Abstract
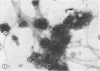
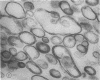
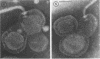
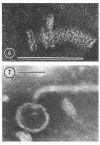
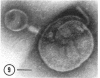
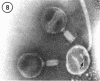
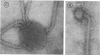
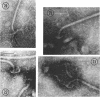
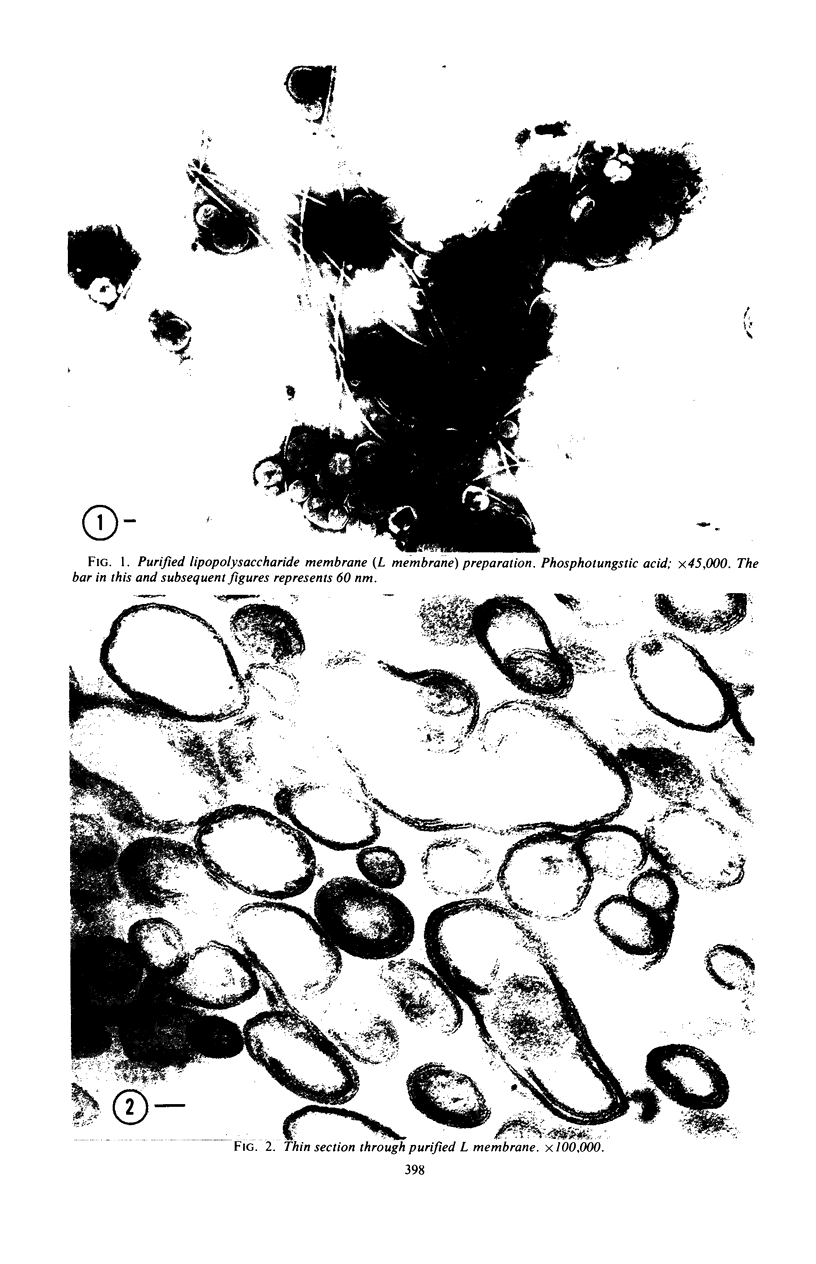
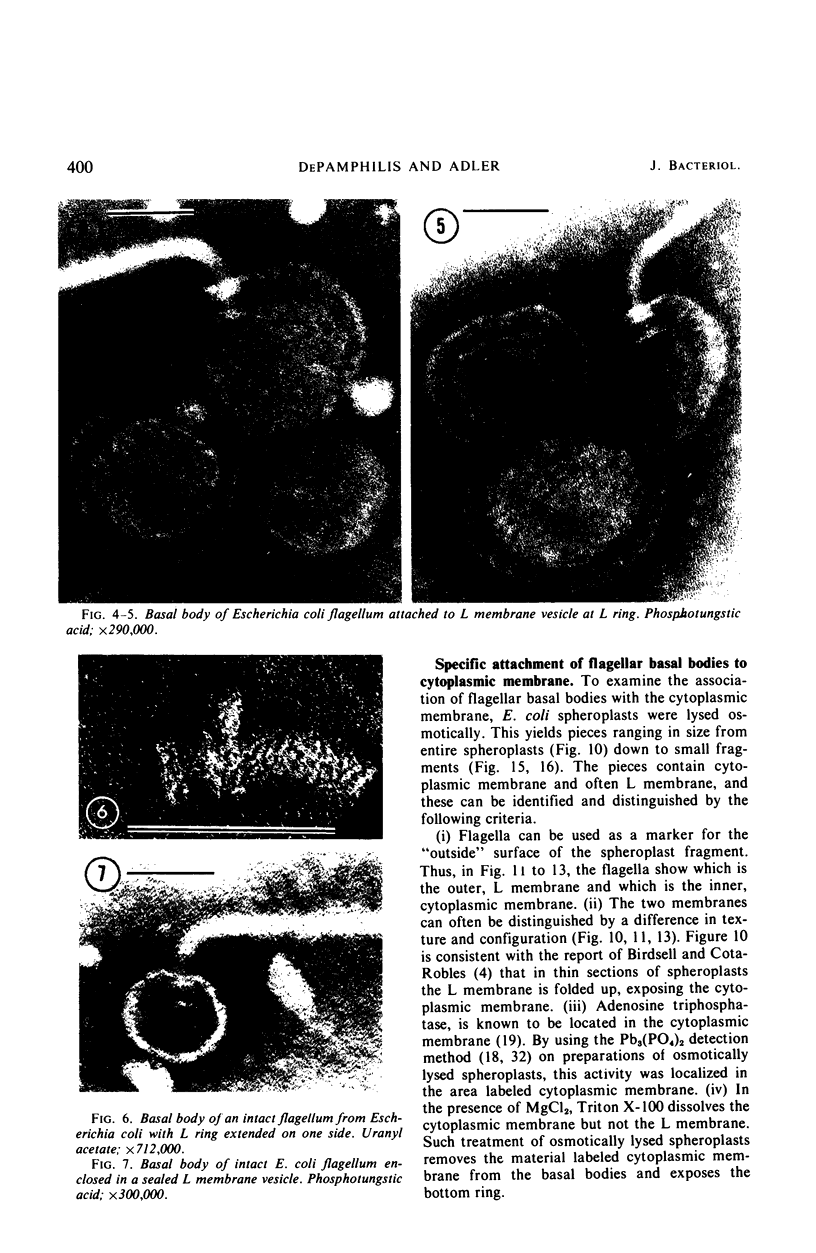
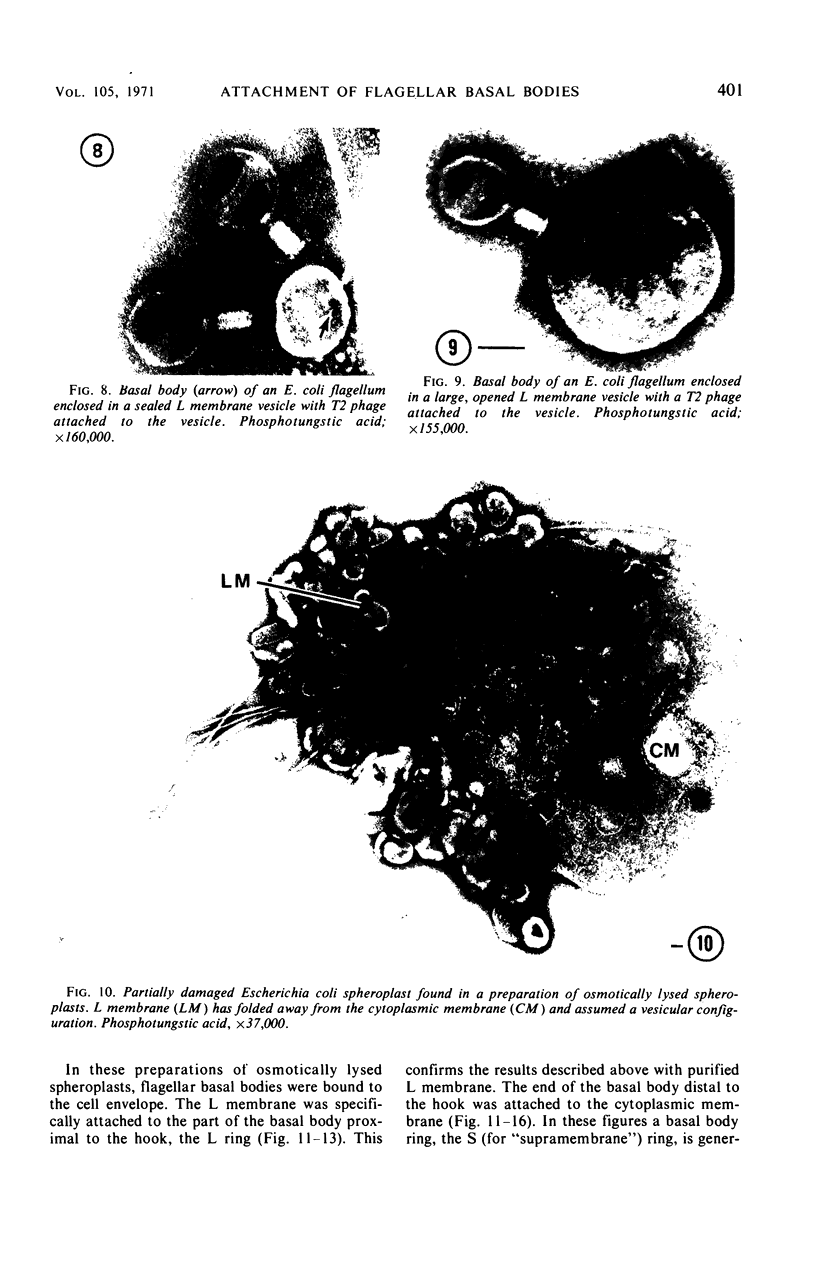
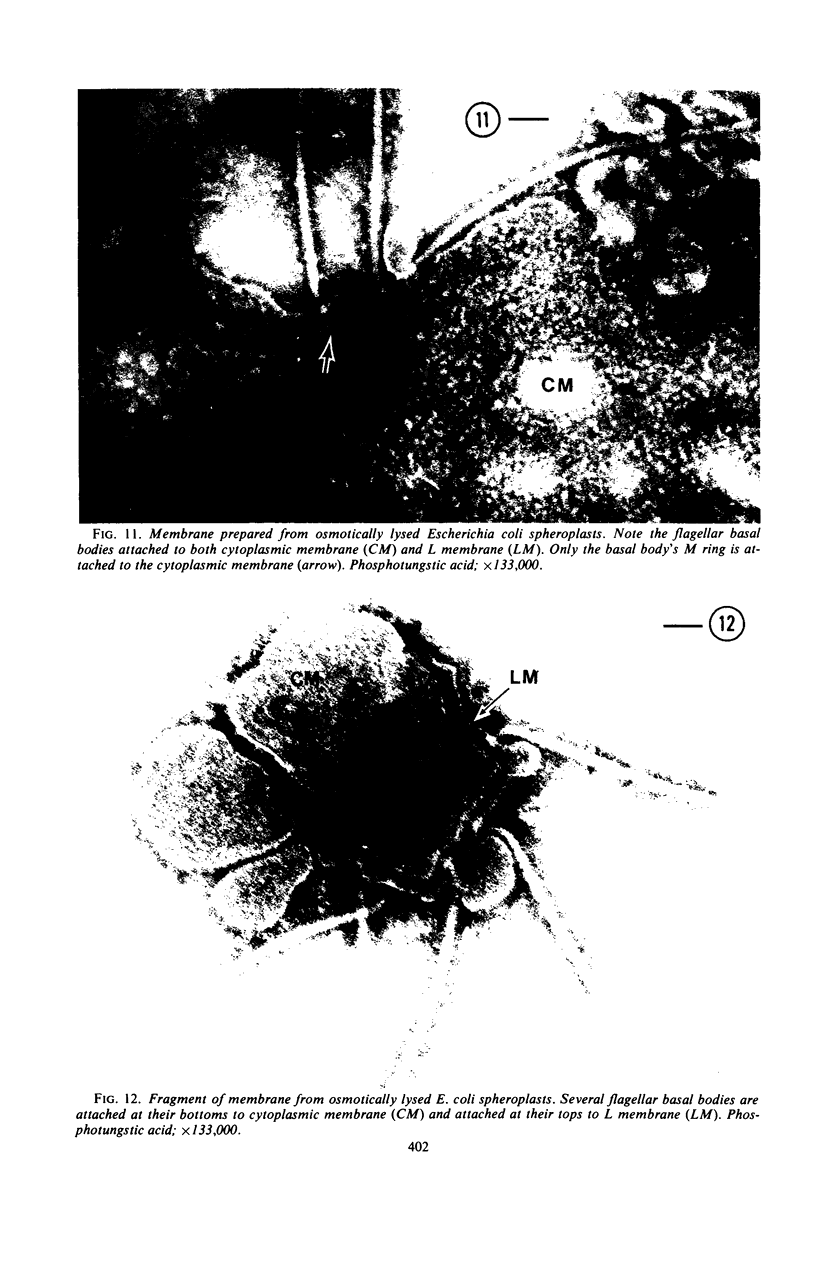
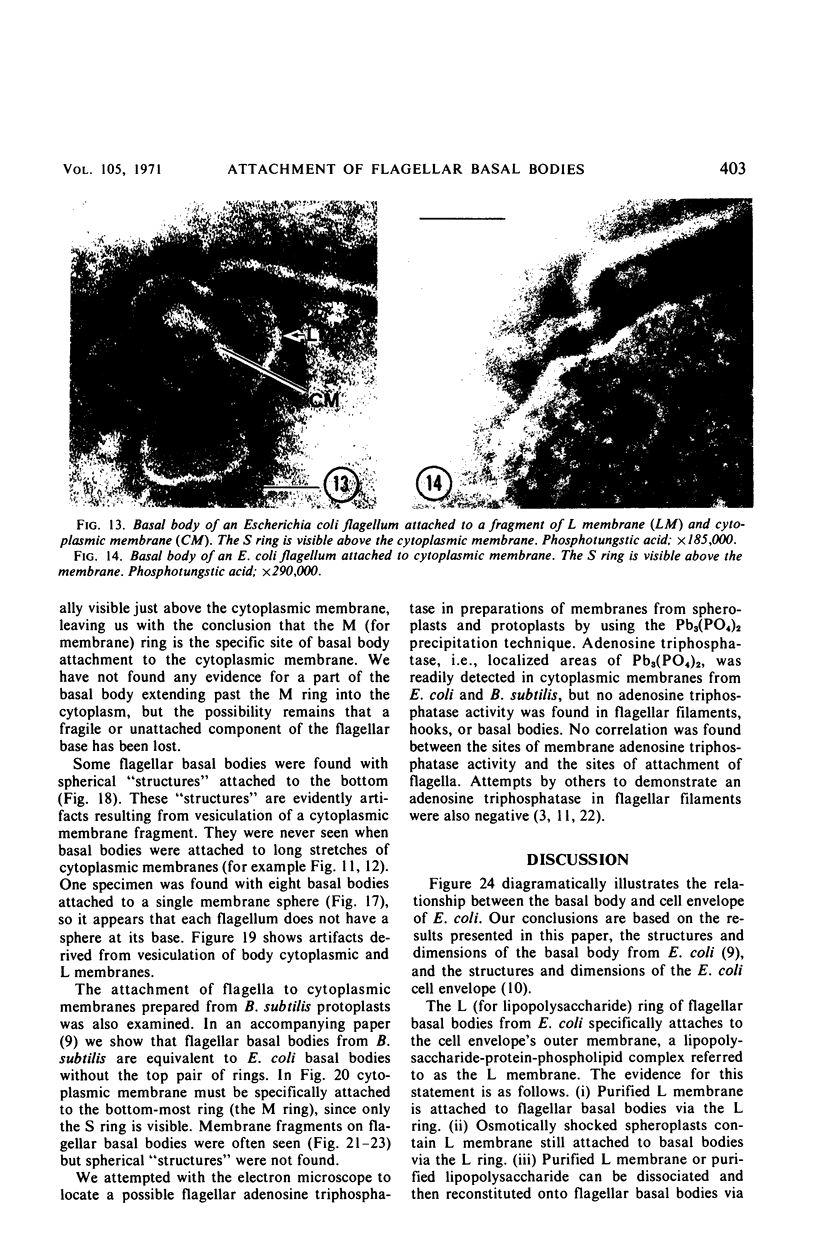
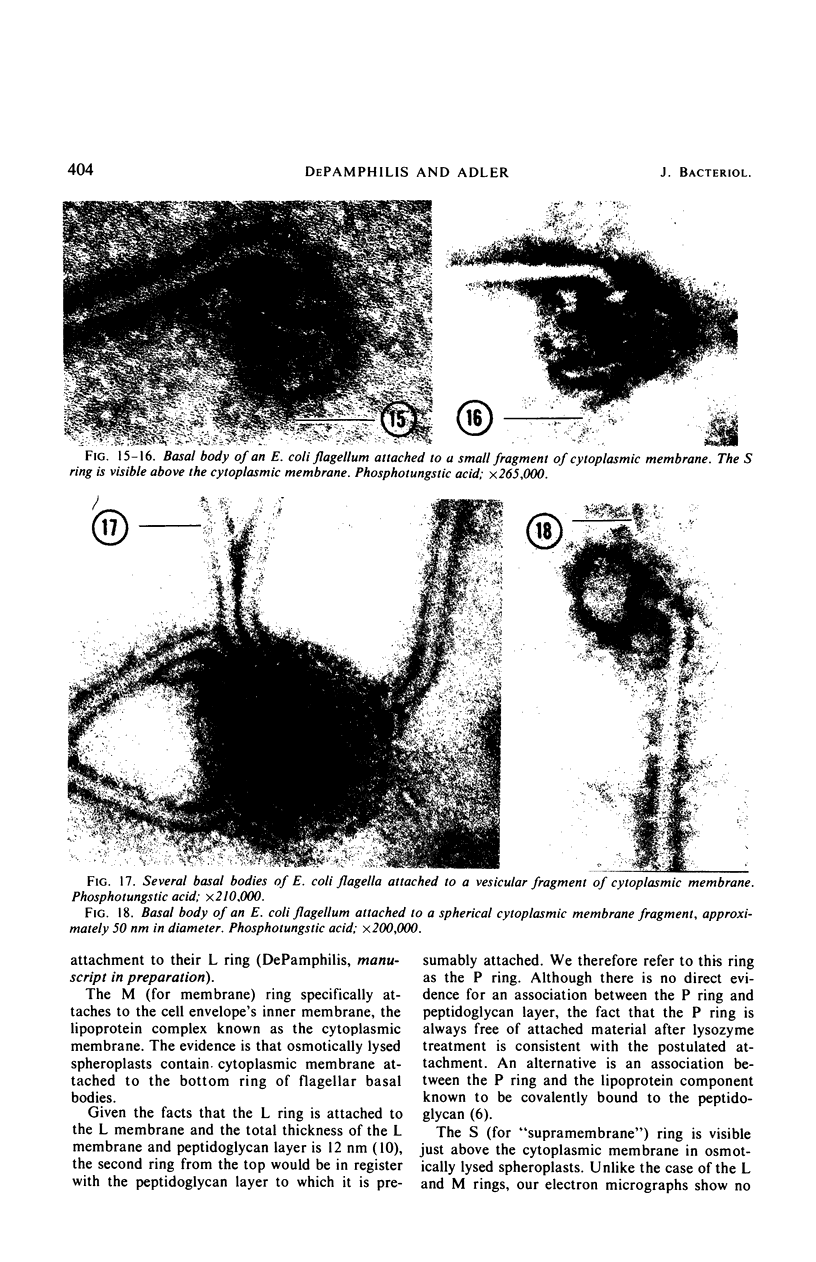
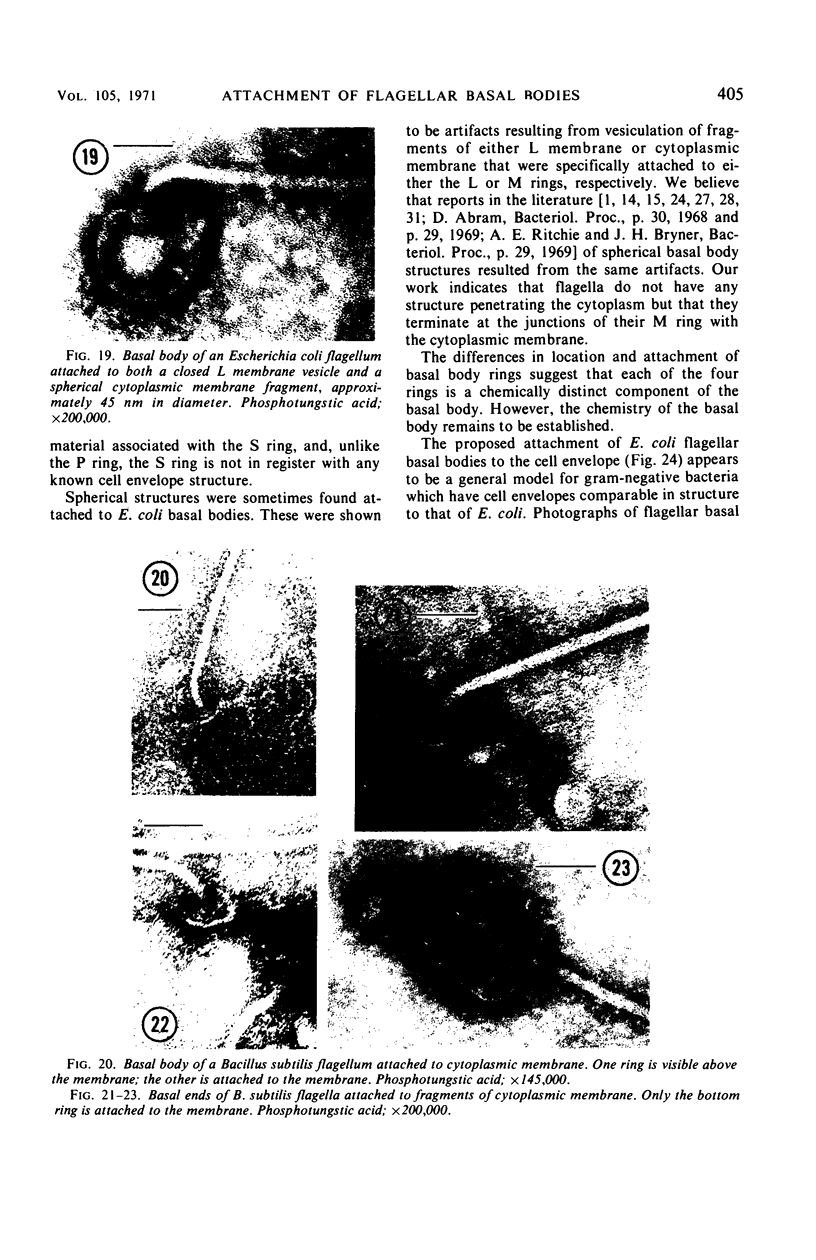
A procedure is described for the purification of the Escherichia coli outer membrane (lipopolysaccharide or L membrane) with flagella still attached. The resulting lipopolysaccharide membrane was in the form of vesicles that had a trilaminar structure in thin section and contained about 55% lipopolysaccharide and 45% protein. T2 or T4 phage preadsorbed to E. coli were found attached to the purified lipopolysaccharide membrane. Flagella were bound to the purified lipopolysaccharide membrane specifically at the basal body ring closest to the hook (the L ring). The cytoplasmic membrane in preparations from osmotically lysed E. coli spheroplasts or Bacillus subtilis protoplasts was specifically attached to flagella at the basal body ring farthest from the hook (the M ring). In the E. coli preparation, lipopolysaccharide membrane was also present and was attached to the L ring. From these data and a knowledge of the structure and dimensions of the E. coli flagellar basal body and cell envelope, a model for flagellar attachment is deduced.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram D., Koffler H., Vatter A. E. Basal structure and attachment of flagella in cells of Proteus vulgaris. J Bacteriol. 1965 Nov;90(5):1337–1354. doi: 10.1128/jb.90.5.1337-1354.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram D., Vatter A. E., Koffler H. Attachment and structural features of flagella of certain bacilli. J Bacteriol. 1966 May;91(5):2045–2068. doi: 10.1128/jb.91.5.2045-2068.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow G. H., Blum J. J. On the "Contractility" of Bacterial Flagellae. Science. 1952 Nov 21;116(3021):572–572. doi: 10.1126/science.116.3021.572-a. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Lysis of spheroplasts of Escherichia coli by a non-ionic detergent. Biochem Biophys Res Commun. 1968 May 10;31(3):438–446. doi: 10.1016/0006-291x(68)90496-8. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bazire G., London J. Basal organelles of bacterial flagella. J Bacteriol. 1967 Aug;94(2):458–465. doi: 10.1128/jb.94.2.458-465.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Fine structure and isolation of the hook-basal body complex of flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):384–395. doi: 10.1128/jb.105.1.384-395.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):376–383. doi: 10.1128/jb.105.1.376-383.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M. Genetic studies of paralyzed mutant in Salmonella. I. Genetic fine structure of the mot loci in Salmonella typhimurium. Genetics. 1966 Sep;54(3):715–726. doi: 10.1093/genetics/54.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., KERRIDGE D., HORNE R. W. THE FINE STRUCTURE AND MODE OF ATTACHMENT OF THE SHEATHED FLAGELLUM OF VIBRIO METCHNIKOVII. J Cell Biol. 1963 Aug;18:327–336. doi: 10.1083/jcb.18.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRACE J. B. Some observations on the flagella and blepharoplasts of Spirillum and Vibrio spp. J Gen Microbiol. 1954 Apr;10(2):325–327. doi: 10.1099/00221287-10-2-325. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Hoeniger J. F., Van Iterson W., Van Zanten E. N. Basal bodies of bacterial flagella in Proteus mirabilis. II. Electron microscopy of negatively stained material. J Cell Biol. 1966 Dec;31(3):603–618. doi: 10.1083/jcb.31.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Marchesi V. T., Palade G. E. The localization of Mg-Na-K-activated adenosine triphosphatase on red cell ghost membranes. J Cell Biol. 1967 Nov;35(2):385–404. doi: 10.1083/jcb.35.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation and properties of outer and cytoplasmic membranes in Escherichia coli. Biochim Biophys Acta. 1969;193(2):268–276. doi: 10.1016/0005-2736(69)90188-6. [DOI] [PubMed] [Google Scholar]
- Morowitz H. J., Terry T. M. Characterization of the plasma membrane of Mycoplasma laidlawii. V. Effects of selective removal of protein and lipid. Biochim Biophys Acta. 1969 Jul 15;183(2):276–294. doi: 10.1016/0005-2736(69)90084-4. [DOI] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- PEASE P. Some observations upon the development and mode of attachment of the flagella in Vibrio and Spirillum species. Exp Cell Res. 1956 Feb;10(1):234–237. doi: 10.1016/0014-4827(56)90092-1. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara J. The root of flagella of Vibrio cholerae. Jpn J Microbiol. 1965 Mar;9(1):49–54. doi: 10.1111/j.1348-0421.1965.tb00274.x. [DOI] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaituzis Z., Doetsch R. N. Relationship between cell wall, cytoplasmic membrane, and bacterial motility. J Bacteriol. 1969 Oct;100(1):512–521. doi: 10.1128/jb.100.1.512-521.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Iterson W., Hoeniger J. F., Van Zanten E. N. Basal bodies of bacterial flagella in Proteus mirabilis. I. Electron microscopy of sectioned material. J Cell Biol. 1966 Dec;31(3):585–601. doi: 10.1083/jcb.31.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]