Abstract
Immune thrombocytopenia purpura (ITP) is a bleeding disorder in which platelet-specific autoantibodies cause a loss of platelets. In a subset of patients with ITP and infected with Helicobacter pylori, the number of platelets recovers after eradication of H. pylori. To examine the role of H. pylori infection in the pathogenesis of ITP, the response of 34 ITP patients to treatment with a standard H. pylori eradication regimen, irrespective of whether they were infected with H. pylori, was evaluated. Eradication of H. pylori was achieved in all H. pylori–positive patients, and a significant increase in platelets was observed in 61% of these patients. By contrast, none of the H. pylori–negative patients showed increased platelets. At baseline, monocytes from the H. pylori–positive patients exhibited an enhanced phagocytic capacity and low levels of the inhibitory Fcγ receptor IIB (FcγRIIB). One week after starting the H. pylori eradication regimen, this activated monocyte phenotype was suppressed and improvements in autoimmune and platelet kinetic parameters followed. Modulation of monocyte FcγR balance was also found in association with H. pylori infection in individuals who did not have ITP and in mice. Our findings strongly suggest that the recovery in platelet numbers observed in ITP patients after H. pylori eradication is mediated through a change in FcγR balance toward the inhibitory FcγRIIB.
Introduction
Immune thrombocytopenia purpura (ITP) is an autoimmune disorder caused by increased platelet clearance by anti-platelet autoantibodies (1). In 1998, Gasbarrini et al. reported increase in platelet count in ITP patients infected with Helicobacter pylori after successful eradication of this bacterium (2). Recent accumulating evidence in Italy and Japan indicates that the eradication of H. pylori is effective in increasing the platelet count in nearly half of H. pylori–infected patients with idiopathic ITP (3, 4). In addition, a recent report showed that this platelet response lasts for years and cases of relapse are few (5). Based on its efficacy, good safety profile, and low cost, H. pylori eradication therapy for adult ITP is becoming very popular in several countries. Some investigators have suggested that the efficacy of H. pylori eradication in ITP patients may be mediated by H. pylori–independent mechanisms, such as immunomodulatory effects of the drugs used for the regimen (3), but we recently reported its complete lack of efficacy in H. pylori–uninfected ITP patients in a prospective study in which the patients were treated with a standard H. pylori eradication regimen irrespective of their H. pylori infection status (6). This finding clearly indicates that the platelet recovery observed in ITP patients after the eradication regimen results from the disappearance of H. pylori itself.
Several hypotheses have been proposed regarding the mechanism by which H. pylori might induce the development of ITP. One is that Abs to H. pylori components cross-react with platelet surface antigens. In this regard, Takahashi et al. reported that platelet eluates from H. pylori–positive ITP patients recognized the cytotoxin-associated gene A (CagA), one of the H. pylori–derived proteins that determine bacterial virulence (7), although another group demonstrated that platelet eluates from H. pylori–positive ITP patients that reacted with glycoprotein IIb/IIIa (GPIIb/IIIa) or GPIb failed to recognize H. pylori antigens (8). Another potential mechanism is modulation of the host’s immune system by H. pylori in a manner that promotes the emergence of autoreactive B cells (9). However, no significant difference between H. pylori–positive and H. pylori–negative individuals has been found for non–organ-specific autoantibody responses, such as anti-nuclear, anti-microsome, or anti–smooth muscle Abs (10). Despite these findings, the role of H. pylori infection in the pathogenesis of ITP remains obscure. These previous studies focused on anti-platelet Ab production in association with H. pylori infection, but potential effects of H. pylori infection on the platelet clearance process in ITP patients have not been assessed. In this study, to elucidate the mechanism responsible for platelet recovery in ITP patients after the successful eradication of H. pylori, we conducted a prospective study in which factors potentially associated with the pathogenic processes of ITP, i.e., autoimmune responses to the major platelet antigen GPIIb/IIIa, parameters associated with platelet turnover, and the phenotypic and functional properties of phagocytes, were serially measured in ITP patients who were treated with a standard eradication regimen, irrespective of their H. pylori infection status. Our findings demonstrate that the platelet recovery observed in ITP patients after H. pylori eradication is associated with modulation of the monocyte Fcγ receptor balance toward the inhibitory Fcγ receptor IIB (FcγRIIB).
Results
Patient characteristics.
Thirty-four consecutive patients with ITP (14 males and 20 females, aged 24 to 73 yr) were enrolled in this open-label, prospective study. H. pylori infection was detected in 23 patients (68%). Comparison of the pretreatment clinical characteristics of the H. pylori–positive and –negative patients (Table 1) showed the H. pylori–positive patients tended to be older than the H. pylori–negative patients (P = 0.08). Some patients took low-dose prednisolone (≤7.5 mg daily), but there was no difference in the frequency or the mean dosage between H. pylori–positive and –negative patients. A history of splenectomy was significantly less frequent in the H. pylori–positive patients compared with the H. pylori–negative patients, indicating that refractory cases were more common in the H. pylori–negative patients.
Table 1 .
Clinical characteristics of 34 ITP patients according to the presence or absence of H. pylori infection
Immunologic and platelet turnover parameters before treatment in ITP patients with and without H. pylori infection.
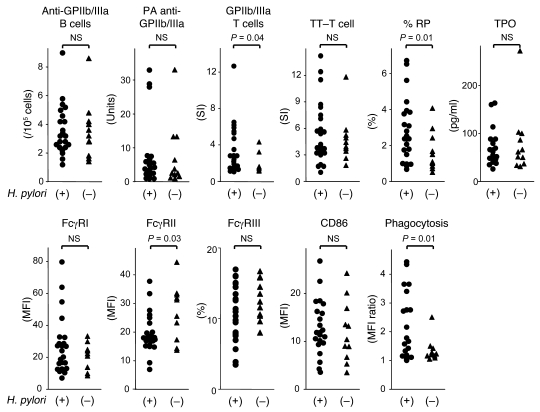
A total of 11 immunologic and platelet turnover parameters, including anti-GPIIb/IIIa Ab–producing B cells, platelet-associated anti-GPIIb/IIIa Abs, T cell responses induced by GPIIb/IIIa and tetanus toxoid, the proportion of reticulated platelets, the circulating thrombopoietin (TPO) level, the relative expression levels of FcγRI, FcγRII (FcγRIIA plus FcγRIIB), FcγRIII, and CD86 on circulating monocytes, and the nonspecific phagocytosis of circulating monocytes were compared between ITP patients with and without H. pylori infection (Figure 1). We evaluated the proportion of monocytes expressing FcγRIII instead of its expression level because only a small subset of the peripheral blood monocytes expressed this FcγR.
Figure 1. Immunologic and platelet turnover parameters prior to treatment in ITP patients with or without H. pylori infection.
The anti-GPIIb/IIIa Ab–producing B cells, platelet-associated (PA) anti-GPIIb/IIIa Abs, GPIIb/IIIa-specific T cell response, tetanus toxoid–specific (TT-specific) T cell response, proportion of reticulated platelets (%RP), circulating TPO level, expression levels of FcγRI and FcγRII (FcγRIIA plus FcγRIIB) on monocytes, proportion of FcγRIII-positive monocytes, expression level of CD86 on monocytes, and nonspecific phagocytosis of monocytes were compared among 23 ITP patients infected with H. pylori and 11 ITP patients who were not infected with H. pylori. Expression of FcγRII was examined using mAb clone FLI8.26, which reacts with both FcγRIIA and FcγRIIB. The differences between the 2 groups were analyzed using the Mann-Whitney U test.
There was no difference in the frequency of anti-GPIIb/IIIa Ab–producing B cells or platelet-associated anti-GPIIb/IIIa Ab levels between the 2 groups. In contrast, H. pylori–positive patients showed a more prominent T cell response to GPIIb/IIIa compared with H. pylori–negative patients, and there was no difference in the degree of the response to tetanus toxoid, an irrelevant foreign recall antigen. The proportion of reticulated platelets was significantly greater in the H. pylori–positive patients than in the H. pylori–negative patients. In circulating monocytes, the expression of FcγRII, including both activating FcγRIIA and inhibitory FcγRIIB, was lower in the H. pylori–positive patients than the H. pylori–negative patients. Moreover, the expression of activating FcγRI tended to be upregulated in the H. pylori–positive patients (P = 0.06). Finally, monocytes from the H. pylori–positive patients exhibited an enhanced phagocytic capacity compared with those from the H. pylori–negative patients, suggesting an activated monocyte phenotype predominated in the H. pylori–positive patients.
Platelet response to the H. pylori eradication regimen.
All 34 patients completed a 7-day standard eradication regimen that consisted of amoxicillin, clarithromycin, and lansoprazole regardless of whether they were positive for H. pylori infection. Adverse events potentially related to the therapy were observed in 10 patients (29%): abdominal pain and/or diarrhea in 9 and skin rash in 1. All the symptoms resolved quickly after the regimen ended. Eradication was successful in all 23 H. pylori–positive patients, and 14 (61%) of them were classified as responders. In contrast, none of the 11 H. pylori–negative patients showed an increased platelet count. These frequencies were significantly different (P = 0.001). The platelet counts 0, 12, and 24 wk after initiation of the eradication regimen in H. pylori–positive responders and nonresponders, and in H. pylori–negative patients are shown in Figure 2. Some H. pylori–positive nonresponders showed a slight increase in platelet count, but almost no fluctuation in platelet count was observed in the H. pylori–negative patients. During the 24-wk period, prednisolone dosage was reduced in 2 responders at 12 wk (7.5 to 5 mg and 5 to 2.5 mg), but the stable dosage was given in the remaining patients.
Figure 2. Serial platelet counts before and after initiation of the H. pylori eradication regimen in 14 H. pylori–positive ITP responders, 9 H. pylori–positive ITP nonresponders, and 11 H. pylori–negative ITP nonresponders.
Changes in the absolute values 12 and 24 wk from the baseline value taken at wk 0 were assessed by paired t test. *P < 0.01 compared with wk 0.
Serial changes in immunologic and platelet turnover parameters after H. pylori eradication.
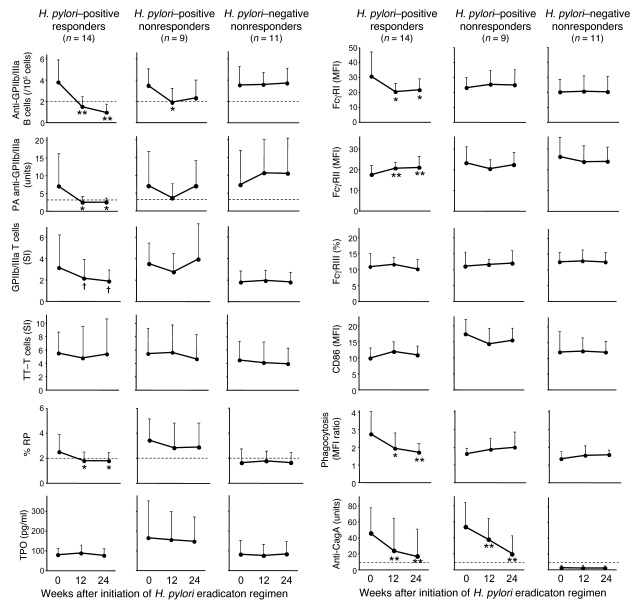
Immunologic and platelet turnover parameters measured prior to treatment were serially examined 12 and 24 wk after initiation of the eradication regimen in the H. pylori–positive responders and nonresponders and in the H. pylori–negative patients (Figure 3). The anti-GPIIb/IIIa Ab–producing B cells and platelet-associated anti-GPIIb/IIIa Abs were significantly reduced at 12 and 24 wk in the H. pylori–positive responders. The proportion of reticulated platelets was also significantly reduced in the H. pylori–positive responders. Similar but weak trends were observed in the H. pylori–positive nonresponders but not in the H. pylori–negative patients. The peripheral blood T cell response to GPIIb/IIIa tended to be suppressed in the H. pylori–positive responders (P = 0.09), while there was no fluctuation in the response to tetanus toxoid. In monocytes from H. pylori–positive responders, their baseline upregulated FcγRI expression decreased and the downregulated FcγRII (FcγRIIA plus FcγRIIB) expression increased to the levels of monocytes from H. pylori–negative patients after H. pylori eradication. In contrast, the proportion of FcγRIII-positive monocytes and CD86 expression levels on monocytes were stable in all 3 groups during the study period. The enhanced phagocytic capacity of monocytes in H. pylori–positive responders was significantly reduced after H. pylori eradication, reaching the level of H. pylori–negative patients. These changes in the monocyte phenotype were not observed in the H. pylori–positive nonresponders or in the H. pylori–negative patients. The IgG anti-CagA Ab level gradually reduced following the eradication regimen in the H. pylori–positive patients irrespective of the platelet response, but samples were still positive for anti-CagA Ab even at 12 and 24 wk, when the platelet count had nearly fully recovered.
Figure 3. Serial measurements of immunologic and platelet turnover parameters before and after the H. pylori eradication in 14 H. pylori–positive ITP responders, 9 H. pylori–positive ITP nonresponders, and 11 H. pylori–negative ITP nonresponders.
Anti-GPIIb/IIIa Ab–producing B cells, platelet-associated anti-GPIIb/IIIa Abs, GPIIb/IIIa-specific T cell response, tetanus toxoid–specific T cell response, proportion of reticulated platelets, circulating TPO level, expression levels of FcγRI and FcγRII (FcγRIIA plus FcγRIIB) on monocytes, proportion of FcγRIII-positive monocytes, expression level of CD86 on monocytes, nonspecific phagocytosis of circulating monocytes, and anti-CagA Ab levels were measured prior to treatment and 12 and 24 wk after initiation of the H. pylori eradication regimen. Expression of FcγRII was examined using mAb clone FLI8.26, which reacts with both FcγRIIA and FcγRIIB. Results are shown as the mean + SD. Changes in the values at 12 and 24 wk from the baseline value measured at wk 0 were assessed by paired t test. Dotted lines indicate the cut-off values: 2.0 for anti-GPIIb/IIIa Ab–producing B cells; 3.3 U for platelet-associated anti-GPIIb/IIIa Abs; 2.0% for % RP; and 7.5 U for anti-CagA Ab. †P < 0.1, *P < 0.05, and **P < 0.01 compared with wk 0.
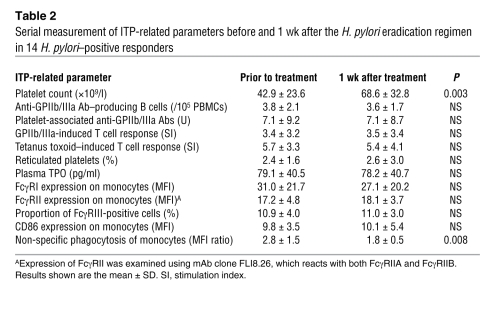
In the H. pylori–positive responders, after the eradication of H. pylori, the parameters for T and B cell responses to platelet antigens and platelet turnover improved, and the activated monocyte phenotype was suppressed, as the platelet count increased. To examine which parameter changed first, we further evaluated these parameters at 1 wk, when the eradication regimen had just ended, in the 14 H. pylori–positive responders (Table 2). The platelet count was significantly increased at 1 wk, consistent with a previous report (5). At this time point, the phagocytic capacity of monocytes was the only parameter that had changed from its pretreatment level.
Table 2 .
Serial measurement of ITP-related parameters before and 1 wk after the H. pylori eradication regimen in 14 H. pylori–positive responders
In 3 H. pylori–positive patients who were untreated and whose platelet count was >50 × 109/l at study entry, 2 were responders and the remaining 1 was a nonresponder. When these 3 patients were excluded from the analysis, concordant results regarding changes in the monocyte phenotype were obtained.
Change in the FcγRIIA/IIB balance of monocytes after H. pylori eradication.
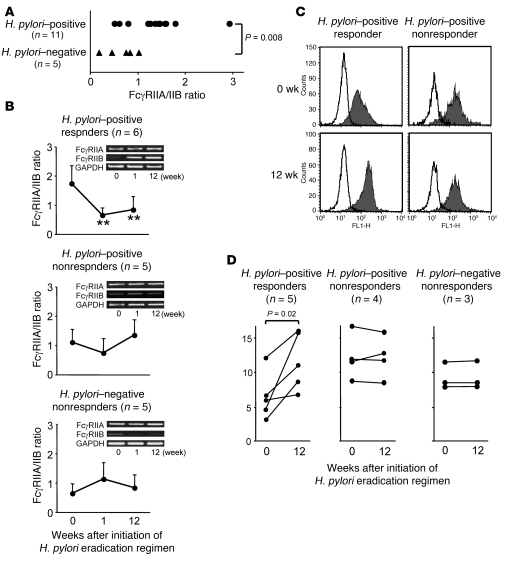
In humans, 2 types of FcγRII are expressed on monocytes: an activating receptor, FcγRIIA, and an inhibitory receptor, FcγRIIB (11). Because the extracellular domains of FcγRIIA and FcγRIIB are highly homologous, we measured the mRNA expression levels of FcγRIIA and FcγRIIB on sorted monocytes independently, by quantitative PCR, using specific primers for the intracellular domains of these FcγRs. The results revealed that the FcγRIIA/IIB mRNA expression ratio was significantly higher in the H. pylori–positive than the H. pylori–negative patients prior to treatment (Figure 4A). In the H. pylori–positive responders, the FcγRIIA/IIB ratio was significantly decreased at 1 and 12 wk, but this change was not observed in the H. pylori–positive nonresponders or in the H. pylori–negative patients (Figure 4B). When the protein expression of FcγRIIB on monocytes was examined by flow cytometry using mAbs specific to the intracellular domain of this molecule, FcγRIIB expression was increased at 12 wk in a representative H. pylori–positive responder but not in a nonresponder (Figure 4C). As a result, FcγRIIB expression was significantly upregulated at 12 wk in H. pylori–positive responders, but this change was not observed in the H. pylori–positive nonresponders or in the H. pylori–negative patients (Figure 4D). These findings indicate that a change in the FcγR balance toward the inhibitory FcγRIIB, together with a diminished phagocytic capacity of the monocytes, is the first event that occurs as a result of the H. pylori eradication in responders.
Figure 4. Change in FcγRIIB expression levels in circulating monocytes from ITP patients before and after initiation of the H. pylori eradication.
(A) FcγRIIA/IIB mRNA expression ratio on monocytes prior to treatment was determined by quantitative TaqMan PCR in 11 ITP patients infected with H. pylori and 5 uninfected ITP patients. The difference between the 2 groups was analyzed using the Mann-Whitney U test. (B) The FcγRIIA/IIB expression ratio on monocytes was serially measured prior to treatment and at 1 and 12 wk after initiation of the H. pylori eradication regimen in 6 H. pylori–positive ITP responders, 5 H. pylori–positive ITP nonresponders, and 5 H. pylori–negative ITP nonresponders. Results are shown as the mean + SD. Changes in the values at 1 and 12 wk from the baseline value taken at wk 0 were assessed by paired t test. **P < 0.01 compared with pre-treatment. Representative RT-PCR results for the expression of FcγRIIA, FcγRIIB, and GAPDH are shown for each patient group. (C) The protein expression of FcγRIIB on monocytes prior to treatment and 12 wk after initiation of the H. pylori eradication regimen in a representative H. pylori–positive responder and nonresponder. Open histograms show the cells stained with isotype-matched control Ab, and closed histograms show anti-FcγRIIB mAb–treated cells. (D) Expression levels of FcγRIIB on monocytes were measured prior to treatment and 12 wk after initiation of the H. pylori eradication regimen in 5 H. pylori–positive ITP responders, 4 H. pylori–positive ITP nonresponders, and 3 H. pylori–negative ITP nonresponders.
We further examined a potential role of Th1/Th2 balance in regulating FcγR expression profiles on monocytes in association with H. pylori infection (11), but there was no substantial change in the ratio of IFN-γ+CD4+ T cells to IL-4+CD4+ T cells in circulation prior to treatment and 12 wk after initiation of the eradication regimen in the H. pylori–positive responders (2.6 ± 1.3 and 2.7 ± 1.3, respectively), the H. pylori–positive nonresponders (3.1 ± 0.6 and 3.7 ± 1.0, respectively), or the H. pylori–negative patients (3.4 ± 1.1 and 3.5 ± 1.5, respectively).
Parameters that predict the platelet response to H. pylori eradication.
To examine which parameters predict the platelet response to the eradication of H. pylori in ITP patients, patient characteristics, the immunologic and platelet turnover parameters listed in Figure 1, as well as the IgG anti-CagA Ab level and FcγRIIA/IIB mRNA expression ratio in monocytes at pretreatment were compared between the H. pylori–infected responders and nonresponders. The FcγRII expression level on monocytes was significantly lower in the responders than in nonresponders (17.2 ± 4.8 versus 23.4 ± 8.2 MFI, P = 0.03). In addition, the phagocytic capacity and FcγRIIA/IIB expression ratios of the monocytes were significantly higher in the responders than in the nonresponders (2.8 ± 1.5 versus 1.3 ± 0.3, P = 0.02; and 1.7 ± 0.6 versus 1.1 ± 0.4, P = 0.04).
Phenotypic and functional properties of monocytes before and after H. pylori eradication in non-ITP subjects.
To evaluate whether changes in the phenotypic and functional properties of circulating monocytes after the eradication of H. pylori were specific to ITP patients, 9 non-thrombocytopenic volunteers infected with H. pylori were treated with the standard eradication regimen. Eradication was successful in 7 subjects, but not in 2. As expected, there was no substantial change in the platelet count during the observation period in any subject. In monocytes from the successfully treated individuals, there were trends toward downregulated FcγRI expression and phagocytic capacity and toward upregulated FcγRII (FcγRIIA plus FcγRIIB) expression 12 wk after the eradication, as observed in the H. pylori–infected ITP responders, but these changes were not statistically significant (Figure 5). The lack of significance appears to be due to the highly variable responses among individuals. It is of note that the subjects with an activated monocyte phenotype, i.e., high FcγRI expression, low FcγRII expression, and enhanced phagocytic capacity, at pretreatment were more likely to respond with improvements in these conditions upon the eradication of H. pylori.
Figure 5. Serial measurements of the phenotype of circulating monocytes and the anti-CagA Ab level before and after initiation of the H. pylori eradication regimen in 9 non-ITP individuals infected with H. pylori according to the outcome of the regimen.
Expression levels of FcγRI, FcγRII (FcγRIIA plus FcγRIIB), and CD86, non-specific phagocytosis of circulating monocytes, and anti-CagA Ab level were measured prior to treatment and 12 wk after initiation of the H. pylori eradication regimen. Expression of FcγRII was examined using mAb clone FLI8.26, which reacts with both FcγRIIA and FcγRIIB. Results for 7 individuals in which H. pylori was successfully eradicated and for 2 individuals in whom the eradication failed are shown separately. A dotted line indicates the cut-off for anti-CagA Abs (7.5 U).
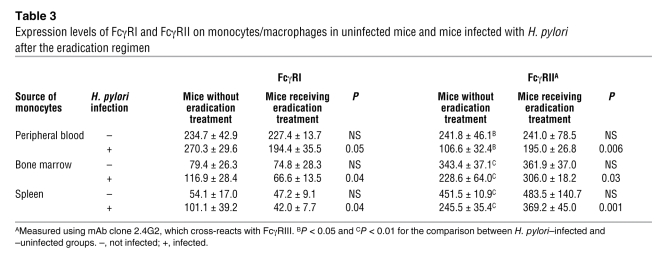
FcγR phenotype on monocytes/macrophages in H. pylori–infected and uninfected mice.
We prepared 4 groups of mice (n = 5 in each group): H. pylori–infected mice, uninfected mice, H. pylori–infected mice that had received eradication treatment, and uninfected mice that had received eradication treatment. In mice, there is only one FcγRII, which corresponds to human FcγRIIB. As shown in Table 3, the expression of FcγRII measured using mAb clone 2.4G2 on the monocytes/macrophages derived from peripheral blood, bone marrow, and spleen was significantly lower in mice infected with H. pylori than in uninfected mice, while the FcγRI expression tended to be higher in mice infected with H. pylori (P < 0.1). Both the infected and uninfected mice were given the triple H. pylori eradication regimen, and successful eradication was confirmed in all the infected mice by bacterial culture of the stomach at 6 wk. In the monocytes/macrophages derived from the peripheral blood, bone marrow, and spleen from these H. pylori–eradicated mice, the FcγRII expression was upregulated and FcγRI expression was downregulated after the eradication. Since mAb clone 2.4G2 cross-reacts with FcγRIII, peripheral blood samples were further examined using a mAb specific to FcγRII (clone Ly17.2) (12). The FcγRII expression level was again significantly lower in H. pylori–infected mice compared with mock-treated mice (3.2 ± 0.4 versus 3.9 ± 0.2 MFI, P = 0.01). In H. pylori–infected mice, the FcγRII expression level was increased after the eradication treatment (3.2 ± 0.4 versus 3.9 ± 0.3 MFI, P = 0.02).
Table 3 .
Expression levels of FcγRI and FcγRII on monocytes/macrophages in uninfected mice and mice infected with H. pylori after the eradication regimen
Discussion
In this comprehensive analysis, we demonstrated that the platelet recovery observed in a subset of H. pylori–infected ITP patients after H. pylori eradication is likely to be mediated through a change in the FcγR balance on monocytes/macrophages. An expression level of FcγRII on monocytes was first identified as one of factors associated with H. pylori infection in ITP patients by flow cytometric analysis using a mAb that reacts with both FcγRIIA and FcγRIIB, but this association was later shown to be primarily attributable to inhibitory FcγRIIB based on 2 different assays: quantitative PCR for assessment of mRNA expression and flow cytometric analysis using a mAb specific to the intracellular domain of FcγRIIB. The functional properties of monocytes/macrophages, including phagocytosis and antigen presentation, are controlled by the balance between activating FcγRs and an inhibitory receptor, FcγRIIB (13). Circulating monocytes from H. pylori–infected ITP patients exhibited an activated phenotype with enhanced phagocytic capacity, which potentially resulted from downregulated FcγRIIB, and this phenotype reverted to that of H. pylori–uninfected ITP patients after the eradication of H. pylori, but only in the responders. In addition, this change in monocyte phenotype preceded the improvements in autoimmune and platelet kinetic parameters. Therefore, H. pylori infection plays an important role in ITP pathogenesis by altering the FcγR balance of monocytes/macrophages in favor of activating FcγRs, through downregulation of the inhibitory receptor FcγRIIB.
Our recent studies on T cells that were autoreactive to GPIIb/IIIa in ITP patients revealed that the pathogenic process of ITP can be explained by a continuous loop in which B cells produce IgG anti-platelet autoantibodies, macrophages in the reticuloendothelial system (RES) phagocytose opsonized platelets via FcγRs and present GPIIb/IIIa-derived antigenic peptides, and GPIIb/IIIa-reactive T cells are activated and exert their helper activity (14, 15). It has been shown that anti-GPIIb/IIIa Abs in ITP patients are predominant of the IgG1 subclass (16), which has the highest affinity for FcγRII (13). Thus, FcγRs expressed on macrophages in the RES apparently play a central role in 2 aspects of the pathogenic process: platelet destruction and sustained autoimmune responses to the platelet antigens. It is still debated which activating FcγR is predominantly involved in ITP pathogenesis (17), but the increase in the expression of inhibitory FcγRIIB relative to the activating FcγRs could eventually result in the attenuation of the pathogenic continuous loop. Therefore, platelet recovery mediated through the upregulated FcγRIIB expression on monocytes after the eradication of H. pylori in ITP patients involves 2 consequent steps: the rapid platelet increase observed at 1 wk is the result of the blockade of platelet clearance by macrophages in the RES, while the sustained platelet increase results from the suppression of antigen presentation by macrophages and the subsequent inhibition of T and B cell responses to platelet antigens. To assess the role of H. pylori infection on platelet clearance, it is interesting to evaluate whether H. pylori infection boosts platelet destruction in mice treated with platelet-depleting mAbs. A similar suppressive effect on the FcγRIIB expression on macrophages is reported for intravenous immunoglobulin (IVIG). Samuelsson et al. demonstrated that IVIG requires the presence of FcγRIIB to prevent thrombocytopenia in a murine model of passive ITP (18). In this model, FcγRIIB expression on macrophages was upregulated upon IVIG treatment.
The pathogenesis of ITP in association with H. pylori infection is most likely multifactorial. Our mechanism does not exclude other proposed mechanisms for platelet recovery after eradication of H. pylori in ITP, such as molecular mimicry between CagA and platelet surface antigens (7). Moreover, it has been shown that some strains of H. pylori induce platelet aggregation that is dependent on von Willebrand factor and IgG Abs specific for H. pylori interacting with their corresponding receptors GPIb and FcγRIIA on platelets (19). In this model, Abs specific for H. pylori are capable of opsonizing platelets through binding to H. pylori, von Willebrand factor, and GPIb, as are anti-platelet autoantibodies. Thus, decrease in H. pylori–specific Ab levels after the eradication treatment may also suppress presentation of platelet antigens by macrophages. These Ab-mediated mechanisms appear to play a role in the later phase of platelet recovery after the H. pylori eradication, since the change in monocyte phenotype preceded the improvements in the Ab responses to GPIIb/IIIa and CagA.
Platelet recovery after the eradication of H. pylori is observed only in a subgroup of H. pylori–infected ITP patients (3, 4). Our results here indicate that ITP patients with an activated monocyte phenotype, such as an enhanced phagocytic capacity and reduced FcγRIIB expression, are likely to respond to H. pylori eradication, suggesting that the activated monocyte/macrophage phenotype contributes to driving the pathogenic loop that maintains the anti-platelet autoimmune condition in such responders. This finding is clinically important, since an activated monocyte phenotype can predict platelet recovery after the eradication of H. pylori in ITP patients. The activation status of circulating monocytes was also heterogeneous in non-ITP individuals infected with H. pylori, and subjects with an activated monocyte phenotype were responsive to H. pylori eradication, which changed the FcγR balance. Thus, whether H. pylori infection activates the host’s monocytes/macrophages differs among individuals independently of the presence or absence of ITP. There is great variability in the efficacy of the H. pylori eradication therapy in ITP patients among ethnic groups. Cohorts from Japan and Italy report response rates of 39%–100%, while those from Spain and the United States document little to no platelet response (20). The reason for such variability among countries is not clear, but this could be explained by differences in epidemic H. pylori strains or genetic backgrounds among populations. In this regard, H. pylori genotypes were different between the eastern Asian strain and the European strain (21). In addition, genetic factors that regulate the expression of FcγRs may contribute to this difference. Several polymorphisms exist within the human FcγR genes that exhibit altered affinities for IgG, potentially resulting in specific allele–dependent clearance rates of immune complexes (22). In particular, one SNP within the FcγRIIB gene alters its receptor signaling (23, 24) and predicts the development of chronic disease in children with acute ITP (25). Another polymorphism in the promoter region of the FcγRIIB gene downregulates its transcriptional activity (26).
Potential patient selection bias is one of the limitations of this study. Because our hospital is a referral center, many patients had relatively long disease duration and had been treated with prednisolone and/or splenectomy. In addition, our patients were a relatively mild subset, since we excluded patients with active bleeding to minimize dropouts during the study period. We have to consider this potential bias upon interpreting the results, but there was no apparent difference in patient characteristics between H. pylori–infected responders and nonresponders. In addition, the high frequency of splenectomized patients in the H. pylori–negative group might affect the phenotypic and functional properties of circulating monocytes, although there was no apparent difference in the FcγR expression levels and the phagocytic capacity of monocytes among the H. pylori–negative ITP patients regardless of their history of splenectomy (data not shown). Another limitation is the use of peripheral blood monocytes instead of macrophages in the RES in the analysis. However, in mice, H. pylori infection changed the FcγR balance similarly in monocytes/macrophages derived from peripheral blood, bone marrow, and spleen, suggesting that circulating monocytes potentially represent the whole monocyte/macrophage system.
The modulation of the monocyte FcγR balance associated with H. pylori infection was detected in ITP and non-ITP individuals as well as in mice, indicating that this response might be a normal host immune response against microorganisms that establish chronic infection lasting several decades. It is known that H. pylori colonizes the mucous layer of the stomach and does not invade the gastric epithelium, but H. pylori infection induces local and systemic responses through stimulation of the innate and adaptive immune systems (27). The molecular events that induce the change in the properties of monocytes/macrophages still remain unclear. We failed to show the role of Th1 response related with H. pylori infection in the monocyte phenotype change. On the other hand, it is reported that components of H. pylori are released and are responsible for the activation of dendritic cells and macrophages through toll-like receptor signaling (28, 29). In this regard, high frequencies of some specific H. pylori genotypes are reported in ITP patients compared with non-ITP subjects (5), suggesting potential roles for the bacterium’s structure and virulence profiles in the pathogenesis of ITP.
Previous studies demonstrated a causal link between infection and autoimmune diseases, such as intestinal infections by Campylobacter jejuni and Guillian-Barré syndrome. In this case, Abs cross-reacting with lipooligosaccharides present in C. jejuni and in ganglioside GM1 are responsible for development of this disease (30). On the other hand, in ITP patients, H. pylori infection enhanced immunogenicity of platelet-specific autoantigens secondary to infection-mediated FcγR balance change of macrophages. A similar mechanism is shown in the animal model for Coxsackie B4 virus–induced type I diabetes (31), in which the virus is thought to act by increasing immunogenicity of autoantigens secondary to local inflammation. In addition, infections may also protect from autoimmune diseases. Onset of type I diabetes was inhibited by helminth parasite infection in animal models (32). A recent randomized, double-blind, placebo-controlled trial demonstrated improvement of active ulcerative colitis by infection with Trichuris suis (33). Effects of infectious agents on the pathogenesis of autoimmunity appear to be variable among diseases, and underlying mechanisms are multiple and complex, probably different according to pathogens.
In summary, H. pylori infection plays an important role in the pathogenesis of ITP by altering the FcγR balance of monocytes/macrophages in favor of activating FcγRs, through the downregulation of FcγRIIB. The efficacy and safety profiles of the H. pylori eradication make this regimen an attractive option, but it is indicated only for H. pylori–infected patients in certain ethnic groups. The mechanism that induced platelet recovery after H. pylori eradication reported here indicates that the FcγR balance on monocytes/macrophages may be a reasonable therapeutic target for ITP. In this case, small molecules that inhibit the downstream signal of activating FcγR could have beneficial effects in ITP patients (34). Further studies to evaluate how H. pylori infection modulates monocyte/macrophage function may be useful for understanding the pathogenesis of ITP and for developing new therapeutic strategies for ITP.
Methods
Patients and controls.
This was a single-center, open-label, prospective study involving consecutive 34 patients with ITP. The diagnosis of ITP was based on thrombocytopenia persisting longer than 6 mo, normal or increased bone marrow megakaryocytes without morphologic evidence of dysplasia, and no secondary immune or non-immune disease that could account for the thrombocytopenic state (35). All the patients were referred to Keio University Hospital, Tokyo, Japan, and had been treated for at least 6 mo. Platelet counts were <100 × 109/l during the preceding 3 mo. The exclusion criteria included age (< 18 yr), active bleeding, pregnancy or lactation, a history of potential adverse effects associated with penicillins, current or previous treatment with proton pump inhibitors, or other debilitating illness (e.g., cancer). Nine healthy volunteers infected with H. pylori served as control subjects. The study protocol conformed to the ethical principles of the World Medical Association Declaration of Helsinki as reflected in a priori approval from the Keio University Institutional Review Board, and written informed consent was obtained from each participant.
Assessment of H. pylori infection.
To evaluate H. pylori infection, we performed a 13C urea breath test using a UBiT tablet (Otsuka Assay), analyzed serum IgG H. pylori–specific Abs using a commercially available kit (Kyowa Medex Company), and analyzed H. pylori antigen in stool samples using ImmunoCard STAT! HpSA (Meridian Bioscience Inc.), in all patients (36). Patients positive for the urea breath test plus at least one additional test were regarded as H. pylori positive, whereas those negative for all 3 tests were considered H. pylori negative. To minimize false-negative results, patients had not received antacids or antibiotics for at least 2 wk before the tests. Successful eradication was defined as a negative result for the urea breath test 12 wk after the eradication regimen.
H. pylori eradication regimen and follow-up.
All patients and controls were given amoxicillin (750 mg twice daily), clarithromycin (400 mg twice daily), and lansoprazole (30 mg twice daily) for 7 d regardless of their H. pylori infection status and were then followed for the subsequent 24 wk. The patients were allowed to continue their other therapies during the study period, provided the dosages of drugs were maintained at a constant level until the study was completed, except for prednisolone, which could be decreased or discontinued. A therapeutic response was defined as a platelet count higher than 50 × 109/l and doubling of the baseline at 24 wk after initiation of the eradication regimen.
Cell preparation.
Heparinized peripheral blood samples were obtained from all subjects at 0 (pre-treatment), 12, and 24 wk, and from some patients at 1 wk, after initiation of the eradication regimen. After the platelet-rich plasma was isolated, the residual cell components were subjected to Lymphoprep (Nycomed Pharma AS) density gradient centrifugation to isolate the PBMCs. Freshly isolated PBMCs were resuspended in RPMI 1640 containing 10% heat-inactivated FBS, 2 mmol/l l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin and were immediately used in the following experiments. Platelet-poor plasma was isolated from platelet-rich plasma by centrifugation.
Evaluation of anti-GPIIb/IIIa Ab response.
B cells producing IgG anti-GPIIb/IIIa Abs were detected using the enzyme-linked immunospot assay, as previously described (37). Each experiment was conducted in 5 independent wells, and the results represent the mean of the 5 values. The frequency of circulating anti-GPIIb/IIIa Ab–producing B cells was calculated as the number per 105 PBMCs, and the cut-off value was defined as 2.0 cells. IgG anti-GPIIb/IIIa Abs in platelet eluates (from 5 × 107 platelets) were measured by ELISA using purified human GPIIb/IIIa as the antigen (38). Ab units were calculated from the optical density at 450 nm (OD450) results, based on a standard curve obtained from serial concentrations of pooled plasma with high-titer IgG anti-GPIIb/IIIa Abs. All samples were examined in duplicate, and the results were calculated as the mean of the 2 values. The cut-off for platelet-associated anti-GPIIb/IIIa Abs was 3.3 U.
Evaluation of T cell response to GPIIb/IIIa.
The antigenic specificity of T cells was determined by antigen-induced T cell proliferation as previously described (39). Briefly, PBMCs were cultured in the presence or absence of antigen for 7 d. After a final 16-h incubation with 0.5 μCi/well 3H-thymidine, the cells were harvested, and the 3H-thymidine incorporation was determined in a TopCount microplate scintillation counter (Packard). Antigens used were trypsin-digested GPIIb/IIIa, mock-treated PBS, and tetanus toxoid (List Biological Laboratories) used at a concentration of 5 μg/ml. All the cultures were prepared in triplicate, and all the values represent the mean of triplicate determinations. Antigen-specific T cell response was expressed as the stimulation index, which was calculated as the cpm incorporated into cultures with trypsin-digested GPIIb/IIIa or tetanus toxoid divided by the cpm incorporated into cultures with mock treatment with PBS.
Evaluation of platelet turnover.
Reticulated platelets were detected by staining freshly isolated platelets with thiazole orange (Retic-COUNT; Becton Dickinson) followed by flow cytometric analysis, as described previously (40). The cut-off for the percentage of reticulated platelets was 2.0%. The plasma TPO level was measured using a commercially available ELISA kit (Quantikine; R&D Systems) according to the manufacturer’s protocol.
Evaluation of phenotypic and functional properties of circulating monocytes.
Unfixed PBMCs were stained with FITC-conjugated anti-FcγRI/CD64 (clone 10.1), anti-FcγRII/CD32 (clone FLI8.26), anti-FcγRIII/CD16 (clone 3G8) (BD Biosciences), or anti-CD86 mAbs (clone BU63; Ancell), in combination with PC5-conjugated anti-CD14 mAb (clone RMO52; Beckman Coulter). For FcγRIIB staining, the cells were fixed and permeabilized using a buffer containing paraformaldehyde and saponin (BD Biosciences) and subsequently stained with mAbs to the C-terminal portion of FcγRIIB (clone EP888Y; Epitomics), followed by incubation with FITC-labeled anti-rabbit IgG F(ab′)2 (Beckman Coulter) and PC5-conjugated anti-CD14 mAb. The negative controls were cells incubated with fluorescent-labeled isotype-matched mouse mAbs against an irrelevant antigen. The cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences) using CellQuest software. Viable cells were selected by the exclusion of apoptotic cells stained with propidium iodide (Sigma-Aldrich). Relative expression levels of FcγRI, FcγRII (FcγRIIA plus FcγRIIB), FcγRIIB, FcγRIII, and CD86 on gated CD14+ monocytes were expressed as MFI, which was calculated based on the intensity of the cells incubated with appropriate isotype-matched control mAb as a reference. The proportion of cells positive for FcγRIII in CD14+ monocytes was also determined. Each assay included a quality control PBMC sample derived from a single healthy donor, which had been stored in aliquots at –80°C. The nonspecific phagocytic capacity of monocytes was examined as described previously (41). In brief, PBMCs were cultured with neutral FITC–dextran with a molecular weight of 2,000 kDa (Sigma-Aldrich) for 30 min at 0°C or 37°C and analyzed on a flow cytometer. The uptake of FITC-labeled dextran was evaluated by MFI on gated CD14+ monocytes. The phagocytic capacity was expressed as an MFI ratio, which was calculated as the MFI obtained at 37°C divided by the MFI at 0°C. Consistent settings for detector sensitivity, compensation, and scatter gating were used in the analyses of all the samples.
Th1/Th2 balance.
Proportions of IFN-γ+ cells and IL-4+ cells in peripheral blood CD4+ T cells were evaluated using flow cytometry–based Intracellular Cytokine Staining Kit Human (BD Biosciences) according to the manufacturer’s protocol. The results were expressed as the ratio of IFN-γ+CD4+ T cells to IL-4+CD4+ T cells.
Anti-CagA Abs.
Anti-CagA Abs of the IgG isotype were measured in duplicate in plasma samples using a commercially available ELISA kit (ravo Diagnostika). The cut-off of 7.5 U was based on the manufacturer’s information.
Gene expression of FcγRIIA and FcγRIIB on monocytes.
Circulating CD14+ monocytes were isolated from PBMCs using an anti-CD14 mAb coupled to magnetic beads (Miltenyi Biotech) followed by MACS column separation. Flow cytometric analysis revealed that the sorted fractions contained >95% CD14+ cells. Total RNA was extracted from monocytes using the RNeasy kit (Qiagen), and first-strand cDNA synthesized from the total RNA was subjected to PCR using a panel of specific primers (FcγRIIA: sense primer, 5′-CTGACTGTGCTTTCCGAATG-3′, and antisense primer, 5′-TGGATGAGAACAGCGTGTAG-3′; FcγRIIB: sense primer, 5′-ACAAGCCTCTGGTCAAGGTC-3′, and antisense primer, 5′-TTCCCTGCACTCAGGGTATC-3′; and GAPDH: sense primer, 5′-TGAACGGGAAGCTCACTGG -3′, and antisense primer, 5′-TCCACCACCCTGTTGCTGTA-3′). The PCR products were resolved by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining. In addition, mRNA expression levels were quantitatively assessed using the TaqMan real-time PCR system (Applied Biosystems). A combination of primers and a probe specific for human FcγRIIA and FcγRIIB was purchased from Applied Biosystems. The relative expression levels were calculated from a standard curve generated by plotting the amount of PCR product against the serial amount of input PBMC cDNA, and the FcγRIIA/IIB ratio was calculated from the relative expression levels in the same sample.
Infection and eradication of H. pylori in mice.
Six-week-old, specific pathogen–free male mice (C57BL/6; Sankyo Lab Service) were given irradiated food and autoclaved distilled water ad libitum. The Sydney strain of H. pylori SS1, which was grown at 37°C under microaerobic conditions, was used for oral inoculation, as described previously (42, 43). Suspensions of H. pylori (2 × 107 CFU/ml, 15 ml/kg) were administered to mice after overnight starvation, twice with a 2-wk interval, while control mice received suspensions of buffer solution alone (mock infection). Twelve weeks later, half of the mice in the H. pylori–inoculated group and the control group were sacrificed for examination. The remaining H. pylori–inoculated and control mice were treated with the H. pylori eradication treatment consisting of lansoprazole (10 mg/kg), amoxicillin (3 mg/kg), and clarithromycin (30 mg/kg) suspended in 0.5% carboxymethyl cellulose sodium salt solution, once a day for 2 d (44). Six weeks later, all the mice were sacrificed for examination. H. pylori infection in the excised stomach was evaluated by microaerobic bacterial culture, as previously described (45). Mononuclear cells were isolated from the peripheral blood, spleen, and bone marrow and stored at –80°C, and all samples were subjected to flow cytometric analysis on the same day. Cells were incubated with a Cy5-labeled anti-mouse CD11b mAb (clone M1/70; Beckman-Coulter) in combination with a biotin-labeled anti-mouse FcγRI polyclonal Ab (R&D Systems) or a rat mAb reactive with both mouse FcγRII (clone 2.4G2; BD Biosciences — Pharmingen) and biotin-labeled anti-rat IgG (Jackson ImmunoResearch Laboratories Inc.), followed by treatment with FITC-streptavidin (Beckman Coulter). In some instances, we used a mouse mAb specific to FcγRII (clone Ly17.2) (Sloan-Kettering Cancer Institute) in combination with FITC-labeled anti-mouse IgG F(ab′)2. All mouse procedures were approved by the Keio University Animal Research Committee.
Statistics.
All continuous values are shown as the mean ± SD. Comparisons to determine the statistical significance between 2 groups were performed using the Fisher’s Exact test or Mann-Whitney U test, as appropriate. Changes in the absolute values at different time points from the baseline value taken at week 0 were compared by paired t test.
Acknowledgments
We thank Ulrich Hammerling (Sloan-Kettering Institute) for coordinating acquisition of mAb clone Ly17.2 and Masahiro Kizaki and Mitsuru Murata for recruiting the patients for this study. This work was supported by a grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology, a research grant on intractable diseases from the Japanese Ministry of Health, Labour and Welfare, and the Nagao Memorial Fund.
Footnotes
Nonstandard abbreviations used: CagA, cytotoxin-associated gene A; FcγRIIB, Fcγ receptor IIB; GPIIb/IIIa, glycoprotein IIb/IIIa; ITP, immune thrombocytopenia purpura; RES, reticuloendothelial system; TPO, thrombopoietin.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:2939–2949 (2008). doi:10.1172/JCI34496
See the related Commentary beginning on page 2677.
References
- 1.Cines D.B., Blanchette V.S., Mannucci P.M., Remuzzi G., Clines D.B. Immune thrombocytopenic purpura. N. Engl. J. Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 2.Gasbarrini A., et al. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. . Lancet. 1998;352:878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 3.Fujimura K., et al. Is eradication therapy useful as the first line of treatment in Helicobacter pylori–positive idiopathic thrombocytopenic purpura? Analysis of 207 eradicated chronic ITP cases in Japan. . Int. J. Hematol. 2005;81:162–168. doi: 10.1532/IJH97.04146. [DOI] [PubMed] [Google Scholar]
- 4.Franchini M., Veneri D. Helicobacter pylori-associated immune thrombocytopenia. . Platelets. 2006;17:71–77. doi: 10.1080/09537100500438057. [DOI] [PubMed] [Google Scholar]
- 5.Emilia G., et al. Helicobacter pylori infection and chronic immune thrombocytopenic purpura: long-term results of bacterium eradication and association with bacterium virulence profiles. . Blood. 2007;110:3833–3841. doi: 10.1182/blood-2006-12-063222. [DOI] [PubMed] [Google Scholar]
- 6.Asahi A., et al. Effects of Helicobacter pylori eradication regimen on anti-platelet autoantibody response in infected and uninfected patients with idiopathic thrombocytopenic purpura. . Haematologica. 2006;91:1436–1437. [PubMed] [Google Scholar]
- 7.Takahashi T., et al. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. . Br. J. Haematol. 2004;124:91–96. doi: 10.1046/j.1365-2141.2003.04735.x. [DOI] [PubMed] [Google Scholar]
- 8.Michel M., et al. Autoimmune thrombocytopenic purpura and Helicobacter pylori infection. . Arch. Intern. Med. 2002;162:1033–1036. doi: 10.1001/archinte.162.9.1033. [DOI] [PubMed] [Google Scholar]
- 9.Yamanishi S., et al. Implications for induction of autoimmunity via activation of B-1 cells by Helicobacter pylori urease. . Infect. Immun. 2006;74:248–256. doi: 10.1128/IAI.74.1.248-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellicano R., et al. Prevalence of non-organ-specific autoantibodies in patients suffering from duodenal ulcer with and without Helicobacter pylori infection. . Dig. Dis. Sci. 2004;49:395–398. doi: 10.1023/B:DDAS.0000020491.78450.82. [DOI] [PubMed] [Google Scholar]
- 11.Pricop L., et al. Differential modulation of stimulatory and inhibitory Fc receptors on human monocytes by Th1 and Th2 cytokines. J. Immunol. 2001;166:531–537. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- 12.Schiller C., et al. Mouse FcγRII is a negative regulator of FcγRIII in IgG immune complex-triggered inflammation but not in autoantibody-induced hemolysis. Eur. J. Immunol. 2000;30:481–489. doi: 10.1002/1521-4141(200002)30:2<481::AID-IMMU481>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Takai T. Roles of Fc receptors in autoimmunity. Nat. Rev. Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 14.Kuwana M., Ikeda Y. The role of autoreactive T-cells in the pathogenesis of ITP. Int. J. Hematol. 2005;81:106–112. doi: 10.1532/IJH97.04176. [DOI] [PubMed] [Google Scholar]
- 15.Kuwana M., Kawakami Y., Ikeda Y. Splenic macrophages maintain the anti-platelet autoimmune response via uptake of opsonized platelets in patients with chronic ITP [abstract]. Blood. 2005;106(Suppl.):68a. doi: 10.1111/j.1538-7836.2008.03161.x. [DOI] [PubMed] [Google Scholar]
- 16.Chan H., Moore J.C., Finch C.N., Warkentin T.E., Kelton J.G. The IgG subclasses of platelet-associated autoantibodies directed against platelet glycoproteins IIb/IIIa in patients with idiopathic thrombocytopenic purpura. Br. J. Haematol. 2003;122:818–824. doi: 10.1046/j.1365-2141.2003.04509.x. [DOI] [PubMed] [Google Scholar]
- 17.Crow A.R., Lazarus A.H. Role of Fcγ receptors in the pathogenesis and treatment of idiopathic thrombocytopenic purpura. J. Pediatr. Hematol. Oncol. 2003;25(Suppl. 1):S14–S18. doi: 10.1097/00043426-200312001-00004. [DOI] [PubMed] [Google Scholar]
- 18.Samuelsson A., Towers T.L., Ravetch J.V. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 19.Byrne M.F., et al. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. . Gastroenterology. 2003;124:1846–1854. doi: 10.1016/S0016-5085(03)00397-4. [DOI] [PubMed] [Google Scholar]
- 20.Kuwana M., Ikeda Y. Helicobacter pylori and immune thrombocytopenic purpura: unsolved questions and controversies. . Int. J. Hematol. 2006;84:309–315. doi: 10.1532/IJH97.06188. [DOI] [PubMed] [Google Scholar]
- 21.Azuma T. Helicobacter pylori CagA protein variation associated with gastric cancer in Asia. . J. Gastroenterol. 2004;39:97–103. doi: 10.1007/s00535-003-1279-4. [DOI] [PubMed] [Google Scholar]
- 22.van der Pol W., van de Winkel J.G. IgG receptor polymorphisms: risk factors for disease. Immunogenetics. 1998;48:222–232. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]
- 23.Li X., et al. A novel polymorphism in the Fcγ receptor IIB (CD32B) transmembrane region alters receptor signaling. Arthritis Rheum. 2003;48:3242–3252. doi: 10.1002/art.11313. [DOI] [PubMed] [Google Scholar]
- 24.Floto R.A., et al. Loss of function of a lupus-associated FcγRIIb polymorphism through exclusion from lipid rafts. Nat. Med. 2005;11:1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 25.Bruin M., et al. Platelet count, previous infection and FCGR2B genotype predict development of chronic disease in newly diagnosed idiopathic thrombocytopenia in childhood: results of a prospective study. Br. J. Haematol. 2004;127:561–567. doi: 10.1111/j.1365-2141.2004.05235.x. [DOI] [PubMed] [Google Scholar]
- 26.Su K., et al. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcγRIIb alters receptor expression and associates with autoimmunity. II. Differential binding of GATA4 and Yin-Yang1 transcription factors and correlated receptor expression and function. . J. Immunol. 2004;172:7192–7199. doi: 10.4049/jimmunol.172.11.7192. [DOI] [PubMed] [Google Scholar]
- 27.Ernst P.B., Gold B.D. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. . Annu. Rev. Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 28.Ferrero R.L. Innate immune recognition of the extracellular mucosal pathogen, Helicobacter pylori. . Mol. Immunol. 2005;42:879–885. doi: 10.1016/j.molimm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Rad R., et al. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. . Gastroenterology. 2007;133:150–163. doi: 10.1053/j.gastro.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 30.Yuki N., et al. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barré syndrome. . Proc. Natl. Acad. Sci. U. S. A. 2004;101:11404–11409. doi: 10.1073/pnas.0402391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horwitz M.S., et al. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 32.Saunders K.A., Raine T., Cooke A., Lawrence C.E. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Summers R.W., Elliott D.E., Urban J.F., Jr., Thompson R.A., Weinstock J.V. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. . Gastroenterology. . 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Braselmann S., et al. R406, an orally available spleen tyrosine kinase inhibitor blocks Fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 35.George J.N., et al. Idiopathic thrombocytopenic purpura: A practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88:3–40. [PubMed] [Google Scholar]
- 36.Suzuki H., Hibi T., Marshall B.J. Helicobacter pylori: present status and future prospects in Japan. . J. Gastroenterol. 2007;42:1–15. doi: 10.1007/s00535-006-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuwana M., Okazaki Y., Kaburaki J., Ikeda Y. Detection of circulating B cells secreting platelet-specific autoantibody is a sensitive and specific test for the diagnosis of autoimmune thrombocytopenia. Am. J. Med. 2003;114:322–325. doi: 10.1016/S0002-9343(02)01522-X. [DOI] [PubMed] [Google Scholar]
- 38.Kuwana M., Okazaki Y., Kaburaki J., Kawakami Y., Ikeda Y. Spleen is a primary site for activation of platelet-reactive T and B cells in patients with immune thrombocytopenic purpura. J. Immunol. 2002;168:3675–3682. doi: 10.4049/jimmunol.168.7.3675. [DOI] [PubMed] [Google Scholar]
- 39.Kuwana M., et al. Immunodominant epitopes on glycoprotein IIb-IIIa recognized by autoreactive T cells in patients with immune thrombocytopenic purpura. Blood. 2001;98:130–139. doi: 10.1182/blood.V98.1.130. [DOI] [PubMed] [Google Scholar]
- 40.Kuwana M., et al. Initial laboratory findings useful for predicting the diagnosis of idiopathic thrombocytopenic purpura. Am. J. Med. 2005;118:1026–1033. doi: 10.1016/j.amjmed.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 41.Takahara K., et al. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int. Immunol. 2004;16:819–829. doi: 10.1093/intimm/dxh084. [DOI] [PubMed] [Google Scholar]
- 42.Lee A., et al. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. . Gastroenterology. 1997;112:1386–1397. doi: 10.1016/S0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H., et al. Attenuated apoptosis in H. pylori-colonized gastric mucosa of Mongolian gerbils in comparison with mice. . Dig. Dis. Sci. 2002;47:90–99. doi: 10.1023/A:1013219621422. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu N., et al. Eradication diminishes enhancing effects of Helicobacter pylori infection on glandular stomach carcinogenesis in Mongolian gerbils. . Cancer Res. 2000;60:1512–1514. [PubMed] [Google Scholar]
- 45.Suzuki H., et al. H. pylori-associated gastric pro- and anti-oxidant formation in Mongolian gerbils. . Free Radic. Biol. Med. 1999;26:679–684. doi: 10.1016/S0891-5849(98)00248-2. [DOI] [PubMed] [Google Scholar]