Abstract
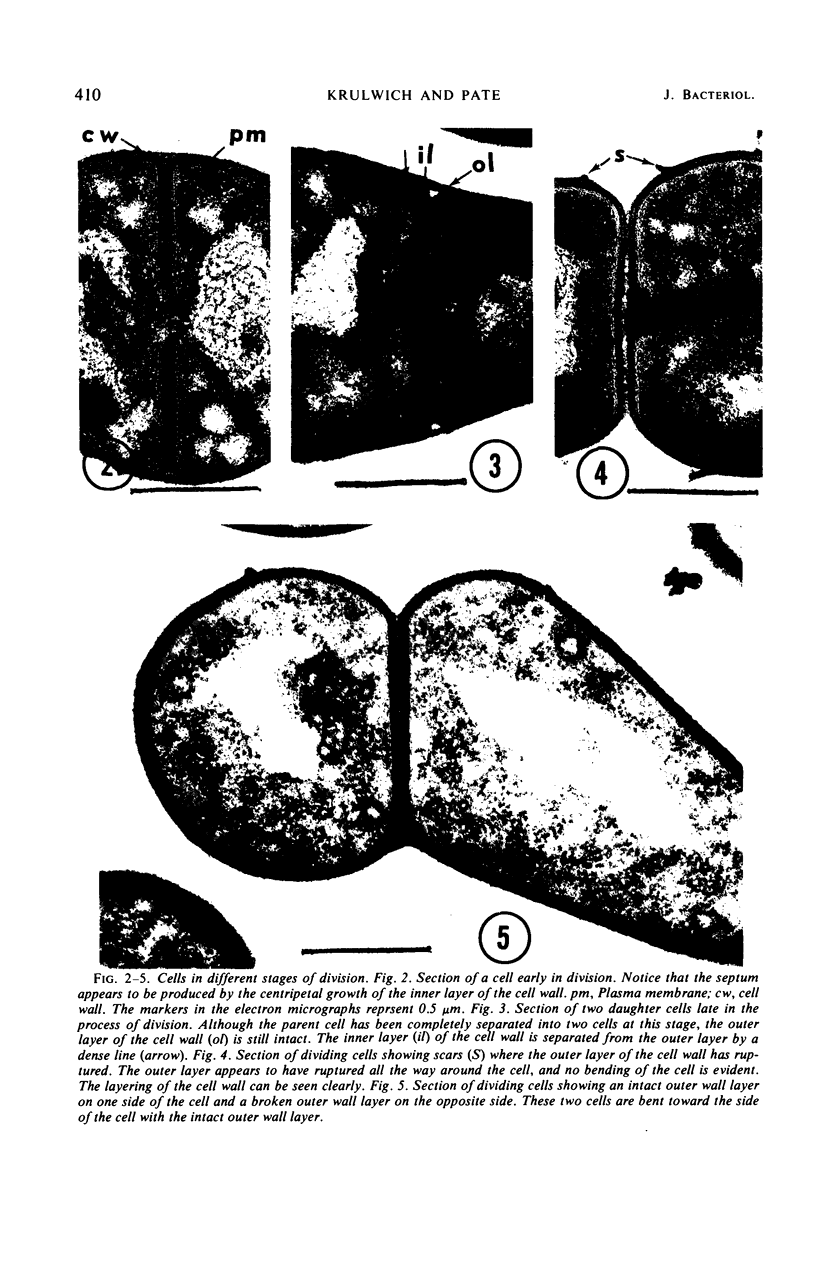
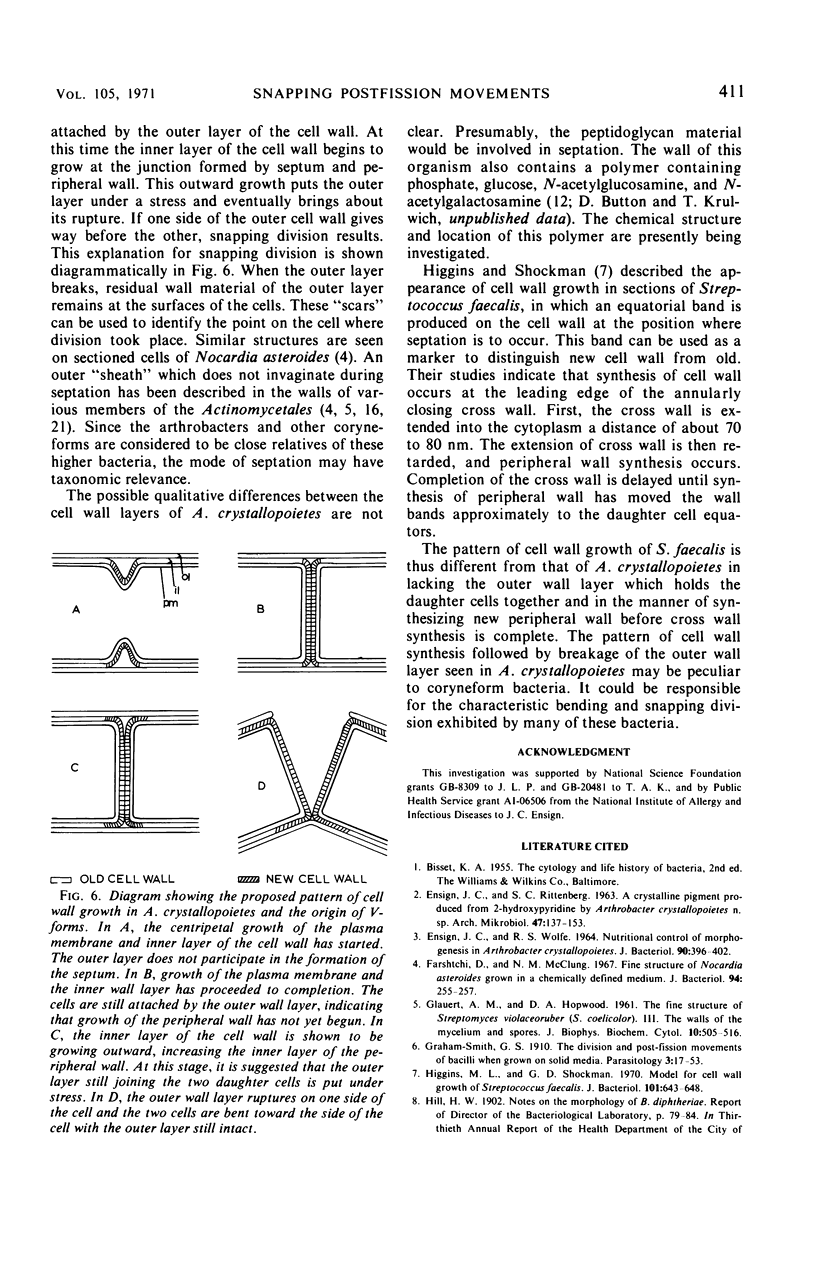
The ultrastructure of dividing rod-stage cells of Arthrobacter crystallopoietes was examined by electron microscopy. The cell walls consist of two layers. During cell division, the inner layer invaginates to form the septum. The outer layer does not participate in septum formation. After septum formation is completed, the two daughter cells remain attached by the outer layer of the cell wall. It appears that localized rupture of the outer layer during further wall growth is responsible for the phenomenon known as “snapping division” or “snapping postfission movement.”
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ENSIGN J. C., RITTENBERG S. C. A CRYSTALLINE PIGMENT PRODUCED FROM 2-HYDROXYPYRIDINE BY ARTHROBACTER CRYSTALLOPOIETES N.SP. Arch Mikrobiol. 1963 Dec 10;47:137–153. doi: 10.1007/BF00422519. [DOI] [PubMed] [Google Scholar]
- Farshtchi D., McClung N. M. Fine structure of Nocardia asteroides grown in a chemically defined medium. J Bacteriol. 1967 Jul;94(1):255–257. doi: 10.1128/jb.94.1.255-257.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces violaceoruber (S. coelicolor). III. The walls of the mycelium and spores. J Biophys Biochem Cytol. 1961 Aug;10:505–516. doi: 10.1083/jcb.10.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman H., Frank M. E. Time-Lapse Photomicrography of Lashing, Flexing, and Snapping Movements in Escherichia coli and Corynebacterium Microcultures. J Bacteriol. 1965 Sep;90(3):789–795. doi: 10.1128/jb.90.3.789-795.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANCOURT M. W., LECHEVALIER H. A. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF SPINY CONIDIA IN SPECIES OF STREPTOMYCES. Can J Microbiol. 1964 Jun;10:311–316. doi: 10.1139/m64-042. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SGUROS P. L. New approach to the mode of formation of classical morphological configurations by certain coryneform bacteria. J Bacteriol. 1957 Dec;74(6):707–709. doi: 10.1128/jb.74.6.707-709.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARR M. P., KUHN D. A. On the origin of V-forms in Arthrobacter atrocyaneus. Arch Mikrobiol. 1962;42:289–298. doi: 10.1007/BF00422046. [DOI] [PubMed] [Google Scholar]
- Wildermuth H., Hopwood D. A. Septation during sporulation in Streptomyces coelicolor. J Gen Microbiol. 1970 Jan;60(1):51–59. doi: 10.1099/00221287-60-1-51. [DOI] [PubMed] [Google Scholar]