Abstract
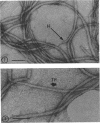
Highly purified axial filaments have been prepared from the spirochete Treponema zuelzerae, which possess a fine structure similar to the “beaded” form of bacterial flagella. The preparations consist largely of protein but also contain small amounts of hexose (less than 1%). The buoyant density of these filaments is 1.29 g/cm3. At pH 4.3, in the presence of 4 m urea and 10−3m ethylenediaminetetraacetic acid, filament protein migrates as a single band in acrylamide gel electrophoresis. Filaments dissociate to subunits in acid, alkali, urea, guanidine or with heating, indicating that these subunits are not covalently bonded in the organized structure. This is consistent with amino acid analysis which reveals that, like bacterial flagella, the filaments are completely lacking in half-cystine. Sedimentation equilibrium measurements on dissociated axial filaments in 6 m guanidine show that the subunits are homogeneous with respect to molecular weight. A weight-average molecular weight of 37,000 ± 1,600 daltons is obtained from these measurements. The amino acid composition of axial filaments is similar to that of various types of flagellin molecules, but the filament protein is somewhat richer in tyrosine, phenylalanine, and proline than flagellin. Tryptic peptide maps of axial filaments are consistent with the amino acid composition calculated for a molecular weight of 37,000 daltons. No amino terminal end group could be detected by the dansyl chloride method, suggesting that this end group might be blocked in the axial filament protein. The results obtained show that the axial filaments of T. zuelzerae are similar chemically to bacterial flagella and suggest that they are composed of aggregates of a single species of protein subunit.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., KOFFLER H. IN VITRO FORMATION OF FLAGELLA-LIKE FILAMENTS AND OTHER STRUCTURES FROM FLAGELLIN. J Mol Biol. 1964 Jul;9:168–185. doi: 10.1016/s0022-2836(64)80098-x. [DOI] [PubMed] [Google Scholar]
- ASAKURA S., EGUCHI G., IINO T. RECONSTITUTION OF BACTERIAL FLAGELLA IN VITRO. J Mol Biol. 1964 Oct;10:42–56. doi: 10.1016/s0022-2836(64)80026-7. [DOI] [PubMed] [Google Scholar]
- ATFIELD G. N., MORRIS C. J. Analytical separations by highvoltage paper electrophoresis. Amino acids in protein hydrolysates. Biochem J. 1961 Dec;81:606–614. doi: 10.1042/bj0810606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharier M. A., Eiserling F. A., Rittenberg S. C. Eletron microscopic observations on the structure of Treponema zuelzerae and its axial filaments. J Bacteriol. 1971 Jan;105(1):413–421. doi: 10.1128/jb.105.1.413-421.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharier M. A., Rittenberg S. C. Immobilization effects of anticell and antiaxial filament sera on Treponema zuelzerae. J Bacteriol. 1971 Jan;105(1):430–437. doi: 10.1128/jb.105.1.430-437.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Y., Brown D. M., Glazer A. N. Characterization of the subunits of the flagella of Proteus vulgaris. J Biol Chem. 1969 Oct 10;244(19):5196–5200. [PubMed] [Google Scholar]
- Easley C. W. Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta. 1965 Sep 13;107(2):386–388. doi: 10.1016/0304-4165(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Canale-Parola E. Fine structure of Spirochaeta stenostrepta, a free-living, anaerobic spirochete. J Bacteriol. 1968 Sep;96(3):822–835. doi: 10.1128/jb.96.3.822-835.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T. Genetics and chemistry of bacterial flagella. Bacteriol Rev. 1969 Dec;33(4):454–475. doi: 10.1128/br.33.4.454-475.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LOWY J., HANSON J. ELECTRON MICROSCOPE STUDIES OF BACTERIAL FLAGELLA. J Mol Biol. 1965 Feb;11:293–313. doi: 10.1016/s0022-2836(65)80059-6. [DOI] [PubMed] [Google Scholar]
- Lowy J., Spencer M. Structure and function of bacterial flagella. Symp Soc Exp Biol. 1968;22:215–236. [PubMed] [Google Scholar]
- MARTINEZ R. J. A METHOD FOR THE PURIFICATION OF BACTERIAL FLAGELLA BY ION EXCHANGE CHROMATOGRAPHY. J Gen Microbiol. 1963 Oct;33:115–120. doi: 10.1099/00221287-33-1-115. [DOI] [PubMed] [Google Scholar]
- MARTINEZ R. J., ROSENBERG E. THERMAL TRANSITION OF SPIRILLUM SERPENS FLAGELLA. J Mol Biol. 1964 May;8:702–707. doi: 10.1016/s0022-2836(64)80119-4. [DOI] [PubMed] [Google Scholar]
- Martinez R. J., Brown D. M., Glazer A. N. The formation of bacterial flagella. 3. Characterization of the subunits of the flagella of Bacillus subtilis and Spirillum serpens. J Mol Biol. 1967 Aug 28;28(1):45–51. doi: 10.1016/s0022-2836(67)80076-7. [DOI] [PubMed] [Google Scholar]
- Nauman R. K., Holt S. C., Cox C. D. Purification, ultrastructure, and composition of axial filaments from Leptospira. J Bacteriol. 1969 Apr;98(1):264–280. doi: 10.1128/jb.98.1.264-280.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll H. Chain initiation and control of protein synthesis. Science. 1966 Mar 11;151(3715):1241–1245. doi: 10.1126/science.151.3715.1241. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- Stephens R. E. Thermal fractionation of outer fiber doublet microtubules into A- and B-subfiber components. A- and B-tubulin. J Mol Biol. 1970 Feb 14;47(3):353–363. doi: 10.1016/0022-2836(70)90307-4. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]