Abstract
Background
Neuroblastomas are characterized by hemizygous 1p deletions, suggesting that a tumor suppressor gene resides in this region. We previously mapped the smallest region of consistent deletion to a 2-Mb region of 1p36.31 that encodes 23 genes. Based on mutation analysis, expression pattern, and putative function, we identified CHD5 as the best tumor suppressor gene candidate.
Methods
We determined the methylation status of the CHD5 gene promoter in NLF and IMR5 (with 1p deletion) and SK-N-SH and SK-N-FI neuroblastoma cell lines using methylation-specific sequencing and measured CHD5 mRNA expression by reverse transcription polymerase chain reaction in cells treated with or without 5-aza-2-deoxycytidine, an inhibitor of DNA methylation. We transfected the cells with CHD5 and antisense (AS) CHD5 DNA to assess the effect of CHD5 overexpression and suppression, respectively, on colony formation in soft agar and growth of xenograft tumors in athymic mice. We also analyzed the association of CDH5 expression with outcomes of 99 neuroblastoma patients. Statistical tests were two-sided.
Results
CHD5 expression was very low or absent in neuroblastoma cell lines. The CHD5 promoter was highly methylated in NLF and IMR5 lines, and CHD5 expression increased after treatment with 5-aza-2-deoxycytidine. Clonogenicity and tumor growth were abrogated in NLF and IMR5 cells overexpressing CHD5 compared with antisense CHD5 (clonogenicity: mean no. of colonies per plate, NLF-CHD5, 43 colonies, 95% confidence interval [CI] = 35 to 51 colonies, vs NLF-CHD5-AS, 74 colonies, 95% CI = 62 to 86 colonies, P < .001; IMR5-CHD5, 11 colonies, 95% CI = 2 to 20 colonies, vs IMR5-CHD5-AS, 39 colonies, 95% CI = 17 to 60 colonies, P = .01; tumor growth, n = 10 mice per group: mean tumor size at 5 weeks, NLF-CHD5, 0.36 cm3, 95% CI = 0.17 to 0.44 cm3, vs NLF-CHD5-AS, 1.65 cm3, 95% CI = 0.83 to 2.46 cm3, P = .002; IMR5-CHD5, 0.28 cm3, 95% CI = 0.18 to 0.38 cm3, vs IMR5-CHD5-AS, 1.15 cm3, 95% CI = 0.43 to 1.87 cm3; P = .01). High CHD5 expression was strongly associated with favorable event-free and overall survival (P < .001), even after correction for MYCN amplification and 1p deletion (P = .027).
Conclusions
CHD5 is the strongest candidate tumor suppressor gene that is deleted from 1p36.31 in neuroblastomas, and inactivation of the second allele may occur by an epigenetic mechanism.
CONTEXT AND CAVEATS
Prior knowledge
Neuroblastoma is a childhood cancer that is characterized as having genomic deletions at chromosome 1p. A neuroblastoma tumor suppressor gene may lie in this region, and based on previous studies, CHD5 is a candidate.
Study design
CHD5 promoter methylation and expression in human neuroblastoma cell lines and effects of CHD5 overexpression on tumor growth in mouse models were assayed. Associations between CHD5 expression and clinical outcomes of 99 neuroblastoma patients were determined.
Contributions
CHD5 expression was low in the cell lines, and the CHD5 promoter was highly methylated. Overexpression of CHD5 slowed tumor growth in mouse models. CHD5 expression was strongly associated with increased event-free and overall survival of neuroblastoma patients.
Implications
CHD5 may be a neuroblastoma tumor suppressor gene, and its expression may be inhibited by promoter methylation.
Limitations
Other genes that are located in the region of the 1p deletions still need to be studied.
Neuroblastoma, a tumor of the sympathetic nervous system, is the most common childhood extracranial solid tumor, accounting for 8%–10% of childhood cancers and 15% of childhood cancer deaths (1). Neuroblastomas demonstrate clinical heterogeneity, from spontaneous regression to relentless progression. We and others have identified different patterns of genetic change that underlie these disparate clinical behaviors (2). One of the most characteristic genetic changes in neuroblastomas is deletion of the short arm of chromosome 1 (1p) (3,4). We have found the 1p deletion in approximately 35% of all neuroblastomas and in 70%–80% of high-risk tumors (5–11), suggesting the loss of a tumor suppressor gene from this region. We analyzed more than 1200 neuroblastomas and mapped the smallest region of consistent deletion (SRD) to 1p36.31, from D1S2660 distally to D1S214 proximally (approximately 2 Mb) (10,12). The SRD that has been identified by most other groups who have mapped 1p deletions in neuroblastomas overlaps this region (13–21). Furthermore, the proximal and distal boundaries of this SRD are defined by three patients each (10,12), clearly establishing that this region contains at least one neuroblastoma tumor suppressor gene that is deleted from these tumors.
In a previous study (12), we mapped 23 genes to the maximal SRD we defined on 1p36.31. We analyzed 30 neuroblastoma cell lines for mutations in these genes but found no examples of mutational inactivation (ie, deletion, frameshift, or stop codon). We analyzed the expression of these 23 genes in neuroblastoma cell lines: seven had very low expression, two (CHD5, RNF207) had virtually absent expression, five had preferential expression in the nervous system, and seven had been previously associated with cancer pathogenesis (two with neuroblastoma). Only CHD5 had all of these features (12,22).
In the current study, we hypothesized that CHD5 was the best candidate tumor suppressor gene deleted from 1p36.31 in neuroblastomas. We assessed the methylation status of the promoter and determined whether methylation status was associated with the presence or absence of 1p deletion in neuroblastoma cell lines. We transfected CHD5 into neuroblastoma cell lines and assessed the effect on both colony formation in soft agar (clonogenicity) and growth of xenograft tumors in athymic mice. We also compared the expression of 12 genes from the 1p36 SRD we defined with clinical and biologic risk factors in 101 neuroblastomas. Our goal was to determine whether CHD5 is a bona fide tumor suppressor gene and the likely target of the 1p36.31 deletions that characterize neuroblastoma.
Subjects and Methods
Patients
The 101 samples (91 from the Children's Oncology Group [COG] and 10 from the Children's Hospital of Philadelphia [CHOP]) that were used in this study were chosen to be representative of rigorously defined clinical and biologic risk groups that were consistent with those used for COG but included additional genetic variables. The CHOP Institutional Review Board approved this study, and written informed consent was obtained before sample collection. The patient population is described in more detail elsewhere (23). Briefly, for this study, low-risk patients were defined as infants (<1 year of age) with stage 1 or 2 disease by the International Neuroblastoma Staging System (INSS) (24) and favorable biologic features. Intermediate-risk patients were almost all patients with INSS stage 3 disease who were older than 1 year. High-risk patients were defined as those with INSS stage 3 or 4 disease (only two had stage 3 disease) who were older than 1 year. High-risk patients were divided into two subsets: those without MYCN amplification (high risk) and those with MYCN amplification (ultrahigh risk). RNA was obtained from these 101 neuroblastoma tumors and subjected to microarray expression profiling using Affymetrix (Santa Clara, CA) U95A chips, as described (23). Twelve of the 23 genes from the SRD were present on these chips (AJAP1, NPHP4 KCNAB2, CHD5, RPL22, ICMT, ACOT7, HES2, TNFRSF25, KLHL21, THAP3, and CAMT1).
Tumor specimens from newly diagnosed patients were submitted between September 1, 1993, and May 30, 2003, and the median follow-up time of patients who did not experience an event was 3.4 years. More specifically, 41 patients were younger than 1 year and 60 were 1 year or older; 27 were stage 1 according to INSS, one patient was stage 2, 23 were stage 3, and 50 were stage 4. Of these 101 patients, 20 had MYCN amplification, 48 had favorable histology, 66 were hyperdiploid, 26 had 1p deletion, and 40 had 11q deletion. Twenty-eight were low-risk, 21 were intermediate-risk, 32 were high-risk (without MYCN amplification), and 20 were ultrahigh-risk (with MYCN amplification). Survival data were available for 99 of the 101 patients.
Cell Lines and Cell Culture
Neuroblastoma cell lines NLF, IMR5, SK-N-SH, SK-N-FI, CHLA-51, CHLA-79, CHLA-90, CHLA-123, CHLA-150, CHP-134, CHP-901, CHP-902R, KCN, KCNR, LA-N-5, LA-N-6, NB69, NBL-S, NGP, NMB, SK-N-AS, SK-N-BE2, SK-N-DZ, and SMS-KAN were obtained from the CHOP cell line bank and grown in vitro under standard conditions (10,22,25). We analyzed the neuroblastoma cell lines for promoter methylation: two with 1p deletion (NLF and IMR5) and two without (SK-N-SH and SK-N-FI). These cell lines were also used to study the effect of exogenous CHD5 expression on in vitro growth, clonogenicity, and tumorigenicity. Cell growth in vitro was assessed by a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. A multiwell scanner was used to measure the absorbance at 570–630 nm dual wavelengths. The untreated controls were assigned a value of 100%. Apoptosis was assessed by annexin V antibody and propidium iodide staining (Roche Molecular Biochemicals, Indianapolis, IN), according to the manufacturer’s instructions as described previously (26). Apoptosis was then monitored by flow cytometry analysis with a FACScan using CELLQuest software (Becton Dickinson, Mountain View, CA). For the studies of CHD5 re-expression using 5-aza-2-deoxycytidine, we plated 1−5 × 105 cells in 6-well plates, grew cells in the absence or presence of 5-aza-2-deoxycytidine (0.1–1.0 μM), and assessed cell morphology (cell size, shape, and neurite outgrowth), cell number measured by MTT assay, and CHD5 expression by real-time reverse transcription–polymerase chain reaction (RT-PCR) after 0, 1, 4, and 7 days.
CHD5 RNA and Protein Expression
Genomic DNA and total RNA were isolated from the 24 neuroblastoma cell lines above, including NLF, IMR5, SK-N-SH, and SK-N-FI, using DNeasy and RNeasy kits (Qiagen, Valencia, CA), respectively. We analyzed CHD5 expression of these 24 lines by RT-PCR using CHD5 primers provided by the manufacturer (Hs00395930_m1) and the TaqMan Applied Biosystems 7900HT real-time RT-PCR system (Applied Biosystems Inc. (ABI), Foster City, CA). Expression of the glyceraldehyde-3-phosphate dehydrogenase gene using ABI primers (Hs4333764-famMGB) was used for normalization. We isolated RNA from the same 101 primary neuroblastoma samples described above and previously (23). We used a commercially available panel of mRNAs that were derived from 17 normal tissues from Clontech (Mountain View, CA), and fetal brain RNA (Clontech) was used as a positive control. Expression profiling was performed on the Affymetrix U95Av2 microarray (23), and the data are freely available at the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/). We measured differential gene expression based on 1p36 allelic status with the Patterns from Gene Expression (PaGE) algorithm (27).
CHD5 protein expression in NLF and IMR5 parental and transfected lines was detected by immunoblotting with rabbit polyclonal anti-CHD5 antiserum (1 : 5000; Strategic Diagnostics, Inc., Newark, DE) and detected with donkey anti-rabbit polyclonal antiserum (Amersham Biosciences, Piscataway, NJ). Briefly, nuclear pellets were vortexed several times in 0.42 M NaCl buffer with all protease inhibitors present. Samples were kept on ice at all times. Clear supernatants with nuclear proteins were collected after centrifuging extracts at 20 000 × g 10 minutes. Protein concentrations were determined by the Bradford method (Bio-Rad Laboratories, Hercules, CA). All extracts were prepared in duplicate, and at least three independent experiments were conducted. Protein complexes were detected with CHD5 antibody and had a predicted molecular weight of 250–260 kDa (based on the amino acid composition), specifically in NLF and IMR5 cells that were transfected with CHD5 in the sense orientation.
Promoter Methylation Analysis
DNA was isolated from NLF, IMR5, SK-N-SH, and SK-N-FI cells as described above after growing them in standard culture conditions for 1 week. We then treated DNA with sodium bisulfite (4 M) to convert all unmethylated cytosines to uracil, and we subsequently amplified the modified DNA using RT-PCR as described previously (28) with primers specific for modified DNA that were designed to encompass from −840 to +769 from the start of CHD5 exon 1 (primer sequences are provided in Supplementary Table 1, available online). The amplified DNA was cloned using the TA cloning kit (Invitrogen, Carlsbad, CA) and sequenced using automated sequencing (ABI). We made six sets of primers in the CHD5 promoter region (−840 to +769; CHD5 GenBank accession number AL031847) to survey for methylation status. PCR products were purified using agarose gel electrophoresis and the Qiagen purification kit (Qiagen, Valencia, CA) and were cloned using the pGEM-T Easy Vector System (Promega, Madison, WI). At least 10 colonies were selected and sequenced to determine the percent methylation for each CpG. The percent methylation was determined by the number of clones with methylation of the given region (as determined by methylation-specific sequencing) divided by 10 and expressed as a percentage. All experiments were performed in duplicate and repeated twice.
Clonogenicity and Tumor Growth Studies
We next performed clonogenicity assays by measuring colony formation in soft agar. Neuroblastoma cell lines NLF, IMR5, SK-N-SH, and SK-N-FI were stably transfected with a CHD5 sense or antisense (AS) DNA (GenBank accession number AF425231) that had been cloned into the expression vector pcDNA3.1 (Invitrogen, Carlsbad, CA). Expression of CHD5 was confirmed by RT-PCR analysis. Stable clones expressing CHD5 were selected and expanded and then seeded into T75 culture flasks and grown to 60%–70% confluency. Agar (0.35%) medium was prepared by mixing regular DNA-grade agar and RPMI 1640 with 10% fetal bovine serum (FBS); 1.5 mL was added to 60 mm wells. Cells were detached by incubation in trypsin-EDTA, centrifuged at 350×g, and suspended (5 × 103 cells) in 0.25% low melting point agarose (Cambrex, Rockland, ME) that was dissolved in RPMI 1640 containing 10% FBS per well. Cells were then plated on the underlayer of 0.35% agar. Agar plates were incubated at 37°C for 3 weeks, at which time cell colonies were stained with 0.005% crystal violet (Sigma, St. Louis, MO) and scored using an inverted microscope. By convention, cell clusters measuring more than 500 μM in greatest diameter were considered a colony. Each soft agar assay was performed in triplicate and repeated at least twice.
Stable neuroblastoma clones of NLF, IMR5, SK-N-SH, and SK-N-FI cells with either sense (-CHD5) or antisense (-CHD5-AS) were suspended in Matrigel (BD Biosciences, San Jose, CA). Six-week-old female nu/nu mice (Charles River Laboratory, Wilmington, MA; n = 160 total; n = 10 in each group, two independent experiments) were given subcutaneous injections of 1 × 107 cells in the right flank. Tumor size (L × [W]2 × 0.523/1000 = x cm3) and body weight were measured once weekly. Mice were killed 5–6 weeks after injection, when the majority of mice that had been injected with tumors derived from cells transfected with CHD5-AS developed tumors that exceeded 3 cm3. Tumors were removed surgically, fixed in 10% formalin, and stained with hematoxylin and eosin for histological examination. CHD5 expression was confirmed in the neuroblastoma xenografts by RT-PCR. This project was reviewed and approved by the CHOP Institutional Animal Care and Use Committee, and mice were housed and protocols were performed in accordance with institutional guidelines. Euthanasia was performed by the administration of ketamine (150 mg/kg) and xylazine (8 mg/kg) intraperitoneally followed by cervical dislocation after deep anesthesia had been obtained.
Statistical Analysis
We analyzed associations between CHD5 mRNA expression and clinical factors by application of the two-sample t test or analysis of variance. Mean values for CHD5 expression are presented with 95% confidence intervals. The distinction between high and low gene expression was based on the median value. We estimated event-free and overall survival probabilities by Kaplan–Meier analysis and compared survival distributions using a two-sided log-rank test. Time to event for event-free survival was defined as the time from diagnosis until the time of first occurrence of relapse, progression, or death, or until last contact if no event occurred. Time to event for overall survival was defined as the time from diagnosis until the time of death, or until last contact if the patient was still alive. We used Cox regression models to examine the prognostic significance of CHD5 expression, 1p deletion, and MYCN amplification. The assumption of proportional hazards was checked using visual inspection of plots of the log-cumulative hazard vs log of survival time. All statistical tests were two-sided, and P values less than .05 were considered statistically significant unless otherwise indicated.
Results
CHD5 Expression and Promoter Methylation
CHD5 expression is restricted to neural tissues, and expression was very low or absent in all the neuroblastoma cell lines examined (22). We previously examined CHD5 for inactivating mutations in 30 neuroblastoma cell lines but found only one missense mutation (12). Thus, CHD5 is not homozygously inactivated by deletion or mutation with substantial frequency, so homozygous inactivation would require an epigenetic mechanism. Therefore, we investigated whether CHD5 expression was silenced by methylation in these lines.
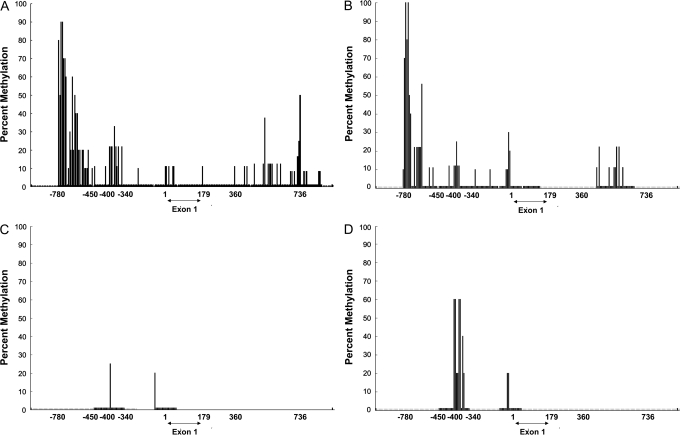
We determined the methylation status of the CHD5 promoter in four cell lines—two with no CHD5expression (both with 1p deletion: NLF, IMR5) and two with low CHD5 expression (both without 1p deletion: SK-N-SH, S-KN-FI)—and in human fetal brain, which has high CHD5 expression. There were strong sites of methylation in NLF and IMR5 (60%–100%) between base pairs −780 to −450 and a secondary site between −400 and −340 bp (Figure 1, A and B). The distal site showed much lower or no methylation in the two cell lines with low CHD5 expression (Figure 1, C and D) or in human fetal brain (data not shown). We grew these lines in increasing concentrations of 5-aza-2-deoxycytidine for up to 7 days and measured normalized CHD5 expression using quantitative RT-PCR. A dose- and time-dependent increase in expression was observed in all lines that was most dramatic (one to two orders of magnitude) in the two cell lines with 1p deletion and no CHD5 expression, compared with only two- to fourfold in the cell lines with readily detectable CHD5 expression (data not shown). Thus, there was a strong association between methylation of the distal promoter site and CHD5 expression, although no well-established regulatory motifs were apparent. Moreover, these data suggest that this site contains an important regulatory domain and they support promoter methylation as a possible mechanism for epigenetic silencing of CHD5 transcription.
Figure 1.
Methylation of the CHD5 promoter and 5′ coding region in four neuroblastoma cell lines. A–B) CHD5 promoter methylation in NLF (A) and IMR5 (B) cells, both of which have hemizygous 1p deletions and virtually no CHD5 expression. C–D) CHD5 promoter methylation in SK-N-SH (C) and SK-N-FI cells (D), which lack 1p deletion and have low expression of CHD5. Methylation at a given GC dinucleotide, as determined by methylation-specific sequencing, is shown as a percentage of sequences analyzed (10 per site). Methylation was determined as the number of clones with methylation of the given region divided by 10 and is expressed as a percentage.
Suppression of Clonogenicity and Tumor Growth
We stably transfected CHD5 sense and antisense vectors into two neuroblastoma cell lines with 1p deletion and MYCN amplification (NLF and IMR5) and two with neither aberration (SK-N-SH and SK-N-FI). We examined the morphology, growth, differentiation, and apoptosis rates of these cell lines, but no major differences were observed (data not shown). We repeated these experiments under low serum conditions but again observed no major differences.
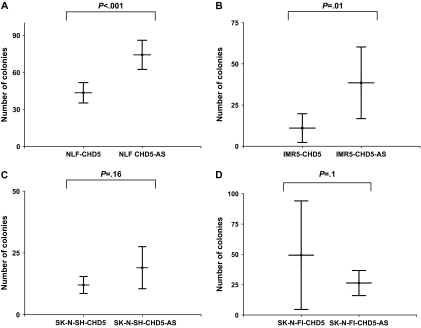
Next, we plated cells from CHD5-transfected and CHD5-AS–transfected lines into soft agar. After 3 weeks of culture, NLF-CHD5-AS clones formed statistically significantly more colonies (mean = 74 large colonies per plate, 95% confidence interval [CI] = 62 to 86 colonies) than NLF-CHD5 clones (mean = 43 colonies, 95% CI = 35 to 51 colonies (P < .001) (Figure 2, A). Similarly, the IMR5-CHD5-AS control cells formed more colonies (mean = 39 colonies, 95% CI = 17 to 60 colonies) than the CHD5-transfected cells (mean = 11 colonies, 95% CI = 2 to 20 colonies) (P = .01) (Figure 2, B). However, there was no difference between the number of colonies formed by CHD5-transfected and CHD5-AS–transfected clones of SK-N-SH (P = .16) and SK-N-FI (P = .1) (Figure 2, C and D). Thus, CHD5 expression caused a statistically significant reduction in colony formation in the CHD5-expressing clones relative to the CHD5-AS clones, but only in cell lines with 1p deletion.
Figure 2.
Effect of altered CHD5 expression on clonogenicity in neuroblastoma cell lines. Plasmids containing CHD5 or antisense CHD5 (CHD5-AS) were stably transfected into NLF and IMR5 neuroblastoma cell lines, both of which have hemizygous 1p deletion and MYCN amplification, and into SK-N-SH and SK-N-FI neuroblastoma cell lines, which have neither. Transfected cells were plated on soft agar, and colonies were counted 3 weeks later. A) NLF cells. CHD5 vs CHD5-AS, P < .001. B) IMR5 cells. CHD5 vs CHD5-AS, P = .001. C) SK-N-SH cells. CHD5 vs CHD5-AS, P = .16. D) SK-N-FI cells. CHD5 vs CHD5-AS, P = .10. Means and 95% confidence intervals (error bars) are shown. P values (two-sided) were calculated using the Student t test. Data are representative of three independent experiments performed in quadruplicate.
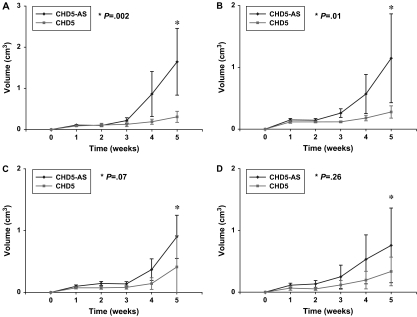
Next, we injected neuroblastoma cells stably expressing CHD5 or CHD5-AS into the flanks of immunosuppressed nu/nu mice. Tumors became palpable within 1–2 weeks, but there was dramatic growth inhibition of CHD5-expressing clones for both NLF and IMR5 neuroblastoma cell lines compared with parental cell lines or CHD5-AS controls (Figure 3, A and B). The mean tumor volume for NLF-CHD5-AS at 5 weeks after injection was 1.65 cm3 (CI = 0.83 to 2.46 cm3), compared with 0.36 cm3 (CI = 0.17 to 0.44 cm3) for NLF-CHD5 (P = .002). The mean tumor size for IMR5-CHD5-AS at 5 weeks after injection was 1.15 cm3 (CI = 0.43 to 1.87 cm3), compared with 0.28 cm3 (CI = 0.18 to 0.38 cm3) for IMR5-CHD5 (P = .01). Both studies were repeated with similar results (data not shown). There was no statistically significant difference in growth rates between CHD5- and CHD5-AS–transfected SK-N-SH and SK-N-FI tumors (Figure 3, C and D), again demonstrating the specificity of tumor growth suppression for neuroblastoma lines with 1p deletion.
Figure 3.
Effect of CHD5 expression on tumor growth in neuroblastoma cell lines. NLF, IMR5, SK-N-SH, and SK-N-FI cells (107 each) stably transfected with plasmids containing CHD5- or CHD5-AS were injected into the flanks of nu/nu mice (n = 10 mice per group), and tumor growth was measured as volume weekly over 5 weeks. A) NLF CHD5-AS vs NLF CHD5, P = .002. B) IMR5 CHD5-AS vs IMR5 CHD5, P = .01. C) SK-N-SH CHD5-AS vs SK-N-SH CHD5, P = .07. D) SK-N-FI CHD5 vs SK-N-FI CHD5-AS, P = .26. Means and 95% confidence intervals (error bars) are shown. All P values (two-sided, comparisons at the 5-week time points) were calculated using a two-sample Student t test. Data are representative of two independent experiments.
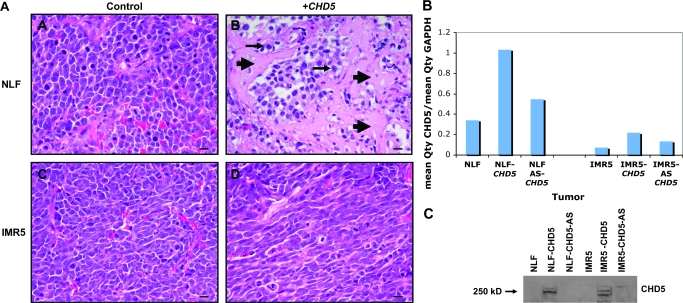
We also observed an effect on histology for the CHD5-transfected tumors. The control tumors for NLF and IMR5 were undifferentiated with scant cytoplasm, as is typical of neuroblastomas (Figure 4, A). The NLF-CHD5 tumors had areas of differentiation and necrosis, whereas the IMR5-CHD5–transfected tumors had a more elongated morphology (Figure 4, A). We examined the mRNA expression of CHD5 in xenograft tumors derived from parental NLF and IMR5 cells, as well as the CHD5-transfected and CHD5-AS–transfected clones, by real-time RT-PCR. CHD5 mRNA expression was higher in the CHD5-transfected clones than their respective parental lines (Figure 4, B), and expression in CHD5-AS cells was intermediate. We also examined CHD5 protein expression in the NLF and IMR5 lines. The CHD5 protein (250–260 kD) was detected only in the lines that were transfected with the CHD5-sense construct (Figure 4, C). In summary, there was clear suppression of both clonogenicity and xenograft tumor growth of CHD5-transfected clones, but only in tumors with 1p deletion.
Figure 4.
Histology and CHD5 expression in xenografts derived from NLF and IMR5 cells transfected with either CHD5 sense or CHD5 antisense constructs. A) Histology of NLF and IMR5 xenograft tumors (see Figure 3) after 5 weeks of growth. NLF-CHD5-AS tumors were composed of undifferentiated cells with scant cytoplasm (top left), whereas NLF-CHD5 tumors showed areas of necrosis (arrowheads) and differentiation (arrows; top right). Cells in the IMR5-CHD5-AS tumors were undifferentiated (bottom left), whereas cells in the IMR5-CHD5 tumors had a more elongated appearance (bottom right). Bar = 20 μm. B) Relative expression of CHD5, normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was determined by real-time reverse transcription–polymerase chain reaction in parental NLF and IMR5 cell lines, as well as the CHD5 sense and CHD5 antisense transfected lines used for the xenograft experiments. The normalized values indicated by each bar graph represent the mean of three measurements. Replicate measurements were within 10% of the mean for each bar shown. C) Expression of CHD5 protein, as detected by immunoblotting, is shown for the NLF and IMR5 parental lines and the corresponding sense- and antisense-transfected cells used in these experiments.
Association With Prognostic Features and Outcome
We analyzed microarray expression data from 101 neuroblastomas representing well-defined risk groups (low, intermediate, high, and ultrahigh) (23) to assess clinical associations of candidate tumor suppressor gene expression from the SRD (10,12). Only 12 of the 23 genes from the SRD were represented on the microarray chip, but these included CHD5, three other leading tumor suppressor gene candidates (TNFRSF25, CAMTA1, and AJAP1) (12), and eight other genes. We compared expression patterns (high vs low) of all genes with clinical and biologic variables currently used in risk stratification of neuroblastoma patients, including patient age and INSS stage, as well as features of the tumor (MYCN amplification, 1p deletion, 11q deletion, tumor cell DNA content/ploidy, Shimada histopathology). These features were also combined into risk groups, as described previously (23,29).
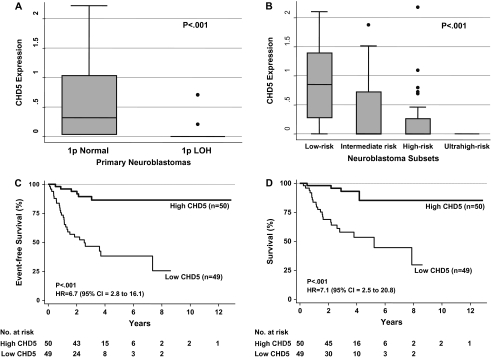
CHD5 expression had the strongest association with all eight clinical and biologic features (Table 1). Expression of ICMT, THAP3, CAMTA1, ACOT7, KCNAB2, RPL22, and KLHL21 had weaker associations and/or associations with fewer variables (two to seven variables). Expression of TNFRSF25 was associated with only one variable, and expression of the remaining three genes (AJAP1, NPHP4, HES2) showed no statistically significant association with any of the variables. High CHD5 expression was strongly associated with favorable clinical and biologic features: younger age (either <12 or <18 months), lower stage (1, 2, 4S vs 3 and 4), non-amplified MYCN, hyperdiploidy, favorable histopathology, and normal 11q (Table 1) (P < .001 for all). CHD5 expression was also statistically significantly lower in tumors with 1p deletion, and expression was inversely proportional to risk group (Figure 5, A and B; P < .001). Together, these data strongly suggest that CHD5 is the tumor suppressor gene deleted from the 1p36 SRD.
Table 1.
Association of candidate tumor suppressor gene expression with prognostic variables and outcome*
| Gene | ||||||||||||
| Characteristic | AJAP4 | NPHP4 | KCNAB2 | CHD5 | RPL22 | ICMT | ACOT7 | HES2 | TNFRSF25 | KLHL21 | THAP3 | CAMTA1 |
| Age <12 mo | NS | NS | NS | <.001 | NS | .007 | NS | NS | NS | NS | .002 | .004 |
| Age <18 mo | NS | .013 | .024 | <.001 | NS | <.001 | .008 | NS | NS | NS | <.001 | <.001 |
| INSS stage | NS | NS | .046 | <.001 | NS | .005 | NS | NS | .033 | NS | <.001 | <.001 |
| MYCN amplified | .031 | NS | .009 | <.001 | .003 | <.001 | <.001 | NS | .008 | NS | .001 | <.003 |
| Ploidy | NS | NS | .013 | <.001 | NS | .001 | NS | NS | .043 | NS | .006 | .037 |
| Shimada | NS | .010 | .003 | <.001 | NS | <.001 | <.001 | NS | NS | NS | <.001 | <.001 |
| Risk group | NS | .019 | .004 | <.001 | .006 | <.001 | .002 | NS | .012 | NS | <.001 | <.001 |
| 1p deletion | NS | NS | .018 | <.001 | NS | <.001 | .001 | NS | NS | .002 | <.001 | <.001 |
| 11q deletion | NS | NS | NS | <.001 | NS | NS | NS | NS | NS | .003 | NS | .033 |
| Log-rank-EFS | NS | NS | .003 | <.001 | NS | NS | NS | NS | NS | NS | .041 | .034 |
| Cox-EFS | NS | NS | .014 | <.001 | NS | .035 | NS | NS | .047 | NS | .032 | .031 |
| Cox-EFS† | NS | NS | NS | .007 | NS | NS | NS | NS | NS | NS | NS | NS |
| Cox-EFS‡ | NS | NS | NS | .010 | NS | NS | NS | NS | NS | NS | NS | NS |
| Cox-EFS§ | NS | NS | NS | .027 | NS | NS | NS | NS | NS | NS | NS | NS |
Genes from the smallest region of deletion that were represented on the Affymetrix platform are shown in order from distal to proximal. For prognostic factors, associations with a P value of <.01 by two-sample t test were considered statistically significant (after a Bonferroni type correction). A P value of <.05 was considered statistically significant for the log-rank test and Cox regression analyses. NS = not significant; INSS = International Neuroblastoma Staging System (24); EFS = event-free survival. All statistical tests were two-sided.
Adjusted for MYCN amplification.
Adjusted for 1p deletion.
Adjusted for MYCN amplification + 1p deletion.
Figure 5.
Association of CHD5 expression with risk factors and outcome in primary neuroblastomas. A) Normalized CHD5 expression in 101 primary neuroblastomas stratified based on the presence (n = 26) or absence (n = 75) of 1p deletion (two-sample t test, P < .001). B) Association of normalized CHD5 expression with the risk group (low, intermediate, high, and ultrahigh) as defined above and in (23). Briefly, for this study, low-risk patients were defined as infants (<1 year of age) with stage 1 or 2 disease by the International Neuroblastoma Staging (INSS) system (24) and favorable biologic features. Intermediate-risk patients were almost all patients with INSS stage 3 disease who were older than 1 year. High-risk patients were defined as those with INSS stage 3 or 4 (only two had stage 3) disease who were older than 1 year. High-risk patients were divided into two subsets: those without MYCN amplification (high risk) and those with MYCN amplification (ultrahigh risk). Twenty-eight were low-risk, 21 were intermediate-risk, 32 were high-risk (without MYCN amplification), and 20 were ultrahigh risk (with MYCN amplification) patients. Survival data were available for 99 of the 101 patients. The association of CHD5 expression with risk group was assessed (analysis of variance, P < .001). The box stretches from the 25th to 75th percentile. The median is shown with a line across the box. The whiskers extend to the highest and lowest observed values that are lower than and higher than 1.5 times the interquartile range from the third and first quartile values. The solid circles are outlying values beyond the whisker edges. C) Association of CHD5 expression with event-free survival in univariate analysis (log-rank, P < .001). D) Association of CHD5 expression with overall survival in univariate analysis (log-rank, P < .001). HR = hazard ratio; CI = confidence interval. All P values are two-sided.
We compared event-free survival and overall survival for 99 of the 101 neuroblastoma patients based on the expression of candidate tumor suppressor genes, with or without adjustment for 1p deletion and MYCN amplification. CHD5 was the only gene whose expression was strongly associated with event-free survival in both univariate and multivariable analyses (Table 1). Expression of the KCNAB2, CAMTA1, THAP3, and TNFRSF25 genes was weakly associated with outcome by log-rank and/or Cox regression, but only in univariate analyses. High chd5 expression was strongly associated with favorable event-free survival and overall survival (event-free survival: HR = 6.7, 95% CI = 2.8 to 16.1, and overall survival: HR = 7.1, 95% CI = 2.5 to 20.8; both P < .001; Figure 5, C). Cox regression analysis showed that, after adjustment for CHD5 expression, 1p deletion was no longer statistically significantly associated with survival. Conversely, after including 1p deletion or MYCN amplification separately or jointly in the models, CHD5 expression remained statistically significantly associated with event-free survival (P = .027) (Table 1). These results demonstrate that CHD5 expression is strongly associated with outcome in neuroblastomas, CHD5 is the likely target of 1p deletions in these tumors, and it may have a direct role in the biology and behavior of neuroblastomas.
Discussion
We previously identified 23 genes in the SRD on 1p36 (10,12) and sequenced every coding exon of these genes in 30 neuroblastoma cell lines (21 with 1p deletion). None of the genes showed evidence of homozygous genetic inactivation by deletion, frameshift, or inactivating mutation (12). However, expression analyses and functional considerations suggested that CHD5 was the most promising tumor suppressor gene candidate. CHD5 was the only gene that had preferential expression in the nervous system (12), virtually absent expression in neuroblastomas with 1p deletion, and prior implication in neuroblastoma pathogenesis (22). We transfected CHD5 into several neuroblastoma cell lines, and there was a limited effect in vitro, yet we observed dramatic suppression of clonogenicity and tumorigenicity. This result suggests that CHD5 functions as a tumor suppressor gene and may be particularly important for anchorage-independent growth. Indeed, the phenomenon of a limited effect on in vitro growth yet suppression of clonogenicity and in vivo tumorigenicity has been seen at times even with classical tumor suppressor genes, such as RB1 and TP53 (30–33). Furthermore, the chromatin remodeling function of CHD5 is consistent with other tumor suppressor genes in human cancers, including SMARCB1 (formerly known as INI1/SNF5) (34–36), SMARCA4 (BRG1/hBRM) (37,38), and BMI1 (39–42). Thus, based on these analyses, CHD5 emerged as the lead tumor suppressor gene candidate from the 1p36 SRD in neuroblastomas.
To provide additional evidence to support CHD5 as a tumor suppressor gene, we analyzed the relationship of gene expression with prognostic variables, event-free survival, and overall survival for 12 of the 23 genes from the SRD in 101 neuroblastomas. Only six genes showed statistically significant associations with three or more risk factors. CHD5 expression showed the strongest association with all eight clinical and biologic variables. High CHD5 expression was also strongly associated with favorable outcome. Indeed, using Cox regression analysis, 1p deletion was no longer associated with outcome after correction for CHD5 expression. Conversely, the association between CHD5 expression and outcome was retained even after correction for both MYCN amplification and 1p deletion (P = .027). These data suggest that CHD5 expression is a potent prognostic variable, that it likely plays a role in neuroblastoma pathogenesis, and that it may play a role even in some tumors without apparent 1p deletion. Our studies also demonstrate that CHD5 expression is not just a surrogate marker for 1p deletion because it was by far most strongly associated with outcome, and the expression of other genes tested from the SRD was weakly or not at all associated with outcome. More detailed studies will be required to determine whether addition of CHD5 expression as a prognostic marker may more rigorously defined neuroblastoma risk groups.
Although we did not find homozygous genetic inactivation of CHD5 (12), we did find strong promoter methylation and transcriptional silencing of the remaining allele in 1p-deleted neuroblastoma lines compared with lines without 1p deletion or with fetal brain tissue. This epigenetic mechanism could inactivate expression or lower it sufficiently to functionally silence the gene. Indeed, there is precedent for transcriptional silencing by methylation to inactivate the second allele of other tumor suppressor genes, including RASSF1A (43,44) and OPCML (45). Also, CHD5 could be re-expressed by growing these cells in 5-aza-2-deoxycytidine, so this approach may have utility in treating neuroblastomas with low or absent CHD5 expression.
We now have convincing evidence that CHD5 is the tumor suppressor gene deleted from the 1p36.31 SRD in neuroblastomas. It is difficult to prove unequivocally that no other gene from the SRD or elsewhere on 1p36 contributes to the pathogenesis of neuroblastomas. However, within this SRD, CHD5 is the only gene that fulfills all tumor suppressor gene criteria. We first described CHD5 as a novel member of this chromatin remodeling gene family that was consistently deleted from 1p36 in neuroblastomas and as a candidate tumor suppressor gene (22). Recently, Bagchi et al. (46) took an independent approach using chromosome engineering to generate mouse models with gain and loss of a region corresponding to the region of human 1p36 that contained mouse Chd5. They identified mChd5 as a gene that controls proliferation, apoptosis, and senescence via the p19Arf/p53 pathway. Their results also suggest that gene dosage is tightly regulated and that homozygous deletion of Chd5 in tumors is rarely seen, which is consistent with our finding that homozygous genetic inactivation of CHD5 is rare in human neuroblastomas. Furthermore, their independent identification of Chd5 as a tumor suppressor gene in a mouse model supports our findings in human neuroblastomas and suggests that CHD5 may have a role in the pathogenesis of other human cancers characterized by 1p36 deletions. Future studies will determine whether the primary consequence of mouse Chd5 inactivation is through p19Arf/p53 and/or other pathways in these tumors.
This study has potential limitations. Only 12 of the 23 genes from the SRD were represented on the expression profiling microarray so we were unable to assess associations between clinical outcomes with expression of 11 of the genes in the SRD. Nevertheless, the four genes that were the most promising candidates, including CHD5, were all included on the microarray. It was beyond the scope of this project to transfect full-length cDNAs of all 23 genes from the SRD into neuroblastoma cell lines with and without 1p deletion and to assess effects on growth, clonogenicity, and tumorigenicity. However, 10 of the genes from this region were tested in the study by Bagchi et al. (46), and only CHD5 was able to restore growth control.
Funding
National Institutes of Health (R01-CA039771 to G.M.B., U10 CA98413-04 to W.B.L.); Audrey E. Evans Endowed Chair (G.M.B.).
Supplementary Material
References
- 1.Brodeur GM, Maris JM. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th ed. Philadelphia, PA: Lippincott; 2006. pp. 933–970. [Google Scholar]
- 2.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3(3):203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM, Green AA, Hayes FA, Williams KJ, Williams DL, Tsiatis AA. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981;41(11 Pt 1):4678–4686. [PubMed] [Google Scholar]
- 4.Brodeur GM, Sekhon G, Goldstein MN. Chromosomal aberrations in human neuroblastomas. Cancer. 1977;40(5):2256–2263. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353(21):2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 6.Hogarty MD, Liu X, Guo C, et al. Identification of a 1-megabase consensus region of deletion at 1p36.3 in primary neuroblastomas. Med Pediatr Oncol. 2000;35(6):512–515. doi: 10.1002/1096-911x(20001201)35:6<512::aid-mpo2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Maris JM, Guo C, Blake D, et al. Comprehensive analysis of chromosome 1p deletions in neuroblastoma. Med Pediatr Oncol. 2001;36(1):32–36. doi: 10.1002/1096-911X(20010101)36:1<32::AID-MPO1009>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.White PS, Maris JM, Beltinger C, et al. A region of consistent deletion in neuroblastoma maps within human chromosome 1p36.2-36.3. Proc Natl Acad Sci USA. 1995;92(12):5520–5524. doi: 10.1073/pnas.92.12.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White PS, Maris JM, Sulman EP, et al. Molecular analysis of the region of distal 1p commonly deleted in neuroblastoma. Eur J Cancer. 1997;33(12):1957–1961. doi: 10.1016/s0959-8049(97)00311-0. [DOI] [PubMed] [Google Scholar]
- 10.White PS, Thompson PM, Gotoh T, et al. Definition and characterization of a region of 1p36.3 consistently deleted in neuroblastoma. Oncogene. 2005;24(16):2684–2694. doi: 10.1038/sj.onc.1208306. [DOI] [PubMed] [Google Scholar]
- 11.White PS, Thompson PM, Seifried BA, et al. Detailed molecular analysis of 1p36 in neuroblastoma. Med Pediatr Oncol. 2001;36(1):37–41. doi: 10.1002/1096-911X(20010101)36:1<37::AID-MPO1010>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Okawa ER, Gotoh T, Igarashi J, et al. Expression and sequence analysis of candidates for the 1p36.31 tumor suppressor gene deleted in neuroblastomas [published online ahead of print July 30, 2007] Oncogene. 2007;27(6):803–810. doi: 10.1038/sj.onc.1210675. [DOI] [PubMed] [Google Scholar]
- 13.Bauer A, Savelyeva L, Claas A, Praml C, Berthold F, Schwab M. Smallest region of overlapping deletion in 1p36 in human neuroblastoma: A 1 Mbp cosmid and PAC contig. Genes Chromosomes Cancer. 2001;31(3):228–239. doi: 10.1002/gcc.1139. [DOI] [PubMed] [Google Scholar]
- 14.Caron H, Peter M, van Sluis P, et al. Evidence for two tumour suppressor loci on chromosomal bands 1p35-36 involved in neuroblastoma: one probably imprinted, another associated with N-myc amplification. Hum Mol Genet. 1995;4(4):535–539. doi: 10.1093/hmg/4.4.535. [DOI] [PubMed] [Google Scholar]
- 15.Caron H, Spieker N, Godfried M, et al. Chromosome bands 1p35-36 contain two distinct neuroblastoma tumor suppressor loci, one of which is imprinted. Genes Chromosomes Cancer. 2001;30(2):168–174. doi: 10.1002/1098-2264(200102)30:2<168::aid-gcc1072>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Cheng NC, Van Roy N, Chan A, et al. Deletion mapping in neuroblastoma cell lines suggests two distinct tumor suppressor genes in the 1p35-36 region, only one of which is associated with N-myc amplification. Oncogene. 1995;10(2):291–297. [PubMed] [Google Scholar]
- 17.Gehring M, Berthold F, Edler L, Schwab M, Amler LC. The 1p deletion is not a reliable marker for the prognosis of patients with neuroblastoma. Cancer Res. 1995;55(22):5366–5369. [PubMed] [Google Scholar]
- 18.Martinsson T, Sjoberg RM, Hedborg F, Kogner P. Deletion of chromosome 1p loci and microsatellite instability in neuroblastomas analyzed with short-tandem repeat polymorphisms. Cancer Res. 1995;55(23):5681–5686. [PubMed] [Google Scholar]
- 19.Martinsson T, Sjoberg RM, Hallstensson K, Nordling M, Hedborg F, Kogner P. Delimitation of a critical tumour suppressor region at distal 1p in neuroblastoma tumours. Eur J Cancer. 1997;33(12):1997–2001. doi: 10.1016/s0959-8049(97)00278-5. [DOI] [PubMed] [Google Scholar]
- 20.Schleiermacher G, Peter M, Michon J, et al. Two distinct deleted regions on the short arm of chromosome 1 in neuroblastoma. Genes Chromosomes Cancer. 1994;10(4):275–281. doi: 10.1002/gcc.2870100409. [DOI] [PubMed] [Google Scholar]
- 21.Takeda O, Homma C, Maseki N, et al. There may be two tumor suppressor genes on chromosome arm 1p closely associated with biologically distinct subtypes of neuroblastoma. Genes Chromosomes Cancer. 1994;10(1):30–39. doi: 10.1002/gcc.2870100106. [DOI] [PubMed] [Google Scholar]
- 22.Thompson PM, Gotoh T, Kok M, White PS, Brodeur GM. CHD5, a new member of the chromodomain gene family, is preferentially expressed in the nervous system. Oncogene. 2003;22(7):1002–1011. doi: 10.1038/sj.onc.1206211. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Diskin S, Rappaport E, et al. Integrative genomics identifies distinct molecular classes of neuroblastoma and shows that multiple genes are targeted by regional alterations in DNA copy number. Cancer Res. 2006;66(12):6050–6062. doi: 10.1158/0008-5472.CAN-05-4618. [DOI] [PubMed] [Google Scholar]
- 24.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 25.Thompson PM, Maris JM, Hogarty MD, et al. Homozygous deletion of CDKN2A (p16INK4a/p14ARF) but not within 1p36 or at other tumor suppressor loci in neuroblastoma. Cancer Res. 2001;61(2):679–686. [PubMed] [Google Scholar]
- 26.Eggert A, Grotzer MA, Zuzak TJ, et al. Resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res. 2001;61(4):1314–1319. [PubMed] [Google Scholar]
- 27.Manduchi E, Grant GR, McKenzie SE, Overton GC, Surrey S, Stoeckert CJ., Jr Generation of patterns from gene expression data by assigning confidence to differentially expressed genes. Bioinformatics. 2000;16(8):685–698. doi: 10.1093/bioinformatics/16.8.685. [DOI] [PubMed] [Google Scholar]
- 28.Eggert A, Brodeur GM, Ikegaki N. Relative quantitative RT-PCR protocol for TrkB expression in neuroblastoma using GAPD as an internal control. Biotechniques. 2000;28(4):681–682. doi: 10.2144/00284st04. 686, 688–691. [DOI] [PubMed] [Google Scholar]
- 29.Castleberry RP, Pritchard J, Ambros P, et al. The International Neuroblastoma Risk Groups (INRG): a preliminary report. Eur J Cancer. 1997;33(12):2113–2116. doi: 10.1016/s0959-8049(97)00202-5. [DOI] [PubMed] [Google Scholar]
- 30.Bookstein R, Shew JY, Chen PL, Scully P, Lee WH. Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science. 1990;247(4943):712–715. doi: 10.1126/science.2300823. [DOI] [PubMed] [Google Scholar]
- 31.Cajot JF, Anderson MJ, Lehman TA, Shapiro H, Briggs AA, Stanbridge EJ. Growth suppression mediated by transfection of p53 in Hut292DM human lung cancer cells expressing endogenous wild-type p53 protein. Cancer Res. 1992;52(24):6956–6960. [PubMed] [Google Scholar]
- 32.Casey G, Lo-Hsueh M, Lopez ME, Vogelstein B, Stanbridge EJ. Growth suppression of human breast cancer cells by the introduction of a wild-type p53 gene. Oncogene. 1991;6(10):1791–1797. [PubMed] [Google Scholar]
- 33.Fung YK, T'ang A, Murphree AL, et al. The Rb gene suppresses the growth of normal cells. Oncogene. 1993;8(10):2659–2672. [PubMed] [Google Scholar]
- 34.Biegel JA, Fogelgren B, Zhou JY, et al. Mutations of the INI1 rhabdoid tumor suppressor gene in medulloblastomas and primitive neuroectodermal tumors of the central nervous system. Clin Cancer Res. 2000;6(7):2759–2763. [PubMed] [Google Scholar]
- 35.Rousseau-Merck MF, Versteege I, Legrand I, et al. hSNF5/INI1 inactivation is mainly associated with homozygous deletions and mitotic recombinations in rhabdoid tumors. Cancer Res. 1999;59(13):3152–3156. [PubMed] [Google Scholar]
- 36.Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394(6689):203–206. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 37.Fukuoka J, Fujii T, Shih JH, et al. Chromatin remodeling factors and BRM/BRG1 expression as prognostic indicators in non-small cell lung cancer. Clin Cancer Res. 2004;10(13):4314–4324. doi: 10.1158/1078-0432.CCR-03-0489. [DOI] [PubMed] [Google Scholar]
- 38.Reisman DN, Sciarrotta J, Wang W, Funkhouser WK, Weissman BE. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: correlation with poor prognosis. Cancer Res. 2003;63(3):560–566. [PubMed] [Google Scholar]
- 39.Shakhova O, Leung C, Marino S. Bmi1 in development and tumorigenesis of the central nervous system. J Mol Med. 2005;83(8):596–600. doi: 10.1007/s00109-005-0682-0. [DOI] [PubMed] [Google Scholar]
- 40.Song LB, Zeng MS, Liao WT, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66(12):6225–6232. doi: 10.1158/0008-5472.CAN-06-0094. [DOI] [PubMed] [Google Scholar]
- 41.Vonlanthen S, Heighway J, Altermatt HJ, et al. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer. 2001;84(10):1372–1376. doi: 10.1054/bjoc.2001.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns A, van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999;13(20):2678–2690. doi: 10.1101/gad.13.20.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hogg RP, Honorio S, Martinez A, et al. Frequent 3p allele loss and epigenetic inactivation of the RASSF1A tumour suppressor gene from region 3p21.3 in head and neck squamous cell carcinoma. Eur J Cancer. 2002;38(12):1585–1592. doi: 10.1016/s0959-8049(01)00422-1. [DOI] [PubMed] [Google Scholar]
- 44.Morrissey C, Martinez A, Zatyka M, et al. Epigenetic inactivation of the RASSF1A 3p21.3 tumor suppressor gene in both clear cell and papillary renal cell carcinoma. Cancer Res. 2001;61(19):7277–7281. [PubMed] [Google Scholar]
- 45.Sellar GC, Watt KP, Rabiasz GJ, et al. OPCML at 11q25 is epigenetically inactivated and has tumor-suppressor function in epithelial ovarian cancer. Nat Genet. 2003;34(3):337–343. doi: 10.1038/ng1183. [DOI] [PubMed] [Google Scholar]
- 46.Bagchi A, Papazoglu C, Wu Y, et al. CHD5 is a tumor suppressor at human 1p36. Cell. 2007;128(3):459–475. doi: 10.1016/j.cell.2006.11.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.