Abstract
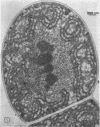
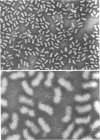
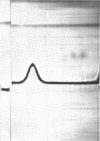
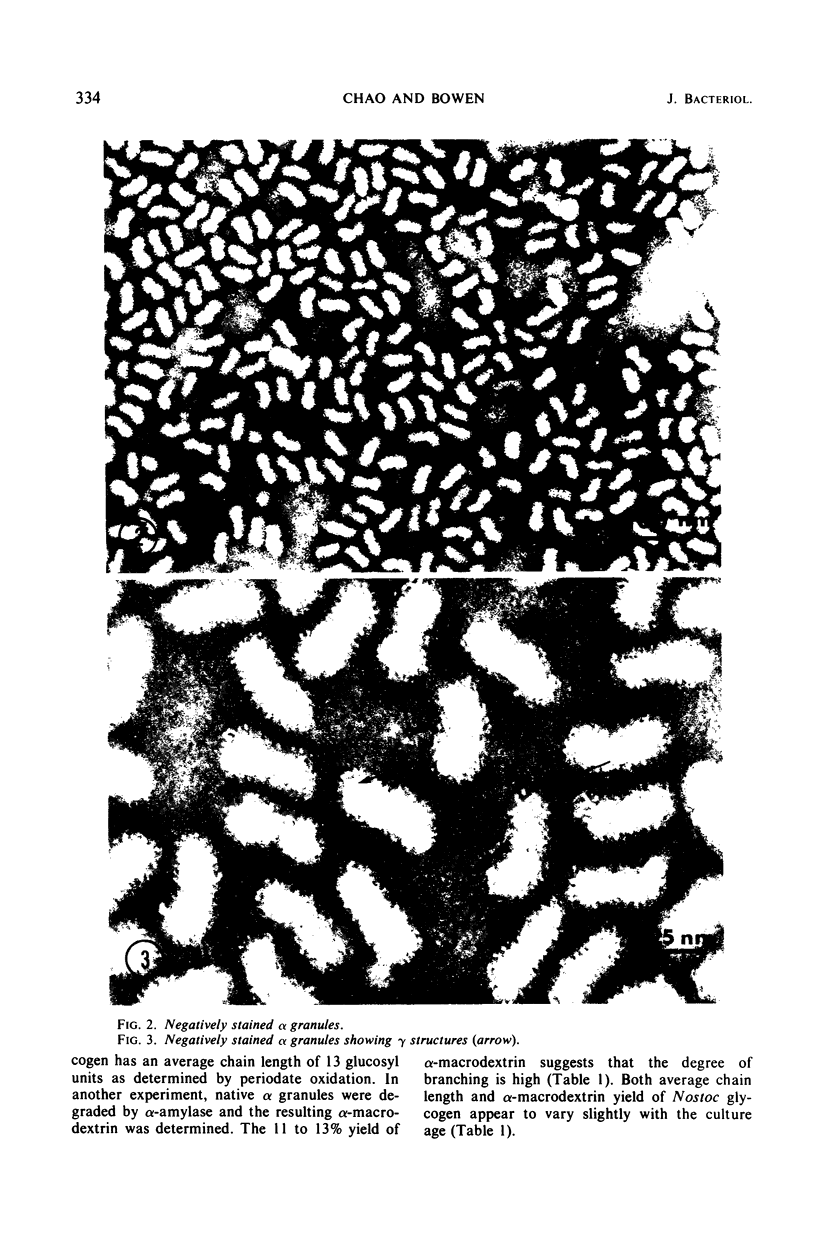
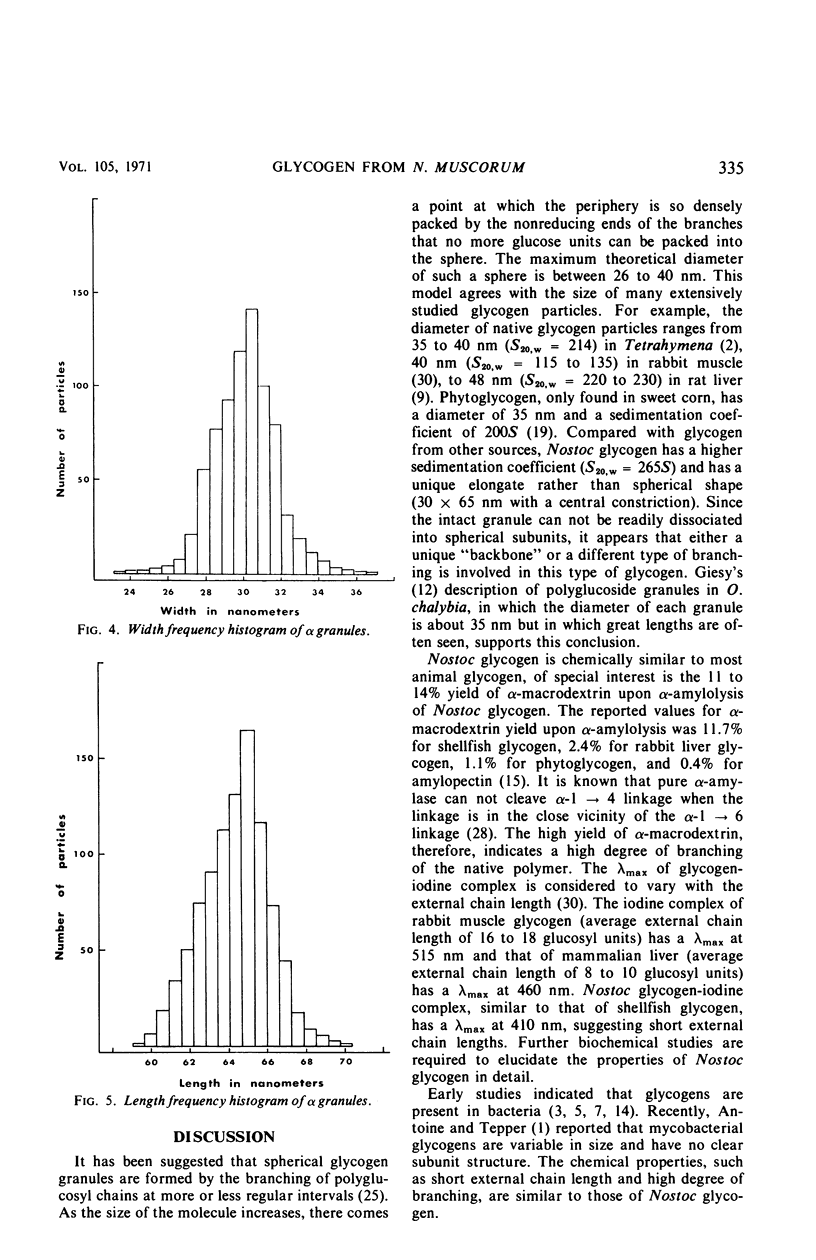
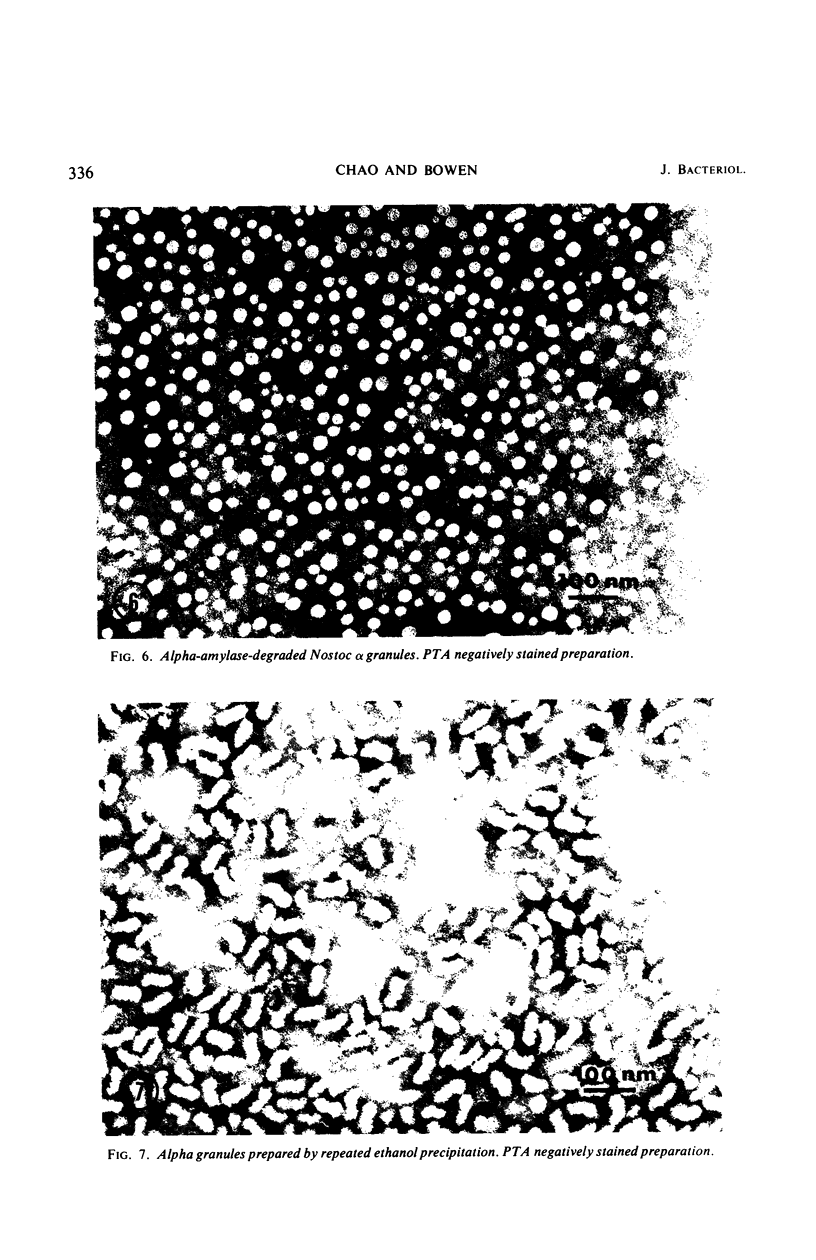
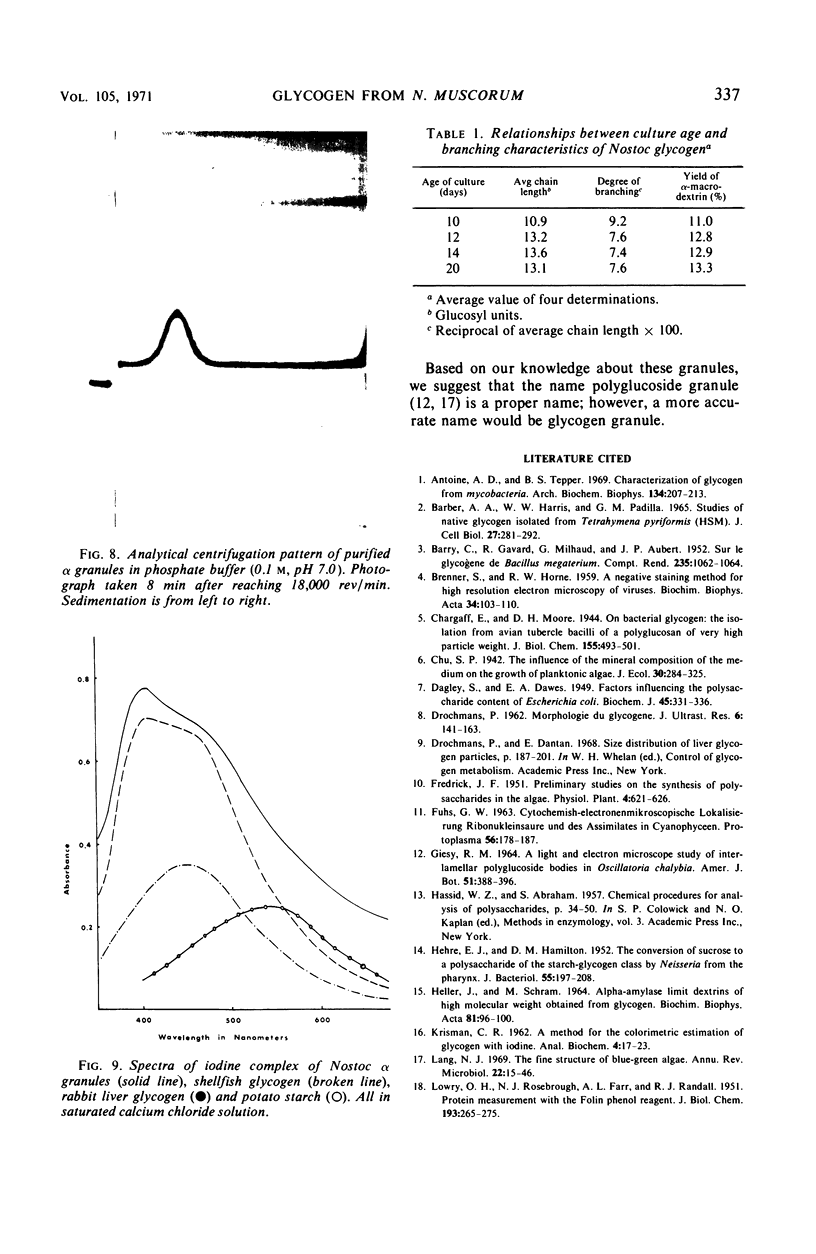
Alpha granules were isolated from a blue-green alga, Nostoc muscorum, in large quantities and high purity by sucrose density gradient centrifugation. Sodium deoxycholate was used to eliminate membrane contamination. Isolated α granules from this species have average dimension of 31 nm in width and 65 nm in length. Each α granule consists of two equal parts. Attempts to dissociate the intact granule under mild conditions into relatively large subunits did not succeed. Analytical centrifugation of the α granules yields a sedimentation coefficient of 265S (S20, w). Chemical analysis reveals that α granules contain highly branched polyglucosyl units with short external chain lengths.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine A. D., Tepper B. S. Characterization of glycogens from mycobacteria. Arch Biochem Biophys. 1969 Oct;134(1):207–213. doi: 10.1016/0003-9861(69)90267-7. [DOI] [PubMed] [Google Scholar]
- BARRY C., GAVARD R., MILHAUD G., AUBERT J. P. Sur le glycogène de Bacillus megatherium. C R Hebd Seances Acad Sci. 1952 Nov 3;235(18):1062–1064. [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Barber A. A., Harris W. W., Padilla G. M. Studies of native glycogen isolated from synchronized Tetrahymena pyriformis (HSM). J Cell Biol. 1965 Nov;27(2):281–292. doi: 10.1083/jcb.27.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., DAWES E. A. Factors influencing the polysaccharide content of Escherichia coli. Biochem J. 1949;45(3):331–337. doi: 10.1042/bj0450331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROCHMANS P. [Morphology of glycogen. Electron microscopic study of the negative stains of particulate glycogen]. J Ultrastruct Res. 1962 Apr;6:141–163. doi: 10.1016/s0022-5320(62)90050-3. [DOI] [PubMed] [Google Scholar]
- Hehre E. J., Hamilton D. M. The Conversion of Sucrose to a Polysaccharide of the Starch-Glycogen Class by Neisseria from the Pharynx. J Bacteriol. 1948 Feb;55(2):197–208. doi: 10.1128/jb.55.2.197-208.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISMAN C. R. A method for the colorimetric estimation of glycogen with iodine. Anal Biochem. 1962 Jul;4:17–23. doi: 10.1016/0003-2697(62)90014-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang N. J. The fine structure of blue-green algae. Annu Rev Microbiol. 1968;22:15–46. doi: 10.1146/annurev.mi.22.100168.000311. [DOI] [PubMed] [Google Scholar]
- MADSEN N. B., CORI C. F. The binding of glycogen and phosphorylase. J Biol Chem. 1958 Dec;233(6):1251–1256. [PubMed] [Google Scholar]
- NIKLOWITZ W., DREWS G. Beiträge zur Cytologie der Blaualgen. IV. Vergleichende elektronenmikroskopische Untersuchungen zur Substruktur einiger Hormogonales. Arch Mikrobiol. 1957;27(2):150–165. [PubMed] [Google Scholar]
- RIS H., SINGH R. N. Electron microscope studies on blue-green algae. J Biophys Biochem Cytol. 1961 Jan;9:63–80. doi: 10.1083/jcb.9.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS P. J., WHELAN W. J. The mechanism of carbohydrase action. 5. Action of human salivary alpha-amylase on amylopectin and glycogen. Biochem J. 1960 Aug;76:246–253. doi: 10.1042/bj0760246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanson J. C., Drochmans P. Rabbit skeletal muscle glycogen. A morphological and biochemical study of glycogen beta-particles isolated by the precipitation-centrifugation method. J Cell Biol. 1968 Jul;38(1):130–150. doi: 10.1083/jcb.38.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]