Abstract
The amygdala is hypothesized to play a critical role in mood regulation, yet its involvement in bipolar disorder remains unclear. The aim of the present study was to compare measurements of amygdala volumes in a relatively large sample of bipolar disorder patients and healthy controls ranging in age from 18 to 49 yrs. Fifty-four adult patients meeting DSM-IV criteria for bipolar disorder and 41 healthy controls matched for age, sex, and education underwent structural 1.5 Tesla MRI scanning. Volumetric measurements of the amygdala were obtained using a manual region-of-interest tracing method with software that allowed simultaneous visualization of the amygdala in three orthogonal planes. The anterior head of the hippocampus was removed in the sagittal plane prior to amygdala volumetry measurement. Multiple regression analysis was computed on amygdala volume measurements as a function of diagnosis, age, sex, and cerebral volume. Bipolar patients showed an age-related reduction of amygdala volume but controls did not. Among bipolar subjects, amygdala volume was unrelated to medication history. There were no significant hemispheric or sex interactions with the main effects. Results support a role for amygdala dysfunction in bipolar disorder which appears most robustly in older relative to younger adult patients. Differential aging effects in bipolar disorder may compromise amygdala integrity and contribute to mood dysregulation.
Keywords: Affect, emotion, depression, neuroimaging, medial temporal lobe
1. Introduction
The amygdala is featured prominently in neurobiological models of mood disorders (Mayberg, 1997; Davidson et al., 2002; Drevets, 2003). Depressed patients tend to show exaggerated amygdala activation to fearful faces, which resolves with treatment (Sheline et al., 2001; Fu et al., 2004). Bipolar patients follow this trend, particularly in unmedicated patients (Yurgelun-Todd et al., 2000; Lawrence et al., 2004; Blumberg et al., 2005) and in mania specifically (Altshuler et al., 2005). The amygdala indexes current mood state in healthy adults as well, with increased activation during sad mood, especially when the mood is reinforced by mood-congruent sensory stimuli (Posse et al., 2003; Wang et al., 2006).
While the importance of the amygdala in mood disorders is undisputed, volumetric studies of the amygdala in bipolar disorder have yielded contradictory findings (summarized in Table 1). In an early study of 48 patients, Swayze et al. (1992) found no significant difference in amygdala volume between bipolar patients and controls. However, this study had poor spatial resolution, having been conducted at 0.5 Tesla using 1-cm thick slices. Subsequent studies have reported either greater (Altshuler et al., 1998, 2000; Strakowski et al., 1999; Brambilla et al., 2003) or smaller (Pearlson et al., 1997; Blumberg et al., 2003; DelBello et al., 2004; Chen et al., 2004; Chang et al., 2005) amygdala volumes with bipolar disorder. These latter experiments had improved structural imaging techniques over Swayze et al. (1992), but also had smaller sample sizes and varying anatomic boundary definitions. The majority of studies that reported amygdala enlargement used a method described by Bartzokis et al. (1998), whereas the other studies did not. Across studies, the age of the patients has also varied widely, ranging from adolescents to middle-age adults. The combination of methodology and age of sample likely contributed to the wide range in mean amygdala volumes reported (1.26 - 4.90 cm3).
Table 1.
Studies of amygdala volumetry in bipolar disorder.
| Study | Sample size
(% Male) |
Bilateral amyg volume (cm3) | Age | Results | Imaging techniques |
|---|---|---|---|---|---|
|
| |||||
| Swayze et al., 1992 | 48 BP (60% M) | 2.19.±0.66 BP | 34.02 BP | No significant difference | 0.5 T |
| 47 C (60% M) | 2.05±0.66 C | 1-cm coronal | |||
|
| |||||
| Pearlson et al., 1997 | 27 BP (59% M) | 1.26±0.21 BP | 34.9±8.6 BP | L amyg smaller | 1.5 T |
| 60 C (72% M) | 1.37±0.19 C | 31.6±8.0 C | 3-mm coronal | ||
|
| |||||
| Altshuler et al., 1998 | 12 BP (100% M) | 1.94±0.36 BP | 50.8±13.3 BP | Overall amyg larger | 1.5 T |
| 18 C (100% M) | 1.69±0.36 C | 53.4±11.1 C | 1.4-mm coronal | ||
|
| |||||
| Strakowski et al., 1999 | 24 BP (71% M) | 3.55±0.55 BP | 27±6 BP | R and L amyg larger | 1.5 T |
| 22 C (59% M) | 3.15±0.4 C | 28±6 C | 1-mm coronal | ||
|
| |||||
| Altshuler et al., 2000 | 24 BP (100% M) | 1.91±0.35 BP | 50.2±12.7 BP | Overall amyg larger | 1.5 T |
| 18 C (100% M) | 1.69±0.36 C | 53.4±11.1 C | 3D 1.4 mm coronal | ||
|
| |||||
| Blumberg et al., 2003 | 36 BP (44% M) | Overall amyg smaller | |||
| (14 adolescent) | 1.80±0.05 BP | 31.0±14.1 BP | 1.5 T | ||
| 56 C (52% M) | 2.14±0.04 C | 28.3±13.7 C | 3D 1.2 mm sagittal | ||
| (23 adolescent) | |||||
|
| |||||
| Brambilla et al., 2003 | 24 BP (63% M) | 2.57±0.69 BP | 35±10 BP | L amyg larger | 1.5T |
| 36 C (61% M) | 2.17±0.58 C | 37±10 C | 3D 1.5 mm coronal | ||
|
| |||||
| DelBello et al., 2004 | 23 BP (61% M) | Overall amyg smaller | |||
| 2.5±0.4 BP | 16.3±2.4 BP | 1.5 T | |||
| 20 C (55% M) | |||||
| 2.75±0.3 C | 17.2±1.9 C | 3D 1 mm axial | |||
| Adolescent | |||||
|
| |||||
| Chen et al., 2004 | 16 BP (50% M) | Age dependent reduction of R amyg | |||
| 1.86±0.41 BP | 16±3 BP | 1.5 T | |||
| 21 C (57% M) | |||||
| 1.99±0.50 C | 17±4 C | 3D 1.5 mm coronal | |||
| Adolescent | |||||
|
| |||||
| Frazier et al., 2005 | 43 BP (54% M) | No significant difference | |||
| 1.67±0.26 BP | 11.3±2.7 BP | 1.5 T | |||
| 20 C (60% M) | |||||
| 1.80±0.30 C | 11.0±2.6 C | 3D 1.5 mm coronal | |||
| Adolescent | |||||
|
| |||||
| Chang et al., 2005 | 20 BP (80% M) | Overall amyg smaller | |||
| 3.93±0.40 BP | 14.6±2.8 BP | 3 T | |||
| 20 C (80% M) | |||||
| 4.39±0.45 C | 14.1±2.8 C | 3D 1.5 mm coronal | |||
| Adolescent | |||||
Note: Amyg = amygdala, BP = bipolar disorder, C = control, L = left hemisphere, R = right hemisphere, M = male.
Given the importance of the amygdala to theories of mood regulation, the present study sought to re-evaluate its role in bipolar disorder using a refined method for amygdala volumetry in a larger sample of young and middle-aged adult patients (N = 54). In addition, the study aimed to examine the impact of age upon amygdala volume, given that results of previous studies may have differed because of age differences in their samples (DelBello et al., 2003, Altshuler et al., 2000).
2. Methods
2.1 Participants
This study used a cross-sectional design. Patients from the psychiatric wards at Duke University Medical Center (Durham, N.C.) and John Umstead Hospital (Butner, N.C.) with bipolar disorder, as well as community dwelling subjects with a history of bipolar disorder, were recruited to participate in this study. Control subjects were recruited from the community by advertisement and supplemented with additional controls identified in the databases of the Duke MHCRC for the Study of Depression in Late-Life. The study was approved by the Institutional Review Boards of both institutions.
Eligibility for subjects was limited to those under age 50 as part of the NIMH study in Late Life Bipolar Disorder. Exclusion criteria included other major psychiatric illnesses, active substance abuse, neurological illness, evidence of dementia (as suggested by 7 or more errors on the Mini-Mental Status Examination), or inability to perform an MRI. At baseline, all subjects completed a SCID interview and underwent a standardized MRI study (see MRI method section below). Subjects with bipolar disorder met diagnostic criteria as defined by the DSM IV. Controls and patients for these analyses were matched by age and sex. Demographics of the sample are provided in Table 2 and described in Section 3.1 below.
Table 2.
Demographics for bipolar patients and healthy controls.
| Variables | Bipolar patients (N=54) | Healthy controls (N=41) |
|---|---|---|
|
| ||
| Age | 30.54 ± 8.98 | 29.91 ± 7.91 |
|
| ||
| Sex | 39 females (72.22%) | 28 females (68.29%) |
|
| ||
| Years of education | 14.72 ± 2.84 | 15.50 ± 2.33 |
|
| ||
| Handedness | 44 right-handed (81.48%) | 30 right-handed (73.17%) |
|
| ||
| Age of onset of current condition (self-report) | 24.05 ± 9.07 | n/a |
|
| ||
| Age of first symptoms of any mood disorder (self-report) | 14.63 ± 7.02 | n/a |
|
| ||
| History of Lithium Use | 38 (70.37%), 7 unclassified (12.96%) | n/a |
|
| ||
| Lithium at Time of Scan | 16 (29.63%), 4 unclassified (7.41%) | n/a |
|
| ||
| History of VPA Use | 35 (64.81%), 12 unclassified (22.22%) | n/a |
|
| ||
| VPA at Time of Scan | 17 (31.48%), 4 unclassified (7.41%) | n/a |
|
| ||
| History of Neuroleptic Use | 31 (57.41%), 6 unclassified (11.11%) | n/a |
|
| ||
| Neuroleptics at Time of Scan | 17 (31.48%), 4 unclassified (7.41%) | n/a |
Note: Values indicate mean (±S.D.) in years except for sex and handedness.
2.2 MRI scanning protocol
All subjects were screened for the presence of cardiac pacemakers, neurostimulators, metallic implants, metal in the orbit, aneurysm clips or any other condition where MRI was contraindicated. Subjects were imaged under an Institutional Review Board approved protocol with a 1.5 Tesla whole-body MRI system (Signa, GE Medical Systems, Milwaukee, WI) using the standard head (volumetric) radiofrequency coil. Padding was used to immobilize the head without causing discomfort. The scanner alignment light was used to adjust the head tilt and rotation so that the axial plane lights passed across the cantho-meatal line and the sagittal lights were aligned with the center of the nose. A rapid sagittal localizer scan was acquired to confirm the alignment.
A dual-echo fast spin-echo (FSE) acquisition was obtained in the axial plane for morphometry of most cerebral structures. An axial IR-prepped 3D series was used for measuring the amygdala. For the FSE series, the pulse sequence parameters were TR = 4000 ms, TE = 30 ms, 32 KHz (±16KHz) full imaging bandwidth, echo train length = 16, 256 × 256 matrix, 3-mm section thickness, 1 Nex and a 20-cm FOV. The images were acquired in two separate acquisitions with a 3-mm gap between sections for each acquisition. The second acquisition was offset by 3 mm from the first so that the resulting data set consisted of contiguous sections. For the 3D series, the pulse sequence parameters were TE = minimum full echo, TI = 300 ms, 16 KHz bandwidth, 256 × 256 matrix, 1.5-mm section thickness, 1 Nex and a 24-cm FOV.
2.3 Whole brain and cerebrum
The NeuroImaging Research Laboratory (NIRL) segmentation protocol has been described previously (Payne et al., 2002). A supervised, semi-automated method used the multiple MR contrasts available to identify different tissue classifications through a seeding process wherein the trained analyst manually selected pixels in each tissue type that was to be identified (gray matter, white matter, cerebrospinal fluid (CSF), background). Once the brain was segmented into tissue types and the non-brain tissue stripped away through a masking procedure, the cerebral hemispheres were measured by tracing.
2.4 Regional tracing procedures
Scans were processed with a semi-automated method for regional measurements using the NIRL software program GRID 6.5. The GRID program allows for viewing and tracing in any of three orthogonal planes, regardless of acquisition plane. Volumes are calculated by multiplying traced area on each slice by slice thickness, and then summing volumes for all (MacFall et al., 1994). Using the procedures defined below, a trained image analyst traced the structures of interest (amygdala or temporal lobe) on every slice where each was found. The analysts were blinded to clinical diagnosis and demographic information.
2.5 Amygdala volumetry
In their seminal review, Brierley et al. (2002) rated the method for amygdala volumetry by Convit et al. (1999) with the highest quality score. Convit and colleagues accomplish consistency in defining amygdala borders in several ways. First, the hippocampal-amygdala border is outlined in the sagittal plane, which is then viewed in the coronal plane where the amygdala is traced. Second, the hippocampal-amygdala transition area is identified in the axial plane, and its border is then projected to the coronal plane. Third, the often difficult-to-define anterior amygdaloid area is simplified by using the optic chiasm as a landmark to identify the most anterior slice to be processed. This method created the cornerstone for our volumetry procedure, which included the following steps: realignment in all three orthogonal planes, removal of the anterior hippocampal head in the sagittal view, and tracing of amygdala in the coronal view. Identification of brain structures was assisted by the Duvernoy (1991) brain atlas.
2.5.1 Realignment
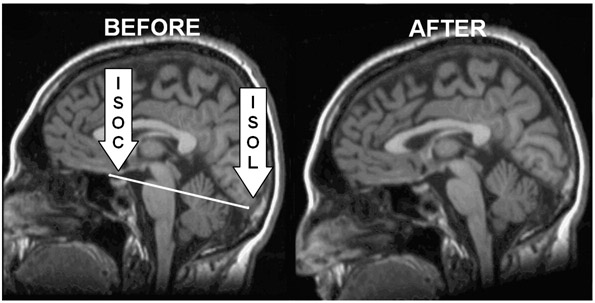
For both the axial and coronal planes, a standard realignment procedure was implemented where the rater aligned to a plane that bisected the brain into right and left cerebral hemispheres. This adjusted the scan in cases of incorrect subject placement in the scanner. Realignment in the sagittal plane was customized for the amygdala method described below and utilized one of the three most medial slices. A line was drawn from the most inferior surface of the orbitofrontal cortex (ISOC) to the most inferior surface of the occipital lobe (ISOL; see Figure 1). GRID oriented the scan so that this plane was horizontal in the sagittal view.
Figure 1.
Realignment of the sagittal plane using the inferior surface of the orbitofrontal cortex (ISOC) and the inferior surface of the occipital lobe (ISOL).
2.5.2 Defining hippocampus in sagittal view
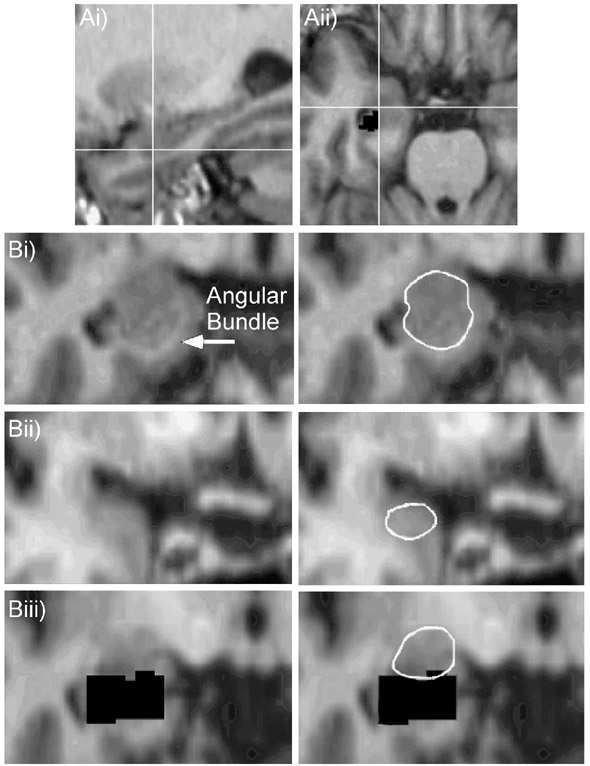
Following Convit et al.'s (1999) delineation of the hippocampal boundaries, our method included removal of the anterior hippocampus by tracing in the sagittal view. The sagittal images contained either a clear white matter boundary (the alveus) or a CSF boundary between the head of the hippocampus and the posterior section of the amygdala. If the white matter boundary was indistinct in one slice of a hemisphere, we utilized GRID's capability to test potential boundaries in the axial plane. If a potential hippocampal boundary fell posterior to or at the level of the temporal horn in the axial plane, then the proposed boundary was used for that sagittal slice (Figure 2A). This method concentrated on the anterior hippocampal section and resulted in a complete removal of the hippocampus in that portion (Figure 2Biii). This removal concentrated only on the anterior hippocampal boundary, was used only to prevent the addition of the hippocampal head to the amygdala volume measure, and did not create a hippocampal volume estimate.
Figure 2.
A. Checking a proposed sagittal hippocampal boundary (Ai) in the axial plane (Aii). The hippocampal head is delineated in the sagittal plane (Figure 2Ai) prior to measuring the amygdala. When the alveus is indistinct in the sagittal plane and the boundary between the amygdala and the hippocampal head is difficult to discern, our method instructs the rater to consult the axial plane (Figure 2Aii). Here the black pixels posterior and lateral to the white cross hairs represent previously removed sections of the anterior hippocampal head. This landmark, combined with the location of the temporal horn of the lateral ventricle, creates a proposed anterolateral border, which can then be identified in the original sagittal image (Figure 2Ai) to best locate the head of the hippocampus. B. Definition of amygdala borders. (Bi) Start point in middle slice where angular bundle is distinct. (Bii) Anterior amygdala border. This slice is the most anterior image where the optic chiasm appears as one continuous structure. (Biii) Posterior amygdala border at cutout of hippocampus (black pixels).
2.5.3 Tracing the amygdala
The analyst began processing in the coronal plane at a middle amygdala slice where the angular bundle clearly separated the medial amygdala border from the entorhinal cortex (Figure 2Bi). Processing continued posteriorly until the end of the amygdala was reached, as signaled by complete replacement of the amygdala by the cutout of the hippocampus (Figure 2Bii). Since we did not follow Convit et al.'s (1999) delineation of the hippocampal amygdalar transition area (HATA) in the axial plane due to our inability to reliably identify this region, this area remained in some of our scans. To remedy the potential problem of including this area in our volume measurements, once a brain slice was reached where the amygdala area did not change size significantly in comparison to the amygdala traced in anterior slices, this slice became the posterior boundary of the structure. As the scans were properly realigned to maximize the shape of the amygdala, the boundary between the amygdala and the HATA was easier to recognize in this final posterior slice. The last coronal slice containing amygdala was obvious because the next slice posterior jumped to only a sliver of gray matter superior to the hippocampus, which was a clear indication that the volume remaining was the HATA and was confirmed by consulting the axial view. Once the most posterior slice was reached, the analyst returned to the angular bundle segment and processed scans in the anterior direction. Tracing the amygdala was complete anteriorly when the optic chiasm ceased to be a continuous structure. The amygdala was typically traced on 8-12 slices using 1.88 mm interpolated slice thickness.
In addition to guidelines on which slices to include, our method included clearly defined intra-slice boundaries. For medial and infero-medial boundaries, in the posterior amygdala the angular bundle and projections of the angular bundle created the border, and in the anterior amygdala the CSF became the new medial border as the angular bundle became indistinct (Figure 2Bii). For these anterior sections, it was important to retain the ovoid shape of the amygdala using the CSF and the lateral and superior-lateral borders (white matter tracts). These white matter tracts served as the superio-lateral border in all slices. However, if these landmarks did not appear distinctly, a border following the ovoid shape created by other distinct landmarks was used. Inferio-lateral boundaries were temporal lobe white matter tracts and extensions of the temporal horn. Most superio-medial boundaries were formed by the CSF, but sometimes this border was created by the white matter tract of the angular bundle. The posterior-inferior border was created by the previously removed hippocampus.
2.6 Reliability
All image analysis technicians received extensive training by experienced analysts. Reliability was established by repeated measurements on multiple (5-10) MR scans before raters were approved to process study data. Intraclass correlation coefficients attained were as follows: total cerebrum = 0.997, left amygdala = 0.91, right amygdala = 0.87.
2.7 Statistical Analysis
Amygdala volume estimates were subject to multiple regression analysis using the general linear model. Estimates from the left and right hemispheres were entered into a single model as the dependent measures. Independent measures were entered simultaneously into the model and included total cerebral volume, diagnosis (bipolar or control), age, sex, as well as the interaction terms diagnosis X age and diagnosis X sex. Subsequent analyses explored the effects of medication history within the bipolar group by regressing amygdala volumes with the independent measures age and drug treatment history (Li, VPA and/or neuroleptics). In these analyses, drug treatments were coded as categorical variables.
3. Results
3.1 Subject characteristics
Scans were obtained from 54 patients (39 female, mean (+SD) age = 30.54 ± 8.98 yrs) and 41 healthy controls (28 female, mean (+SD) age = 29.91 ± 7.91 yrs) (see Table 2). The mean (+ SD) self-reported age of illness onset was 24.05 (± 9.07) yrs. Thirty-eight patients had a history of Lithium (Li) treatment, 35 patients had a history of valproic acid (VPA) treatment, and 31 patients had a history of using neuroleptics. Medication data at the time of scan is presented in Table 2, and six patients (11.11%) were unmedicated at the time of scan. More detailed information regarding duration and dosage of medication history was unavailable for most patients. The frequency of illness cycles was reported by 13 patients to be ‘few’ (i.e. less than 15) and 15 patients to be ‘frequent’ (i.e. more than 15) (26 patients did not provide estimates). Current mood classifications at the time of scanning indicated that 12 patients were stable, 10 patients were depressed, 14 patients were manic, and 1 patient had a mixed episode (17 patients were unclassified).
3.2 Volumetric findings
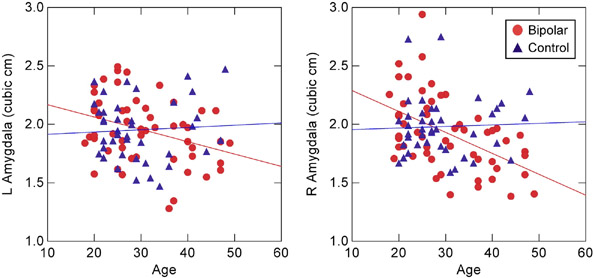
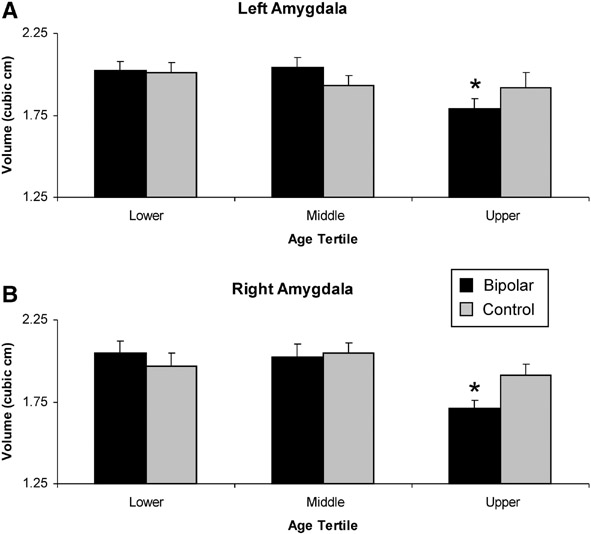
For bipolar patients, the mean (± SD) left amygdala volume was 1.95 (± 0.28) cm3, and right amygdala volume was 1.92 (± 0.32) cm3. For controls, the mean left amygdala volume was 1.95 (± 0.26) cm3, and the mean right amygdala volume was 1.98 (± 0.25) cm3. Results from the multiple regression analysis revealed significant main effects of cerebral volume, F(1, 88) = 11.1, P < 0.001, diagnosis, F(1, 88) = 7.11, P < 0.009, and a marginal trend of age, F(1, 88) = 3.44, P = 0.067. However, the diagnosis and age variables interacted with each other, F(1, 88) = 7.58, P < 0.007. Post-hoc analyses by group showed significant reduction in both left, r(52) = .34, P < 0.011, and right, r(52) = .50, P < 0.00012, amygdala volume with age in bipolar disorder patients, but not in controls (left hemisphere: r(39) = .06, P = 0.71; right hemisphere: r(39) = .04, P = 0.80) (Figure 3). Subsequent analyses on the age interaction showed that only the oldest bipolar patients in the sample had significant volume decreases in both hemispheres relative to controls, as confirmed by analysis of the data stratified into age tertiles, F(1, 30) = 4.18, P < 0.05 (Figure 4). There were no main effects or interactions with the sex or hemisphere variables. In addition, there were no group differences in total cerebral volume. Additional regressions using normalized amygdala volumes produced the same results as the ones reported here (age × diagnosis interaction: F(1, 89) = 6.14, P < 0.015), as did regressions on left and right hemisphere volumes in separate models.
Figure 3.
Amygdala volumes as a function of age in bipolar patients and controls. Regression lines indicate linear decrease in volume with age in bipolar patients but not in controls. L = left hemisphere, R = right hemisphere.
Figure 4.
Amygdala volumes as a function of age tertiles. The bipolar and control samples were divided into the low (age 18-25), middle (age 26-34), and upper thirds (age 36-49) of their respective age distributions. Amygdala volume of bipolar patients in the oldest age cohort is significantly decreased relative to healthy controls in the (a) left and (b) right hemispheres, * P < .05.
3.3 Treatment effects
As a secondary analysis, we considered whether the age effects in bipolar disorder were due to history of Li, VPA or neuroleptic treatment or the presence of these medications at the time of scanning. We conducted two follow-up multiple regressions on the bipolar data using left and right amygdala volumes as dependent measures. The first analysis included the independent variables age and self-reported history of Li, VPA and neuroleptic treatment. Patients who were unsure of their history of drug treatment were not included in the analysis (final N = 35). Results showed that the main effect of age remained when history of medication treatment was accounted for in the regression model, F(1, 30) = 6.97, P < 0.013. The second analysis included the independent variables age and presence of Li, VPA or neuroleptics at the time of scanning. This information was obtained from 48 of the 54 patients, who were included in the analysis. Again, the main effect of age remained even when presence of medications at the time of scanning was accounted for in the regression model, F(1, 43) = 19.61, P < 0.001. Similar results were obtained using normalized volumes (age effect controlling for medication history, F(1, 30) = 4.38, P < 0.045; age effect controlling for medication at the time of scanning, F(1, 43) = 15.53, P < 0.001).
4. Discussion
The principal finding from the present study was an age-dependent reduction of amygdala volume in bipolar disorder. Middle-aged bipolar patients had smaller right and left amygdala volumes relative to younger adult patients and age-matched controls. The aging effects remained even when treatment history and current treatment (Li, VPA, or neuroleptics) were accounted for in the model. There were no effects of sex or hemisphere, and bipolar patients and controls did not differ in cerebral volume estimates.
Previous studies reporting amygdala volumes in bipolar depression patients have yielded conflicting reports (Table 1). Two limitations of existing studies include relatively small sample sizes and mixed volumetry methods. Our finding of age-dependent amygdala atrophy for bipolar depression was calculated from volumes obtained from a larger sample relative to prior studies. This sample size allowed us to model amygdala volumes through the young- to middle-adulthood years. Additionally, these volumes were obtained using a reliable amygdala volumetry procedure which consulted three orthogonal planes to further elucidate potentially indistinguishable boundaries, including the anterior hippocampal head, and therefore avoided including adjacent structures in the volume measurement.
It is unclear why aging in the early-to-middle adult years would impact amygdala volumes in bipolar disorder. One possibility is that overactivation of the amygdala to emotional stimuli, as revealed through functional neuroimaging, is cumulatively excitotoxic. Stress hormones may play a role in this excitotoxicity through a reduction in inhibitory gamma-aminobutyric acid (GABA) transmission, which alters synaptic plasticity in local amygdaloid circuits (Rainnie et al., 2004; Rodriguez Manzanares et al., 2005). Rodent studies have shown that chronic, unpredictable stress has a selective atrophic effect on some neuronal subtypes in the basolateral amygdala (Vyas et al., 2002). Additionally, previous research has concluded that smaller hippocampal volumes as measured in structural MRI scans are associated with depression and might be mediated by high levels of glucocortocoids (Sheline et al., 1996, McEwen, 1997). Further evidence that cortisol levels mediate atrophy in the hippocampus comes from studies of elderly subjects with elevated cortisol levels (Lupien et al., 1998) and patients with Cushing's disease (Starkman et al., 1999). Both unipolar and bipolar disorder are associated with disruption of the hypothalamic–pituitary–adrenal axis and increased basal cortisol levels, regardless of the current mood state of the patient (Holsboer et al., 1987, Cervantes et al., 2001). It is plausible that the heightened cortisol activity in bipolar depression exerted over time is another mechanism of cumulative excitoxicity in the amygdala. Unipolar depression studies have generally found decreased amygdala volumes, which may serve as more evidence for excitotoxic amygdalar atrophy (reviewed in Campbell and MacQueen, 2006)
Researchers have also suggested that amygdalar glial cells are particularly vunerable to this atrophy in depression. In a study of post-mortem brains, atrophy of the amygdala was due to glial cell loss in unipolar depression while no change in glial cell loss was found in the medicated bipolar samples (Bowley et al., 2002). Li and VPA have been found to cause an increase in the cell protective agent BCL-2 (Manji et al., 2000), which could explain why drug treatment appears to have prevented glial cell loss. In addition to Li and VPA, neuroleptics may also alter amygdala morphometry, possibly “normalizing” an enlarged amygdala in schizophrenic patients (Tebartz van Elst et al., 2004).
A majority of bipolar disorder patients in the present study had undertaken drug treatment. After conducting a separate analysis of these patients, we found that Li or VPA treatment history had no significant effect on amygdala volumes, regardless of whether age was included as an independent variable in the analysis. One possible explanation for this result is that the patients may have been taking Li or VPA for a limited period of time, and therefore the neuroprotective effects of the drugs may not have manifested themselves. There is good evidence for this scenario as our patients, for the most part, had been recently diagnosed.
Our finding of an age-related reduction in volume is inconsistent with some previous studies of middle-aged bipolar patients which found larger amygdala in bipolar subjects (Table 1). This inconsistency could be accounted for by population differences and/or methodological differences. Our population was predominantly female (72% of bipolar patients, 68% of healthy controls), while almost all studies listed in Table 1 had majority male populations, overwhelmingly so for those studies reporting enlarged amygdala volumes in bipolar populations. Because we report volumes from the largest adult bipolar sample to date, one can not rule out amygdala enlargement as a localized effect in a small population that does not generalize to a larger population. Methodologically, we were able to use all three planes and remove the anterior hippocampus in advance to improve upon amygdala volumetry estimates. It is worth noting that all studies reporting enlarged amygdala volumes in bipolar patients used the same volumetry method (Bartzokis et al., 1998), which does not include use of orthogonal views in order to verify boundaries. Co-planar verification is especially helpful to ensure the delineation of the hippocampal head from the amygdala, particularly if there are age- or disorder-related changes that affect the appearance of boundary distinctions. If bipolar specific gial cell loss in the amygdala contributes to white matter degradation as seen in structural MRI scans, without the use of orthogonal views adjacent gray matter structures may accidentally be included in the amygdala measurement. Further improvements can be made in future studies by using co-planar verification at higher field strengths for better signal-to-noise ratios.
The present study is limited by the cross-sectional design employed, particularly given that there may have been cohort differences in treatment history. It is possible that the age-associated atrophy found in our subjects is not a direct result of bipolar disorder alone. Regardless of age, patients had similar self-reported durations of bipolar illness, so differences in illness duration cannot account for the aging effect. However, most patients reported that their first psychiatric symptom occurred before the onset of bipolar illness. Therefore, it is possible that even though older patients had similar durations of bipolar disorder as younger patients, they may have been suffering from comorbid psychiatric disorders (or were misdiagnosed) before they were diagnosed with bipolar disorder. Conclusions from the present study are also limited by not including adolescents or geriatric populations to span a broader age range. Provided that the healthy amygdala gradually declines in volume with senescence and that Li or VPA treatment provides possible long-term neuroprotective effects in bipolar disorder, it is conceivable that the group differences found in the present study would diminish or reverse in old age.
The current study also features a predominantly female population, which potentially limits the scope of the results. When explicitly modeled in the regression analyses using either raw or normalized volumes, there were no gender interactions with the effects reported here. Although a recent large-population study found gender differences only in factors which the current study has controlled for (BP II, comorbid thyroid disease, bulimia, and post –traumatic stress disorder) (Baldassano et al 2005), future studies will need to more carefully examine the issue of gender and amygdala volumetry in bipolar patients. Finally, our interpretations are limited in that we focus exclusively on the amygdala here and do not include other regions in the analyses.
In conclusion, results from the current study indicate that age may mediate the relationship between bipolar disorder and amygdala volume across the early-to-middle adulthood years. The present study improves upon prior work by investigating a relatively large population of bipolar disorder patients using a precise amygdala volumetry method that was devised to accommodate the quality of structural scans obtained at 1.5 T. Future studies should be careful to include age explicitly in the model and to employ longitudinal designs in order to confirm etiological relationships.
Acknowledgments
This study was supported in part by NIH grants P50 MH60451 and R01 DA14094, and the Duke University Howard Hughes Neuroscience Forum and Fellowship for Undergraduate Independent Research. The authors thank Dr. James MacFall, Mr. Kulpreet Singh, and Ms. Denise Messer from the Neuropsychiatric Imaging Research Laboratory at Duke University Medical Center for their support and assistance with this project. T.J.D. is now at the Laboratory of Brain and Cognition, NIMH, Bethesda, MD, USA and the Department of Clinical Neuroscience, Karolinska Institutet, Karolinska Hospital, Stockholm, Sweden.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, Bartzokis G, Mintz J, Mazziotta J, Cohen MS. Increased amygdala activation during mania: A function magnetic resonance imaging study. American Journal of Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in Schizophrenia: An MRI study demonstating neuroanatomic specificity. Archives of General Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Baldassano CF, Marangell LB, Gyulai L, Ghaemi SN, Joffe H, Kim DR, Sagduyu K, Truman CJ, Wisniewsk SR, Sachs GS, Cohen LS. Gender differences in bipolar disorder: retrospective data from the first 500 STEP-BP participants. Bipolar Disorders. 7:465–470. doi: 10.1111/j.1399-5618.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Altshuler LL, Greider T, Curran J, Keen B, Dixon WJ. Reliability of medial temporal lobe volume measurements using reformatted 3D images. Psychiatry Research: Neuroimaging Section. 1998;82:11–24. doi: 10.1016/s0925-4927(98)00007-9. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183(3):308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Charney DS, Krystal JH. Frontotemporal neural systems in bipolar disorder. Seminars in Clinical Neuropsychiatry. 2002;7:243–254. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JZ, Gore JC, Charney DS, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of General Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biological Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI investigation of temporal lobe structures in bipolar patients. Journal of Psychiatric Research. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Brierley B, Shaw P, David AS. The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Research Reviews. 2002;39:84–105. doi: 10.1016/s0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Current Opinion in Psychiatry. 2006;19:25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- Cervantes P, Gelber S, Kin FN, Nair VN, Schwartz G. Circadian secretion of cortisol in bipolar disorder. Journal of Psychiatry and Neuroscience. 2001;26(5):411–416. [PMC free article] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(6):565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, Brambilla P, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biological Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Convit A, McHugh P, Wolf OT, de Leon MJ, Bobinski M, De Santi S. MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Research: Neuroimaging. 1999;90:113–123. doi: 10.1016/s0925-4927(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Drevets WC, Farah MJ, Kagan J, McClelland JL, Nolen-Hoeksema S, Peterson BS. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disorders. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Annals of the New York Academy of Sciences. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, three-dimensional surface anatomy and MRI. Springer-Verlag; New York: 1991. [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. American Journal of Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Holsboer F, von Bardeleben U, Wiedemann K, Müller OA, Stalla GK. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression: Implications for pathophysiology of DST nonsuppression. Biological Psychiatry. 1987;22:228–234. doi: 10.1016/0006-3223(87)90237-x. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: Implications for the pathophysiology and treatment of manic-depressive illness. Biological Psychiatry. 2000;48:740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- MacFall JR, Byrum CE, Parashos I, Early B, Charles HC, Chittilla V, Boyko OB, Upchurch L, Krishnan KRR. Relative accuracy and reproducibility of regional MRI brain volumes for point-counting methods. Psychiatry Research: Neuroimaging. 1994;55(3):167–177. doi: 10.1016/0925-4927(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Possible mechanisms for atrophy of the human hippocampus. Molecular Psychiatry. 1997;2(3):255–262. doi: 10.1038/sj.mp.4000254. [DOI] [PubMed] [Google Scholar]
- Payne ME, Fetzer DL, MacFall JR, Provenzale JM, Byrum CE, Krishnan KRR. Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Research: Neuroimaging. 2002;115(12):63–77. doi: 10.1016/s0925-4927(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biological Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore GJ, Schneider F. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage. 2003;18(3):760–768. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. Journal of Neuroscience. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. Journal of Neuroscience. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's Disease. Biological Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain Magnetic Resonance Imaging of Structural Abnormalities in Bipolar Disorder. Archives of General Psychiatry. 1999;56(3):254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Swayze VW, Andreasen NC, Alliger RJ, Yuh WTC, Ehrhardt JC. Subcortical and Temporal Structures in Affective Disorder and Schizophrenia: A magnetic resonance imaging study. Biological Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Bäumer D, Ebert D, Trimble MR. Chronic antidopaminergic medication might affect amygdala structure in patients with schizophrenia. Pharmacopsychiatry. 2004;37:217–220. doi: 10.1055/s-2004-832595. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana R, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, LaBar KS, McCarthy G. Mood alters amygdala activation to sad distractors during an attentional task. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.01.021. in press. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1742–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disorders. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]