methanol and 0.5–1 mL of 0.1 M O-ethylhydroxylamine in 100 mM HEPES (pH 6.5) were added to homogenates (up to 500 µL) or serum (50–200 µL). After vortexing, samples were allowed to stand for 15 min at room temperature. RAL O-ethyloxime was extracted with 10 mL hexane. The organic phase was removed under nitrogen with gentle heating at ~25–30 °C in a water bath. RAL O-ethyloxime samples were resuspended in 120 µL acetonitrile. As little as 10 mg tissue or 50 µL serum produced reliable data, but typically we used 10–80 mg tissue and 50–200 µL serum from adult mice.
Chromatography
Total ROL/RE
ROL and RE were resolved by reverse-phase chromatography (Zorbax SB-C18, 4.6 × 100 mm, 3.5 µm) on a Waters 2695 HPLC system and were quantified by UV absorbance at 325 nm. Analytes were separated at 1 mL/min with 11% water/89% acetonitrile/0.1% formic acid for 9 min followed by a linear gradient over 2 min to 100% acetonitrile. 100% acetonitrile was maintained for 2 min, followed by a linear gradient over 2 min to 5% acetonitrile/1,2-dichloroethane. Final conditions were held for 2 min before returning to initial conditions. Injection volume was 100 µL for all samples, with the exception of liver. To quantify liver RE accurately, a second 10 µL injection was necessary to ensure that the RE signal occurred within the linear detection range. ROL eluted at 4.8 min, retinyl acetate (IS) eluted at 8.9 min, and RE eluted at 16.5 min.
RAL O-ethyloxime
RAL (O-ethyl) oxime was resolved via reverse-phase chromatography (Zorbax SB-C18, 4.6 × 100mm, 3.5 µm) and quantified by UV absorbance at 368 nm. The sum of the syn- and anti- isomers was used to quantify RAL O-ethyloxime. ROL was monitored simultaneously at 325 nm. Analytes were separated at 1 mL/min with a linear gradient from 40% H2O/60% acetonitrile/0.1% formic acid to 5% H2O/95% acetonitrile/0.1% formic acid over 5 min. Final conditions were held for 9 min. RAL O-ethyloximes eluted at 6.6 min (anti-) and 10.9 min (syn-); ROL eluted at 7.2 min.
ROL isomers
This method separates ROL isomers, total RE, and RA isomers. ROL, RE, and RA were resolved by normal-phase chromatography (Zorbax SIL, 4.6 × 250 mm, 5 µm) with a Waters HPLC system (600 series pump, 717 series autosampler, and 2485 UV detector) and were quantified by UV absorbance at 325 nm, 325 nm, and 340 nm, respectively. Analytes were resolved using 0.4% 2-propanol/hexane at 2 mL/min. ROL isomers eluted at: 20.9 min, 13cROL; 27.0 min, 9cROL; 28.9 min, atROL. Other retinoids eluted at: 2.0 min, RE (retinyl palmitate and other retinyl esters); 3.6 min, retinyl acetate; 10.9 min, 13cRA; 12.1 min, 9cRA; 13.1 min, atRA.
Results and Discussion
We have previously developed a quantitative RA assay with attomol detection limits (41,42). Here we discuss methods for RE/ROL and RAL quantification. The diversity in chemical properties and endogenous retinoid concentration ranges (see Figure 1), renders it impractical to perform accurate quantification of RE/ROL/RAL/RA in a single chromatographic run. The vastly different concentrations of RE and ROL in some tissues requires two different volume injections for RE and ROL to be quantifiable in their linear ranges, e.g., a 10 µL injection for RE and a 100 µL injection for ROL in liver samples. In addition, the resuspension solvent must be chosen to accommodate solubility differences between RE and ROL. Acetonitrile solubilizes both RE and ROL, whereas methanol fails to fully solubilize RE. The ROL/RE and RAL reverse-phase methods, as well as the normal-phase method for ROL isomers, have been developed for accurate tissue measurements, but can be applied easily to cell culture or sub-cellular fractions. Many previous assays focus on separations but do not rigorously characterize analytical performance. Other assays focus on identification of retinoid species and are non-quantitative. Here, we aim to provide analytically robust methods to identify and quantify key retinoids of interest.
Sample Preparation
Samples should be harvested, handled, and extracted under yellow or red light to prevent isomerization and/or degradation. A red or yellow filter should be used with dissecting microscopes. Tissues not extracted immediately should be frozen immediately after harvest in liquid N2 and stored whole at −80 °C until assay. Tissue and/or serum can be stored at least one week at −80 °C without significant retinoid degradation. Frozen tissues should be thawed on ice before homogenization, homogenized on ice, and extracted immediately. Homogenized samples will undergo degradation after 2 h at 4 °C from matrix effects (41,44). Samples should not be frozen, thawed, and re-frozen. Extracts resuspended in acetonitrile remain stable in a room temperature autosampler (amber vials/yellow lights and/or shielded from light) for at least three days. Resuspended samples can be stored at −20 °C for one week without significant degradation. Total ROL/RE and ROL isomers use the same extraction method, whereas RAL relies on conversion to a stable oxime derivative for optimal extraction.
Conversion of RAL to RAL-oxime
O-ethylhydroxylamine was used to convert retinal into a stable oxime product for accurate quantification (Figure 2). RAL has a reactive aldehyde group, susceptible to promiscuous reactions in the sample matrix, preventing efficient extraction. Reaction with hydroxylamine or (O-alkyl)hydroxylamines produces syn- and anti- isomers (45–47). It is more desirable to generate RAL oximes from (O-alkyl)hydroxylamines (e.g. (O-ethyl) hydroxylamine) instead of the non-alkylated hydroxylamine, because the anti- retinaloxime isomer of the latter tends to co-elute with retinol and/or elute as a broad asymmetrical peak in both reverse and normal phase HPLC. Not only can co-elution of anti- retinaloxime with ROL interfere with ROL quantification, but also the syn- retinaloxime contributes inconsistently to the total retinal oximes.
Figure 2.
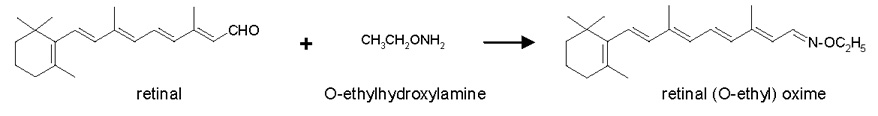
Conversion of RAL into RAL(O-ethyl)oxime.
Chromatography
Three methods were developed to quantify RE, ROL, and RAL in mouse tissues: total ROL and RE; RAL as RAL O-ethyloxime; and ROL isomers. Both total ROL/RE and RAL rely on reverse-phase HPLC separations that use the same mobile phase solvents and analytical column for simplicity and to allow for rapid switching between analyses, with no hardware or solvent reservoir changes. Reverse-phase separation was chosen for its resolution and superior retention time stability, compared to normal-phase. The ROL isomer method was developed for determining the isomeric distribution of retinol. We found that normal-phase HPLC had better resolving power in a shorter analysis time than reverse-phase for ROL isomers. Figure 3 shows examples of retinoid standards for each method with total ROL/RE (Figure 3A), RAL (Figure 3B), and ROL isomers (Figure 3C). All three methods use similar preparation with total ROL/RE and ROL isomers using the same extraction procedure, whereas RAL analysis relies on a simple conversion to a stable oxime derivative before extraction. UV detection after HPLC separation of retinoids offers analysis specificity because very few compounds absorb at wavelengths characteristic of retinoids. The intrinsic absorption of most compounds in the sample milieu is significantly more blue (maxima at shorter wavelength) than that for retinoids. Peak identity/instrument performance for each method is verified daily by injecting a mixture of authentic retinoid standards. Within the course of an analysis, retention times remain stable within ± 0.1 min for all methods.
Figure 3.
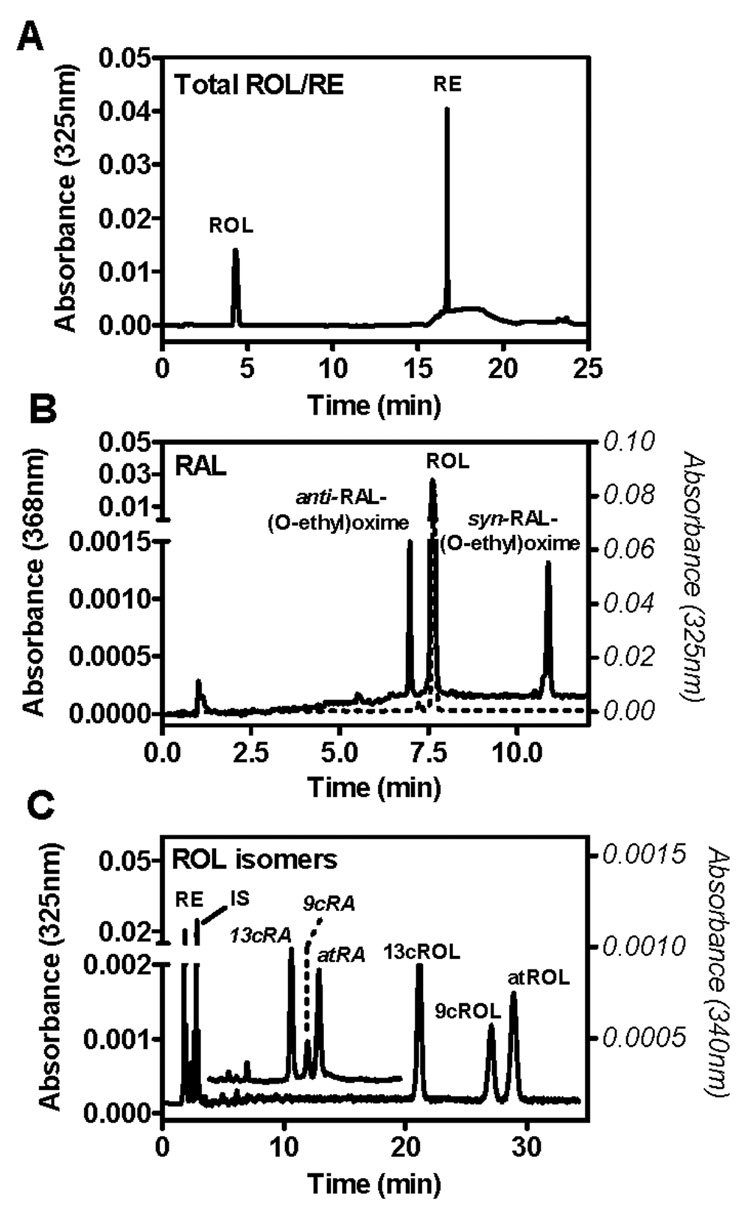
HPLC/UV chromatograms of standard solutions. (A) Total ROL/RE method: ROL (4.8 min) and RE (16.5 min). (B) RAL-oxime method: anti-RAL-(O-ethyl)oxime (6.6 min), ROL (7.2 min), and syn-RAL-(O-ethyl)oxime (10.9 min). The solid line indicates absorbance at 368 nm (left Y-axis); the dashed line indicates absorbance at 325 nm (right Y-axis). (C) ROL isomer method: RE (2.0 min), IS (retinyl acetate, 3.6 min), 13cRA (10.9 min), 9cRA (12.1 min), atRA (13.1 min), 13cROL (20.9 min), 9cROL (27.0 min), and atROL (28.9 min). The RE standard shown is retinyl palmitate. Absorbance of ROH isomers was monitored at 325 nm (left Y-axis); absorbance in the overlay showing RA isomers was monitored at 340 nm (right Y-axis).
Total ROL and RE
The total ROL/RE reverse-phase method was modified from previous methods to use an acetonitrile/water/formic acid mobile phase that transitions to an acetonitrile/dichloroethane mobile phase (31,48). The acetonitrile/water/formic acid mobile phase gives sharper ROL peaks than previous methanol/water-based ROL separations (Figure 4). Water in the mobile phase retains the polar retinol; transition to dichloroethane facilitates elution of the non-polar RE. ROL elutes at 4.8 min, retinyl acetate (IS) elutes at 8.9 min, and RE elutes at 16.5 min (Figure 3A). All endogenous RE are not resolved using this method: it is intended to quantify only total RE. Figure 3A shows retinyl palmitate only, which is up to 90% of the endogenous ester. Retinyl oleate coelutes with retinyl palmitate, whereas other esters, such as retinyl linoleate, retinyl myristate, and retinyl stearate elute just before or after retinyl palmitate. The sum of all ester peaks was used to calculate total RE. Retinyl palmitate is used as the calibrant to calculate total ester, because retinyl palmitate and other retinyl esters have similar absorbance maxima (43).
Figure 4.
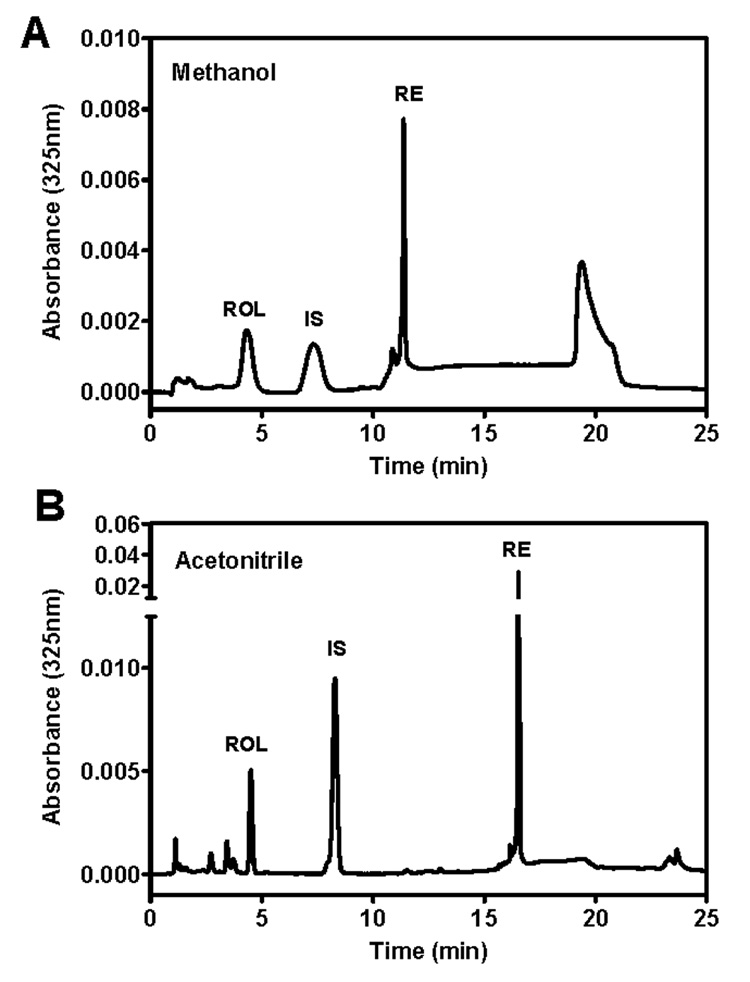
Comparison of (A) methanol-based ROL/RE mobile phase with (B) acetonitrile-based ROL/RE mobile phase. (A/B) are identical mouse kidney samples with ROL (4.5min/4.8 min), IS (retinyl acetate, 7.5 min/8.9 min) and RE (11.7 min/16.6 min). The methanol-based mobile phase in (A) uses the same gradient as the acetonitrile-based mobile phase in (B) with methanol/water/1,2-dichloroethane.
RAL (RAL O-ethyloxime)
This reverse-phase method for RAL quantification uses the same mobile phase solvents and column as the total ROL/RE assay, but with a different gradient. Both anti- and syn- RAL O-ethyloximes are resolved from each other and ROL, are monitored at 368 nm, and elute at 6.8 min and 10.9 min, respectively (Figure 3B). ROL, eluting at 7.6 min, also can be quantified with this method and is monitored concurrently at 325 nm. Syn- and anti- RAL O-ethyloxime isomers are summed and quantified from a calibration curve generated from standard amounts of RAL O-ethylhydroxylamine. Note that the column needs to be flushed periodically when quantifying ester-rich tissue (e.g., liver) to reduce RE accumulation.
ROL isomer
The ROL isomer method is an isocratic normal-phase separation that can quantify the isomeric distribution of retinol, which may be of interest when investigating precursors to RA isomers. This method resolves 13cROL (21.1 min), 9cROL (27.0 min), and atROL (29.0 min) as well as retinyl palmitate (1.9 min) and retinyl acetate (IS, 3.0 min) (Figure 3C). Retinyl palmitate can be quantified using the ROL isomer method, but because of its minimal retention and the possible background contribution from minimally retained matrix components, the total ROL/RE method described here is preferable for RE quantification. RA isomers can be separated using the ROL isomer chromatography method by concurrently monitoring at 340 nm (Figure 3C). RA isomers elute at: 13cRA (10.6 min), 9cRA (12.0 min), and atRA (12.9 min). Although levels of RA in vivo are not detectable above background and/or are the same magnitude as random/interfering peaks, this method is useful for applications in which RA is high, such as enzyme assays. Quantification of in vivo levels of RA is best accomplished with more sensitive detection methods (41,42).
Performance
The analytical performance of each assay was evaluated for: limit of detection (LOD), limit of quantification (LOQ), linearity, intra-assay coefficient of variation, inter-assay coefficient of variation, accuracy, and extraction efficiency. The sensitivity of each method was obtained by measuring standard solutions prepared on the day of use with spectrophotometrically verified concentrations. The LOD is defined as a signal to noise ratio of 3:1, whereas the LOQ is defined as a signal to noise ratio of 10:1 (Table 1). The total ROL/RE and RAL methods have sub-pmol detection limits (0.2 to 0.7 pmol) and are 10-fold more sensitive than the detection limits for the ROL isomer method (3.5 to 4.5 pmol). Similarly, total ROL/RE and RAL methods have lower LOQs (0.4 to 1 pmol), compared to the ROL isomer method LOQ (1 to 5 pmol).
Table 1. Limits of detection and quantification, and reproducibility.
Limit of detection (LOD; S/N = 3) and limit of quantification (LOQ; S/N = 10) measurements were obtained from retinoid solutions prepared on the day of use. Reproducibility was measured by intra-assay (same day) and inter-assay (consecutive day) variation. Variation values were obtained from groups of 3–10 samples from the same mouse liver prepared separately.
| Method | Analyte | LOD (pmol) |
LOQ (pmol) |
Intra-assay CV (%) |
Inter-assay CV (%) |
|---|---|---|---|---|---|
| Total ROL/RE | ROL | 0.2 | 0.4 | 6.8 | 7.0 |
| Total ROL/RE | RE | 0.7 | 1.0 | 5.9 | 10.7 |
| RAL-oxime | RAL | 0.2 | 0.4 | 6.9 | 5.9 |
| RAL-oxime | ROL | --- | 0.5 | 6.4 | 10.8 |
| ROL isomers | atROL | 3.5 | 4.0 | 6.9 | 9.6 |
| ROL isomers | 9cROL | 3.5 | 4.0 | 10.0 | 9.9 |
| ROL isomers | 13cROL | 4.5 | 5.0 | 6.7 | 6.0 |
| ROL isomers | RE | --- | 1.0 | 9.1 | 11.0 |
---, not determined.
Linearity
Figure 5 shows representative calibration curves for each analyte using (A) total ROL/RE method, (B) RAL (RAL (O-ethyl)oxime) method, (C) ROL isomer method. Each point represents either 2–3 replicates and r2 values are >0.99. Linear ranges are: (A) ROL 0.4–1000 pmol, RE 1–1000 pmol; (B) RAL 0.4–600 pmol, ROL 0.5–900 pmol; (C) atROL 4–1000 pmol, 9cROL 4–1000 pmol, 13cROL 4–1000 pmol, RE 1–600 pmol. Each linear detection range spans the physiological range of each analyte (Figure 1 and Figure 5).
Figure 5.
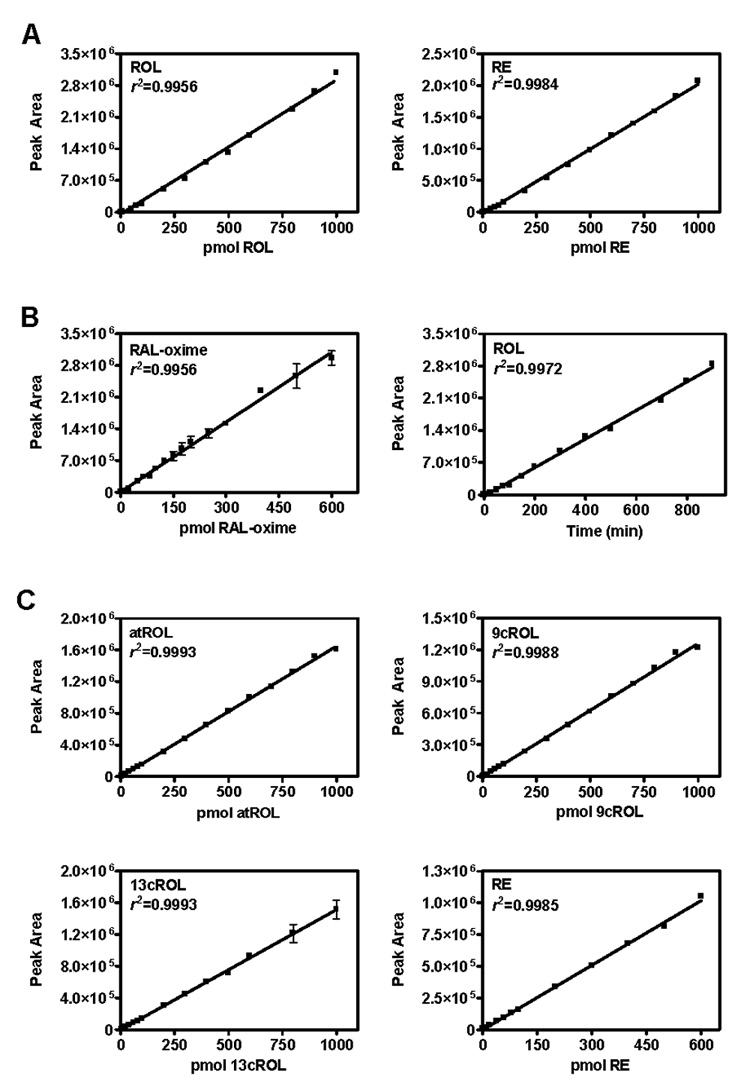
Representative calibration curves for (A) Total ROL/RE method, (B) RAL-oxime method, (C) ROL isomer method. All r2 values were >0.99.
Intra- and inter-assay coefficients of variation
Reproducibility was assessed according to intra-assay (same day) coefficients of variation and inter-assay (consecutive days) coefficients of variation recovered from tissue samples, to reflect assay variability, including sample preparation and chromatographic analysis. These results were generated from groups of 3–10 samples prepared separately from a single mouse liver (Table 1). Intra-assay coefficients of variation ranged from 5.9 to 10.0%, whereas inter-assay coefficients of variation ranged from 5.9 to 11.0%.
Accuracy
Accuracy, the agreement between applied and measured amounts, was >95% for all analytes.
Extraction Efficiency
Retinyl acetate was used as an internal standard to assess handling losses and extraction efficiency for the total ROL/RE. Typical average recoveries (average ± SEM) ranged from 60 ± 8 % (n = 6, adipose) to 92 ± 3 % (n = 9, serum). Retinyl acetate reflected RE recovery accurately for all tissues investigated. For adipose and other lipid-rich tissue tissues, retinyl acetate also accurately reflected the recovery of ROL. However, retinyl acetate did not always accurately reflect ROL recovery from liver and was thus not used to adjust liver ROL values. Exogenous ROL spiked into liver homogenate before extraction was recovered 94 ± 4 % (n = 3). Retinyl acetate accurately reflected RE recovery from liver with a value of 86 ± 10 % (n = 10). Retinyl acetate can be used as an internal standard for the ROL isomer method with similar performance. No internal standard was needed for the RAL O-ethyloxime method, because the recovery of the O-ethyloximes routinely exceeded >95%.
Application
Each method was used to quantify retinoids in mouse tissue. Figure 6 shows representative chromatograms of various tissues: (A) adipose using total ROL/RE method, (B) liver using the RAL O-ethyloxime method, (C) liver using the ROL isomer method. Note that each of the analytes is easily identified. Table 2 summarizes retinoid levels in a variety of tissues from mice fed a diet containing 4 IU vitamin A/g. It should be noted that diet has a profound effect on retinoid levels and the diet of the dam influences the diet of the offspring. Stock diets contain copious vitamin A (~30 IU vitamin A/g—15.4 IU retinyl acetate plus 15.5 IU retinol). The AIN recommends diets with 4 IU vitamin A/g, a slightly higher level than the minimum amount required for mice of 2.4 IU/g (49,50). Mice fed stock diets or bred from mothers fed stock diets will have substantially higher RE and higher ROL levels than those bred from dams fed a 4 IU vitamin A/g diet, which can often complicate and/or obscure effects on retinoid metabolism.
Figure 6.
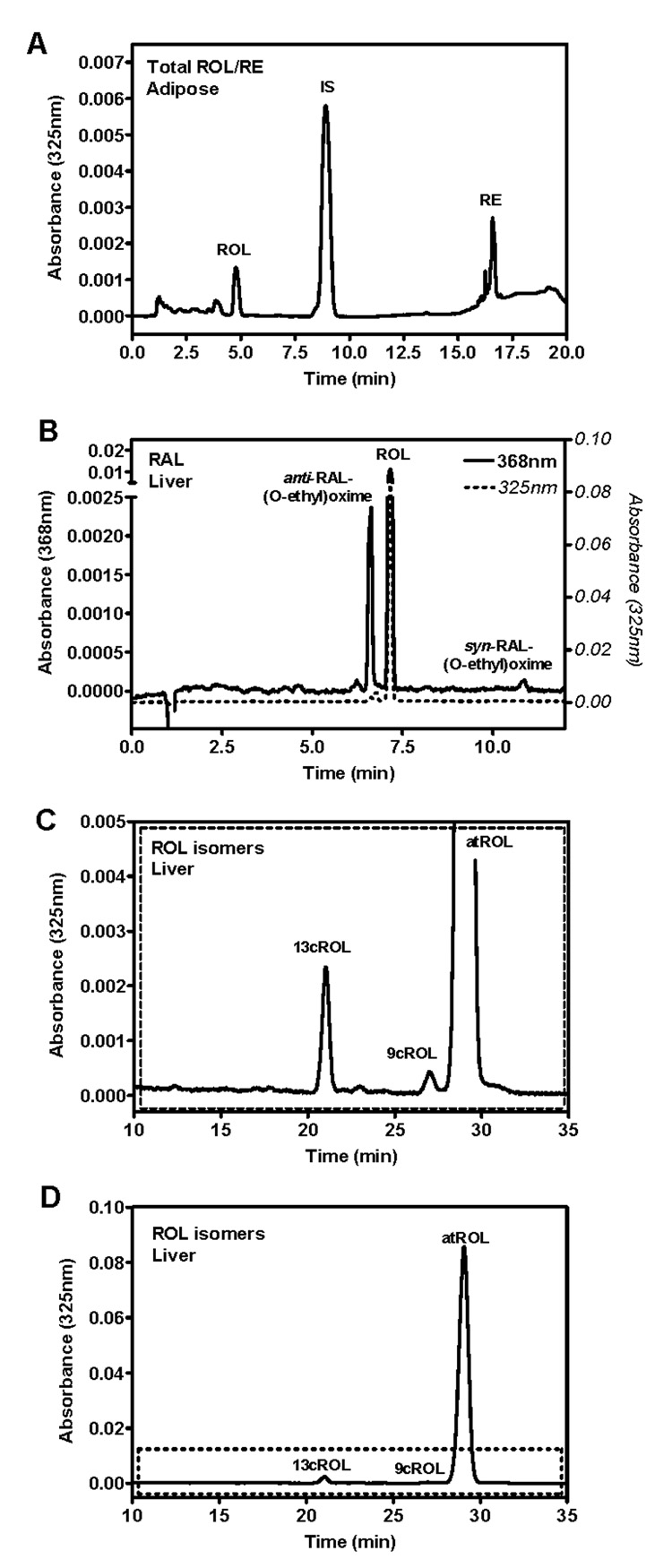
HPLC/UV chromatograms of mouse tissue. (A) Total ROL/RE method measuring adipose: ROL (4.8 min), IS (retinyl acetate, 8.9 min), and RE (16.6 min). (B) RAL-oxime method measuring liver: anti-RAL-(O-ethyl)oxime (6.6 min), ROL (7.2 min), and syn-RAL-(O-ethyl)oxime (10.9 min). (C,D) ROL isomer method measuring liver: 13cROL (20.9 min), 9cROL (27.0 min), atROL (28.9 min). Top panel in (C) is a magnified view showing 13cROL and 9cROL more clearly.
Table 2. Endogenous retinoids in mouse tissues.
| Serum/Tissue | RE (nmol/g) | ROL (nmol/g) | RAL (pmol/g) |
|---|---|---|---|
| &serum# | 0.22 ± 0.02 (69) | 0.81± 0.04 (70) | 32.2 ± 6.2 (6) |
| &liver | 562.6 ± 75.9 (55) | 9.6 ± 0.9 (60) | 160.9 ± 14.3 (26) |
| &kidney | 1.8 ± 0.2 (37) | 0.60 ± 0.04 (37) | 187.3 ± 31.2 (12) |
| &adipose (white) | 0.59 ± 0.09 (29) | 0.63 ± 0.03 (37) | 63.5± 5.2 (12) |
| &adipose (brown) | 0.99 ± 0.21 (5) | 0.64 ± 0.21 (5) | --- |
| &muscle | 0.25 ± 0.03 (31) | 0.15 ± 0.02 (38) | --- |
| &spleen | 1.2 ± 0.1 (26) | 0.60 ± 0.06 (26) | --- |
| &testis | 0.31 ± 0.02 (27) | 0.08 ± 0.01 (27) | 90.7 ± 10.1 (12) |
| &skin | 0.22 ± 0.02 (5) | 0.32 ± 0.01 (5) | --- |
| **brain | 0.84 ± 0.16 (19) | 0.68 ± 0.23 (19) | --- |
| **hippocampus | 0.70 ± 0.05 (27) | 0.30 ± 0.03 (27) | --- |
| **cortex | 0.35 ± 0.04 (18) | 0.08 ± 0.01 (18) | --- |
| **olfactory bulb | 0.63 ± 0.03 (4) | 0.20 ± 0.02 (4) | --- |
| **thalamus | 0.47 ± 0.06 (4) | 0.20 ± 0.04 (4) | --- |
| **cerebellum | 0.73 ± 0.10 (8) | 0.42 ± 0.08 (8) | --- |
| **striatum | 0.41 ± 0.08 (4) | 0.21 ± 0.05 (4) | --- |
Data were obtained from 2 to 4 month-old male SV129 mice fed and bred from dams fed an AIN93G diet with 4 IU vitamin A/g.
Data were obtained from 2 to 4-month-old male C57BL/6 mice fed an AIN93M with 4 IU vitamin A/g from weaning and bred from dams fed a stock diet (>30 IU vitamin A/g). Values are means ± SEM. The numbers in parentheses indicate the number of samples assayed. Each sample was obtained from an individual mouse.
---, not measured.
serum RE and ROL values are expressed in nmol/mL and RAL values are expressed in pmol/mL.
RE values are the highest in liver, the main storage site for vitamin A, with 562.6 ± 75.9 nmol/g. Kidney and spleen have 1.8 ± 0.2 and 1.2 ± 0.1 nmol/g RE, respectively, with 300–500-fold lower RE levels than liver. Other tissues range from 0.25 ± 0.03 nmol/g (muscle) to 0.99 ± 0.21 nmol/g (brown adipose). Circulating RE levels are 0.22 ± 0.02 nmol/mL. RE levels in brain vary by region as do ROL levels, consistent with region-dependent RA levels (41,42).
Compared to RE, ROL levels are higher only in skin (50%) and serum (3.7-fold). Other tissues have ROL levels that range from equivalent (white adipose) to 4-fold lower (cortex), with the exception of liver which has ~60-fold lower ROL than RE. ROL levels were highest in liver at 9.6 ± 0.9 nmol/g. Other tissue ROL levels ranged from 0.08 ± 0.01 nmol/g (testis) to 0.64 ± 0.21 nmol/g (brown adipose), 15- to 120-fold lower than liver ROL levels. Circulating levels of ROL are 0.81 ± 0.04 nmol/mL.
Compared to ROL, RAL levels range from equivalent (testis) to 60-fold lower (liver). RAL levels are highest in liver and kidney with 160.9 ± 14.3 pmol/g and 187.3 ± 31.2 pmol/g RAL, respectively. Testis levels are approximately half (90.7 ± 10.1 pmol/g) and adipose levels are approximately one-third (63.5 ± 5.2 pmol/g) that of liver and kidney. Circulating RAL levels are 32.2 ± 6.2 pmol/g. RAL levels are 4-fold (liver) to 12-fold (kidney) higher than RA levels (42).
Table 3 provides data for ROL isomers in tissues from mice fed a stock diet with 30 IU vitamin A/g. atROL values ranged from 0.90 ± 0.11 nmol/g (serum) to 20.7 ± 2.9 nmol/g (liver). Note that all atROL values are higher than those in Table 2 because of the higher vitamin A content of the mouse diet, by 10%, 2-fold, and 3-fold, respectively, in serum, liver, and adipose. The majority of ROL is present as atROL with 87.4% (adipose) to 94.7% (liver) of total ROL. 9cROL and 13cROL were detected in animals fed diets with 30 IU vitamin A/g at a small percentage of the total ROL: 9cROL, between 1.2% (liver) and 8.7% (adipose) and 13cROL, between 1.4% (liver) and 3.9% (adipose). 9cROL levels are 0.03 ± 0.01 nmol/g (serum) to 0.25 ± 0.01 nmol/g (liver) and 13cROL levels are 0.04 ± 0.01 nmol/g (serum) to 0.30 ± 0.04 nmol/g (liver). 9cROL and 13cROL levels in tissue from mice fed a diet with 4 IU vitamin A/g are lower than those in Table2 or below the limit of quantification/detection for the assay.
Table 3. Endogenous ROL isomers in mouse tissues.
Data were obtained from 2 to 4 month-old male C57BL/6 mice fed and bred from dams fed a stock diet with the equivalent of 30 IU vitamin A/g. Values are means ± SEM. The numbers in parentheses indicate the number of samples assayed. Each sample was obtained from an individual mouse.
| Serum/Tissue | atROL (nmol/g) | 9cROL (nmol/g) | 13cROL (nmol/g) |
|---|---|---|---|
| serum# | 0.90 ± 0.11 (13) | 0.03 ± 0.01 (13) | 0.04 ± 0.01 (13) |
| liver | 20.7 ± 2.9 (18) | 0.25 ± 0.01 (18) | 0.30 ± 0.04 (18) |
| adipose (white) | 1.8 ± 0.3 (4) | 0.18 ± 0.01 (4) | 0.08 ± 0.01 (4) |
serum ROL values are expressed in nmol/mL
To address the possibility that these isomers are artifacts of the extraction process, exogenous retinoids were added to tissue samples before/during homogenization to evaluate isomerization during sample preparation (Figure 7). Addition of atROL increased only atROL, whereas addition of 9cROL increased only 9cROL. Addition of either atRAL or 9cRAL did not cause any increase in either atROL or 9cROL.
Figure 7.
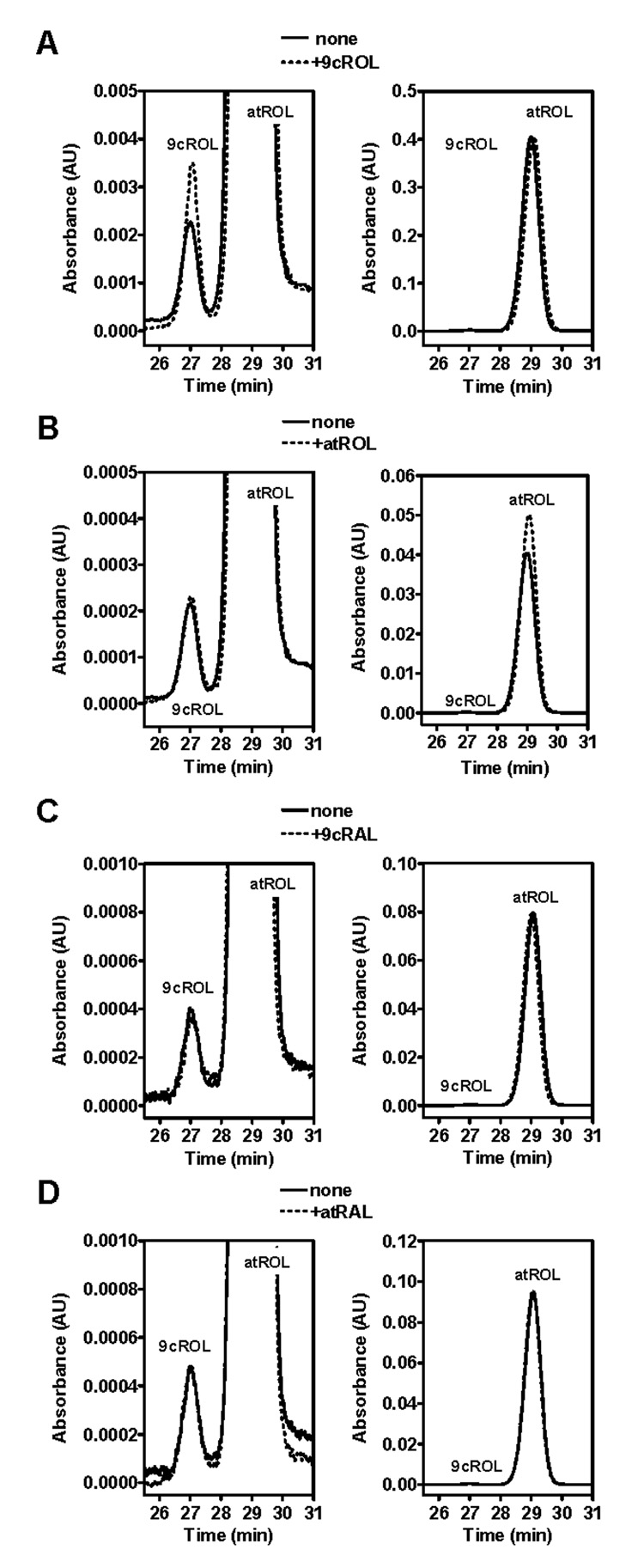
Addition of exogenous retinoids to mouse liver before homogenization to show 9cROL is not formed artifactually. Left panel of each pair is a magnified view of the right panel to show 9cROL more clearly. (A) Addition of 9cROL increases 9cROL only. (B) Addition of atROL increases atROL only. (C, D) Addition of either 9cRAL or atRAL does not increase either 9cROL or atROL.
Comparison to Other Methods
The ROL/RE quantification obtained values similar to those by other methods of analysis. Liver ROL obtained from mice fed a stock diet (20.7 nmol/g, Table 3) was similar to those fed a comparable copious vitamin A diet, ranging from 6–81.5 nmol/g (16,18,22–25,31,32,44,48). Mice fed a 4 IU vitamin A/g diet had 9.6 nmol/g ROL in liver, less than half that of the 30 IU vitamin A/g stock diet, reflecting the lower level of vitamin A in the diet. Serum and adipose ROL in mice fed a stock diet (serum, 0.9 nmol/g; adipose, 1.8 nmol/g; Table 3) was similar to literature values ranging from 0.6–1.58 nmol/g (22,23,44,48,51) and 1.67–2.9 nmol/g (22,23), respectively. Serum and adipose values in mice fed a 4 IU vitamin A/g diet were slightly lower and one-third lower, respectively, of the stock diet. Extrahepatic tissue ROL and RE were comparable to those produced by other methods (22,23). Liver RE in mice fed a 4IU vitamin A/g diet was 562 nmol/g, which was comparable to or lower than RE values reported with a stock diet (473–5500 nmol/g) (16,17,22–25,31,32,44,48). Previous stock diet adipose and liver RAL values were 0.15–0.8 nmol/g and 0.9 nmol/g, respectively, 2- to 6-fold higher than adipose (0.064 nmol/g) and liver (0.161 nmol/g) values obtained from mice fed a 4IU vitamin A/g diet (17,36).
Both the ROL/RE and RAL methods have sensitivity comparable to that reported for HPLC/MS/MS (52) and better than that listed for HPLC/MS (51,53) or HPLC/UV (44). Many assays do not provide information on sensitivity (16,17,19,23–25,30,31,36) or illustrate deficits in sensitivity by reporting large tissue requirements (36). For example, Ziouzenkova, et al. quantified RAL by HPLC with PDA UV detection (RAL MS/MS data reported therein was for analyte ID only) indicating the need to pool adipose fat pad samples for quantification and/or identification (36). Our analysis required only ~60 mg adipose tissue for RAL quantification, a fraction of one typical adipose fat pad. Our other sample requirements ranged from 10–80 mg for tissue and 50–200 µL serum, slightly better than Schmidt et al., who cited typical use of 200–300 mg tissue and 400 µL serum (44).
The ROL isomer method described here was based on previous work (54), focused on separation of various ROL and dehydroretinol isomers, which reported the distribution of isomers in livers of fresh water fish. Our measurements generated similar values for mouse liver. Stancher and Zonta also saw less than 1% isomerization of ROL into other isomers during handling (Figure 7). Wang et al. (53) report data for atROL and 9cROL by LC/MS, but our experience with a similar mobile phase found atROL and 13cROL co-eluted (data not shown). The ROL isomer method described here had sensitivity similar to HPLC/MS detection (53).
Methods described here have several additional advantages. The majority of measurements use the ROL/RE method and the RAL method, which use the same solvents and analytical column, allowing for seamless transition between the two analyses. Both the ROL/RE and the RAL methods have run times of 25 min, including re-equilibration, which is shorter than other methods, for which some separations take >100 min (17,18,22–25,30,31,48,51,53,55). Additionally, our limits of detection/quantification and linear working ranges have been characterized rigorously for each analyte, and the analyte concentration is determined from a standard curve spanning the physiological range of the analyte. External standard methods are far less analytically rigorous and provide only a ratio with a single standard concentration and should be avoided (36).
Sample preparation described here simplifies the sample matrix sufficiently to assay complex matrices (i.e., tissue samples) as well as simple matrices (i.e., serum) without deterioration of analytical performance. Schmidt et al. also provide evidence of an analytically rigorous assay appropriate for tissue retinoid quantification (44), whereas other assays have only been assessed for simpler matrices, such as serum (51,52,55), and/or have not been comprehensively characterized for analytical performance (16,17,19,22–25,30,31,36,54). Simplification of the sample matrix also enhances accuracy and the lives of guard and analytical columns. Other assays cite frequent changing of guard columns (44,52), whereas we typically get greater than 100-fold more injections per typical guard column lifetime.
Analyses which use saponification—an alkaline digestion that frees retinoids from the stabilizing matrix and lipids while hydrolyzing RE to ROL to yield a total ROL measurement—can be problematic. The elevated temperature and exposure to alkali often causes retinoid degradation and isomerization of 4–40% (2). This loss is illustrated by 30–65% lower total ROL values obtained after saponification, compared to the sum of ROL and RE values obtained separately (16,22).
The method of RAL quantification described here converts RAL into a RAL O-ethyloxime, which is optimal for efficient extraction of the reactive and labile aldehyde group and separates both syn- and anti-retinaloxime isomers for accurate quantification. Other methods have used hydroxylamine instead of an (O-alkyl)hydroxylamine, which causes the anti-oxime RAL isomer to co-elute with ROL, and report potentially inaccurate quantification using only one RAL isomer peak (36). Other methods have not converted to an oxime (16,17,48) and/or have not provided in vivo data for RAL (44).
The methods described here provide simple, analytically robust procedures for quantifying retinoids from complex matrices and require only small amounts of tissue sample. These methods should significantly assist elucidation of retinoid function and characterization of alterations in retinoid metabolism and homeostasis during diseases, such as diabetes, obesity, cancer, and Alzheimer’s Disease.
Acknowledgements
This work was supported by NIH Grants DK36870, AG13566, DK46839, and an NIH Kirschstein Individual Fellowship F32 DK066924 (MAK).
Abbreviations
- ROL
retinol
- RE
retinyl ester
- RAL
retinal
- atROL
all-trans-retinol
- 9cROL
9-cis-retinol
- 13cROL
13-cis-retinol
- atRAL
all-trans-retinal
- 9cRAL
9-cis-retinal
- RA
retinoic acid
- atRA
all-trans-RA
- 9cRA
9-cis-RA
- 13cRA
13-cis-RA
- 9,13dcRA
9,13-di-cis-RA
- CV
coefficient of variance
- LOD
limit of detection
- LOQ
limit of quantification
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- PPAR
peroxisome proliferators-activated receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolf G. Multiple functions of vitamin A. Physiol. Rev. 1984;64:873–937. doi: 10.1152/physrev.1984.64.3.873. [DOI] [PubMed] [Google Scholar]
- 2.Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. 2nd Ed. NY: Raven Press; 1994. [Google Scholar]
- 3.Wolf G. Retinoic acid homeostasis: retinoic acid regulates liver retinol esterification as well as its own catabolic oxidation in liver. Nutr. Rev. 2001;59:391–394. doi: 10.1111/j.1753-4887.2001.tb06968.x. [DOI] [PubMed] [Google Scholar]
- 4.Harrison EH. Mechanisms of digestion and absorption of dietary vitamin A. Annu. Rev. Nutr. 2005;25:87–103. doi: 10.1146/annurev.nutr.25.050304.092614. [DOI] [PubMed] [Google Scholar]
- 5.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J. Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 6.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim. Biophys. Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 7.Napoli JL. Retinoic acid: its biosynthesis and metabolism. Prog. Nucleic Acid Res. Mol. Biol. 1999;63:139–188. doi: 10.1016/s0079-6603(08)60722-9. [DOI] [PubMed] [Google Scholar]
- 8.Ruberte E, Friederich V, Chambon P, Moriss-Kay G. Retinoic acid receptors and cellular retinoid binding proteins: their differential pattern of transcription during early morphogenesis in mouse embryos. Development. 1991;111:45–60. doi: 10.1242/dev.111.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Giguere V. Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr. Rev. 1994;15:61–79. doi: 10.1210/edrv-15-1-61. [DOI] [PubMed] [Google Scholar]
- 10.Krezel W, Kastner P, Chambon P. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 1999;89:1291–1300. doi: 10.1016/s0306-4522(98)00342-x. [DOI] [PubMed] [Google Scholar]
- 11.Chung SSW, Wolgemuth DJ. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet. Genome Res. 2004;105:189–202. doi: 10.1159/000078189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 13.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol. Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 14.Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for peroxisome proliferators-activated receptor beta/delta. J. Biol. Chem. 2003;278:41589–41592. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 15.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate action of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghyselinck NB, Bavik C, Sapin V, Mark M, Bonnier D, Hindelang C, Dierich A, Nilsson CB, Hakansson H, Sauvant P, Azais-Braesco V, Frasson M, Picaud S, Chambon P. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang XEL, Lu J, Tso P, Blaner WS, Levin MS, Li E. Increased neonatal mortality in mice lacking cellular retinol-binding protein II. J. Biol. Chem. 2002;277:36617–36623. doi: 10.1074/jbc.M205519200. [DOI] [PubMed] [Google Scholar]
- 19.Vogel S, Mendelsohn CL, Mertz JR, Piantedosi R, Waldburger C, Gottesman ME, Blaner WS. Characterization of a new member of the fatty acid-binding protein family that binds all-trans-retinol. J. Biol. Chem. 2001;276:1353–1360. doi: 10.1074/jbc.M005118200. [DOI] [PubMed] [Google Scholar]
- 20.Gorry P, Lufkin T, Dierich A, Rochette-Egly C, Decimo D, Dolle P, Mark M, Durand B, Chambon P. The cellular retinoic acid binding protein I is dispensable. Proc. Natl. Acad. Sci. USA. 1994;91:9032–9036. doi: 10.1073/pnas.91.19.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampron C, Rochette-Egly C, Gorry P, Dolle P, Mark M, Lufkin T, LeMeur M, Chambon P. Mice deficient in cellular retinoic acid binding protein II (CRABPII) or in both CRABPI and CRABPII are essentially normal. Development. 1995;121:539–548. doi: 10.1242/dev.121.2.539. [DOI] [PubMed] [Google Scholar]
- 22.O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baerh W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J. Biol. Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J. Biol. Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Hu P, Krois CR, Kane MA, Napoli JL. Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse. FASEB J. 2007;21:2886–2896. doi: 10.1096/fj.06-7964com. [DOI] [PubMed] [Google Scholar]
- 25.Hu P, Zhang M, Napoli JL. Ontogeny of rdh9 (Crad3) expression: Ablation causes changes in retinoid and steroid metabolizing enzymes, but RXR and androgen signaling seem normal. Biochim. Biophys. Acta. 2007;1770:694–705. doi: 10.1016/j.bbagen.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 27.Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, FitzPatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinez L, Keating S, Mortier G, Hennekam RCM, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nurnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am. J. Hum. Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misner DL, Jacobs S, Shimizu Y, de Urquiza AM, Solomin L, Perlmann T, DeLuca LM, Stevens CF, Evans RM. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc. Natl. Acad. Sci. USA. 2001;98:11714–11719. doi: 10.1073/pnas.191369798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deltour L, Foglio MH, Duester G. Metabolic deficiencies in alcohol dehydrogenase Adh1, Adh3, and Adh4 null mutant mice. J. Biol. Chem. 1999;274:16796–16801. doi: 10.1074/jbc.274.24.16796. [DOI] [PubMed] [Google Scholar]
- 30.Molotkov A, Duester G. Retinol/ethanol drug interaction during acute alcohol intoxication in mice involves inhibition of retinol metabolism to retinoic acid by alcohol dehydrogenases. J. Biol. Chem. 2002;277:22553–22557. doi: 10.1074/jbc.M201603200. [DOI] [PubMed] [Google Scholar]
- 31.Lei Z, Chen W, Zhang M, Napoli JL. Reduction of all-trans-retinal in the mouse liver peroxisome fraction by the short-chain dehydrogenase/reductase RRD: Induction by the PPAR a ligand clofibrate. Biochemistry. 2003;42:4190–4196. doi: 10.1021/bi026948i. [DOI] [PubMed] [Google Scholar]
- 32.Hoegberg P, Schmidt CK, Fletcher N, Nilsson CB, Trossvik C, Schuur AG, Brouwer A, Nau H, Ghyselinck NB, Chambon P, Hakansson H. Retinoid status and responsiveness to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking retinoid binding protein or retinoid receptor forms. Chem. Bio. Interact. 2005;156:25–39. doi: 10.1016/j.cbi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Staels B. Regulation of lipid and lipoprotein metabolism by retinoids. J. Am. Acad. Dermatol. 2001;45:S158–S167. doi: 10.1067/mjd.2001.113718. [DOI] [PubMed] [Google Scholar]
- 34.Yang Q, Graham TE, Mody N, Preitner F, Peroni QD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 35.Wolf G. Serum retinol-binding protein: a link between obesity, insulin resistance, and type 2 diabetes. Nutr. Rev. 2007;65:251–256. doi: 10.1111/j.1753-4887.2007.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 36.Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Volgel S, Duester G, Plutzky J. Retinaldehyde represses adipogenesis and diet-induced obesity. Nature Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farias EF, Ong DE, Ghyselinck NB, Nakajo S, Kuppumbatti YS, Mira y Lopez R. Cellular retinol-binding protein I, a regulator of breast epithelial retinoic acid receptor activity, cell differentiation, and tumorigenicity. J. Natl. Cancer Inst. 2005;97:21–29. doi: 10.1093/jnci/dji004. [DOI] [PubMed] [Google Scholar]
- 38.Lotan R. A crucial role for cellular retinol-binding protein I in retinoid signaling. J. Natl. Cancer Inst. 2005;97:3–5. doi: 10.1093/jnci/dji031. [DOI] [PubMed] [Google Scholar]
- 39.Fields AL, Soprano DR, Soprano KJ. Retinoids in biological control and cancer. J. Cell. Biochem. 2007;102:886–898. doi: 10.1002/jcb.21530. [DOI] [PubMed] [Google Scholar]
- 40.Goodman AB. Retinoid receptors, transporters, and metabolizers as therapeutic targets in late onset Alzheimer Disease. J. Cell. Phys. 2006;209:598–603. doi: 10.1002/jcp.20784. [DOI] [PubMed] [Google Scholar]
- 41.Kane MA, Chen N, Sparks S, Napoli JL. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J. 2005;388:363–369. doi: 10.1042/BJ20041867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal. Chem. 2008;80:1702–1708. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barua AB, Furr HC. Properties of retinoids. Mol. Biotechnol. 1998;10:167–182. doi: 10.1007/BF02760863. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt CK, Brouwer A, Nau H. Chromatographic analysis of endogenous retinoids in tissues and serum. Anal. Biochem. 2003;315:36–48. doi: 10.1016/s0003-2697(02)00662-0. [DOI] [PubMed] [Google Scholar]
- 45.Tsukida K, Ito M, Tanaka T, Yagi I. High-performance liquid chromatographic and spectroscopic characterization of stereoisomeric retinaloximes. Improvements in resolution and implications of the method. J. Chromatogr. 1985;331:265–272. [Google Scholar]
- 46.Van Kuijk FJGM, Handleman GJ, Dratz EA. Rapid analysis of the major classes of retinoids by step gradient reversed-phase high performance liquid chromatography using retinal (o-ethyl) oxime derivatives. J. Chromatogr. 1985;348:241–251. doi: 10.1016/s0021-9673(01)92458-6. [DOI] [PubMed] [Google Scholar]
- 47.Landers GM, Olson JA. Rapid, simultaneous determination of isomers of retinal, retinal oxime, and retinol by high-performance liquid chromatography. J. Chromatogr. 1988;438:383–392. doi: 10.1016/s0021-9673(00)90269-3. [DOI] [PubMed] [Google Scholar]
- 48.Williams JB, Pramanik BC, Napoli JL. Vitamin A metabolism: analysis of steady-state neutral metabolites in rat tissues. J. Lipid Res. 1984;25:638–645. [PubMed] [Google Scholar]
- 49.Reeves PG. J. Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 50.National Research Council. Nutrient Requirements of Laboratory Animals. 4th Ed. Washington, D.C., USA: National Academy Press; 1995. [Google Scholar]
- 51.van Breeman RB, Nikolic D, Xu X, Xiong Y, van Lieshout M, West CE, Schilling AB. Development of a method for quantitation of retinol and retinyl palmitate in human serum using high-performance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. J. Chrom. 1998;A 794:245–251. doi: 10.1016/s0021-9673(97)01138-2. [DOI] [PubMed] [Google Scholar]
- 52.Gundersen TE, Bastani NE, Blomhoff R. Quantitative high-throughput determination of endogenous retinoids in human plasma using triple-stage liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:1176–1186. doi: 10.1002/rcm.2946. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Chang WY, Prins GS, van Breeman RB. Simultaneous determination of all-trans, 9-cis, 13-cis retinoic acid and retinol in rat prostate using liquid chromatography-mass spectrometry. J. Mass Spectrom. 2001;36:882–888. doi: 10.1002/jms.189. [DOI] [PubMed] [Google Scholar]
- 54.Stancher B, Zonta F. High-performance liquid chromatography of the unsaponifiable from samples of marine and freshwater fish: fractionation and identification of retinol (vitamin A1) and dehydroretinol (vitamin A2) isomers. J. Chrom. 1984;287:353–364. doi: 10.1016/s0021-9673(01)87711-6. [DOI] [PubMed] [Google Scholar]
- 55.Hartmann S, Froescheis O, Ringenbach F, Wyss R, Bucheli F, Bischof S, Bausch J, Wiegand U-W. Determination of retinol and retinyl esters in human plasma by high-performance liquid chromatography with automated column switching and ultraviolet detection. J. Chrom. B. 2001;751:265–275. doi: 10.1016/s0378-4347(00)00481-3. [DOI] [PubMed] [Google Scholar]


