Abstract
Glucose transporter 5 (Glut5) is a high-affinity fructose transporter. It was proposed to be a motor protein or part of the motor complex required for cochlear amplification in outer hair cells (OHCs). Here we show that, in contrast to previous reports, Glut5 is undetectable, and possibly absent, in OHCs harvested from wildtype mice. Further, Glut5-deficient mice display normal OHC morphology and motor function (i.e., nonlinear capacitance and electromotility) and normal cochlear sensitivity. We conclude that Glut5 is not required for OHC motility or cochlear amplification.
Keywords: Hair cell, cochlear amplification, glucose transporter, prestin, electromotility, knockout mice
1. Introduction
Cochlear outer hair cells (OHCs) elongate and contract in response to changes in their membrane potential[8], and are thus able to feed energy back to the basilar membrane in a cycle-by-cycle manner. This feature of OHCs, termed electromotility, has been proposed to form the cellular basis of the cochlear amplifier, an active mechanical amplification mechanism in the cochlea [12,14]. Associated with OHC electromotility is a nonlinear capacitance (NLC) derived from voltage-dependent charge movement within motor proteins in the cell’s lateral membrane [4,35]. A high density of protein particles has also been observed in the plasma membrane of OHCs [16,20,22] and it is assumed that these protein particles constitute the cellular motor [22,36].
Glucose transporter 5 (Glut5 or Slc2a5) has been proposed to be the motor protein or part of the motor-complex of OHCs and thus required for cochlear amplification [5,19]. This proposal is based on the knowledge that fructose can affect the NLC and electromotility of OHCs [19]. Moreover, Glut5 is the only high-affinity fructose transporter detected along the lateral wall of OHCs using immunostaining [5,7,19,29]. It is also abundantly expressed in the epithelial brush border of the small intestine, S3 proximal tubules of kidney, and in the sperm of the testis and epididymis [3,9,28,37].
Discovery of a novel gene prestin has rendered Glut5 a less promising candidate [41]. Prestin is highly expressed in the lateral membrane of the OHCs and is required for OHC electromotility and NLC and for cochlear amplification in vivo [1,25,41]. It is also known that the Glut5 immunosignal during postnatal development is detected after the expression of prestin in OHCs and after the first occurrence of electromotility [7]. Moreover, co-expression of Glut5 with prestin in Chinese Hamster Ovary (CHO) cells does not alter prestin-mediated NLC [26]. Recently, it was also demonstrated that prestin and Glut5 interact in HEK293T cells although the precise subcellular sites of such interactions remain unclear [38]. Hence, the exact roles of Glut5 in OHC electromotility and NLC and in cochlear amplification remain unclear.
Here we report on the generation and characterization of Glut5 knockout mice. Surprisingly, we find that Glut5 is absent or undetectable in wildtype OHCs, which differs from previous reports. Results provide evidence that Glut5 is not required for OHC electromotility or for cochlear amplification.
2. Results
2.1. Generation of Glut5 floxed and null mice
To make a Glut5floxed mouse model, we inserted one loxP site upstream of the predicted promoter and exon 1 and another loxP site downstream of exon 4 of the Glut5 gene which has 14 exons (Fig. 1A). Theoretically, Cre activity can cause the deletion of a total of 6.6 kb genomic DNA that contains: 1) the predicted promoter located upstream of exon1 by Genescan; 2) the ATG translation initiation codon in exon 3; and 3) the N-terminal 43 amino acids including the first transmembrane domain.
Fig. 1. Targeted disruption of the Glut5 gene and distribution of Glut5 in cochleae, testis and epipdidymis.
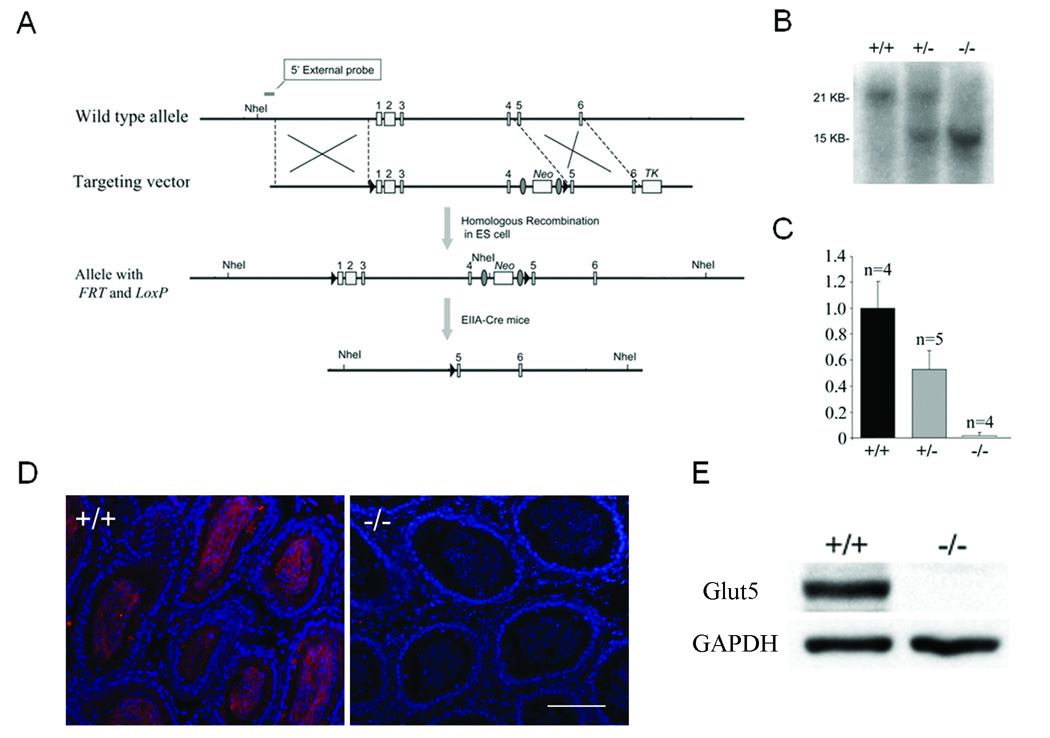
(A) Strategy for targeted deletion of the Glut5 gene. TK, thymidine kinase gene. (B) Tail genomic Southerns (digested with NheI) from wildtype (+/+), Glut5 heterozygous (+/−) and Glut5 homozygous (−/−) N1F3 mice after the floxed Glut5 portion was deleted by crossing with EIIA Cre mice. (C) Real-time RT-PCR analysis of cochlear Glut5 mRNA levels in wildtype (+/+), heterozygous (+/−), and homozygous (−/−) mice at P9. Y axis: relative ratio. Bars: S.E.M. n: the number of mice used in each genotype. Similar results were obtained in kidney. (D) Immunostaining of Glut5 in epididymis of wildtype and Glut5−/− mice. Glut5: red; DAPI: blue. Bar=80µm. Similar results were obtained in epidydimis. (E) Western analysis of Glut5 in testis. Gapdh is used as a loading control.
A Southern blot screen showed that 14 among 160 ES clones picked had undergone homologous recombination. Two of the positive ES clones with normal karyotype were used for blastocyst injection. High chimeric mice from either clone were crossed with C57BL6/J mice to achieve F1 germline transmitted heterozygous mice that carried the Glut5floxed allele.
Intercrosses between Glut5floxed/+ and a ubiquitous Cre expressing line EIIA-Cre [24] resulted in offspring with the expected deletion of the floxed exons 1–4 of Glut5 (namely the Glut5del1–4 allele) (Fig. 1B). Because the Glut5del1–4 allele was subsequently maintained and analyzed in the absence of EIIA-Cre, the deletion of floxed exons 1–4 of Glut5 was indeed transmitted germline.
As expected, no Glut5 transcript was detected in the cochlea or the kidney of Glut5del1–4/del1–4 mice whereas the mRNA levels in cochlea and kidney from Glut5del1–4/+ mice were approximately 0.51 and 0.38 fold, respectively, of those from wildtype littermates (Fig. 1C and data not shown).
It was reported that two splicing variances of Glut5 mRNA exist in mouse [11]. Both splicing variances occur upstream of ATG start codon in exon3 according to updated genomic sequence from www.ensembl.org [11]. Thus, both splicing forms of Glut5 mRNA will be deleted with the 5’untranslated region, the ATG start codon, and the first 43 amino-acids of the coding sequence by our knockout strategy. Fig. 1 also shows results of immunostaining and Western blots using the anti-mouse Glut5-C terminal antibody [38]. In wildtype mice, Glut5 was highly expressed in the sperm of testis and epididymis but no Glut5 was detected from either testis or epididymis in Glut5del1–4/del1–4 mice (Fig. 1D). Similar results were obtained using Western blot analysis of the testis (Fig. 1E). These results indicate that the remaining 3’ coding part of GLUT5 gene does not generate a truncated Glut5 protein. Glut5 protein was, therefore, successfully deleted in Glut5del1–4/del1–4 mice, i.e., Glut5del1–4/del1–4 mice are Glut5-null or Glut5−/−.
2.2. Expression of Glut5 in OHCs
Because of the presence of Glut5 mRNA in the wildtype whole cochleae (Fig. 1C) and previous reports of the presence of Glut5 protein in the OHC’s lateral wall [7,19], we extensively examined whether Glut5 protein is present in mouse OHCs. We first performed immunostaining using the same antibody (anti-huGLUT5-C antibody, #4670-1756, Biogenesis, Brentwood, NH, or catalog #AB1048, Chemicon, Temecula, CA) reported to detect the positive signal along the lateral wall of OHCs from rat and mouse [7,19]. In our hands (both Zuo and Dallos labs), this antibody indeed stained the OHC’s lateral wall in wildtype mice but surprisingly did not stain OHCs in prestin knockout mice [40]. However, this antibody did not label sperm in wildtype mouse testis sections, a well-known positive control for Glut5 (data not shown). Indeed, this antibody was known not to react with rodent Glut5 (Biogenesis data sheet). Furthermore, after 2005, the new batches of antibody with the same catalog number (Biogenesis) and likely derived from a new rabbit, did not label the lateral wall of wildtype OHCs or sperm (data not shown). Therefore, the signal in the OHC’s lateral wall detected by this anti-human GLUT5 antibody is likely a cross-reaction with some unknown protein(s) but not Glut5. Why its signal is lost in prestin knockout OHCs remains a mystery. Furthermore, using our custom-made anti-mouse Glut5-C antibody [38] and the anti-rat Glut5-N antibody (SC-14844), we found no significant differences between wildtype and knockout mice and no signals in OHCs (Fig. 2A).
Fig. 2. Expression of Glut5 in cochleae.
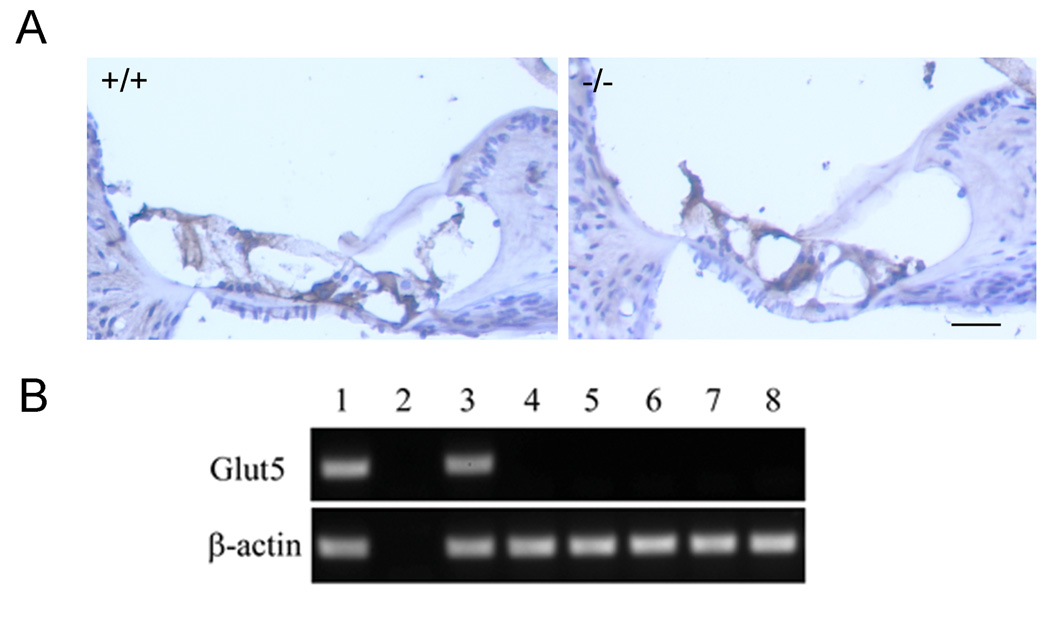
(A) Immunostaining of cochlear sections using the custom-made anti-mouse Glut5-C antibody. No significant differences were observed between wildtype and Glut5−/− mice. Both cochlear sections show similar background staining in some cell types, which suggest some cross reactivity. Identical background staining was observed between both genotypes using different secondary antibodies and antigen-retrieval procedures (data not shown). Bar=40µm. (B) RT-PCR analysis of laser-captured OHCs and other cells in the wildtype mouse cochlear sections (see controls for other cell-type specific markers which were performed on same batches of cochlear sections as in [17]). Lane 1: positive control of cDNA from P9 whole cochleae as the template. Lane 2: negative control of mRNA of P9 whole cochleae without reverse transcription as the template. Lane 3: all cells scraped from cochlear sections. Lanes 4–8: laser-captured (LC) cell types from cochlear sections: 4. inner hair cells; 5. outer hair cells; 6. Deiters cells; 7. Claudius cells; 8. spiral ganglia.
To definitively determine if Glut5 mRNA is present in OHCs, we also performed RT-PCR analysis from isolated wildtype mouse OHCs by using laser-capture microscopy (LCM) [17] and from a mouse OHC cDNA pool made by using LCM method [2]. Both analyses yielded negative results in OHCs, although a Glut5 message is detected in the whole cochlear cDNA (Fig. 2B). Because three pairs of primers from three different regions of Glut5 cDNA were used in these analyses, cochlea-specific alternative splicing in addition to the two known splicing forms is unlikely [11].
2.3. Morphological analysis of Glut5−/− cochleae
The Glut5−/− mice appeared to behave normally and have normal body weight. For analysis of Glut5−/− cochlear morphology, we evaluated plastic sections stained with toluidine-blue and paraffin sections with H&E staining. No obvious defects were found in Glut5−/− cochleae (data not shown). Detailed subcellular structures of the lateral wall of OHCs were further examined using transmission electronic microscopy (Fig. 3A). In the lateral wall of OHCs from Glut5−/− mice, the plasma membrane exhibited the typical wavy surface observed in wildtype OHCs: one layer of subsurface cisternae (SSC) and no apparent abnormalities [39]. In addition, a row of evenly distributed dots, presumably the pillars, were connected between SSC and plasma membrane in homozygotes. These results indicate that the tri-laminar structure appeared intact in Glut5−/− OHCs.
Fig. 3.
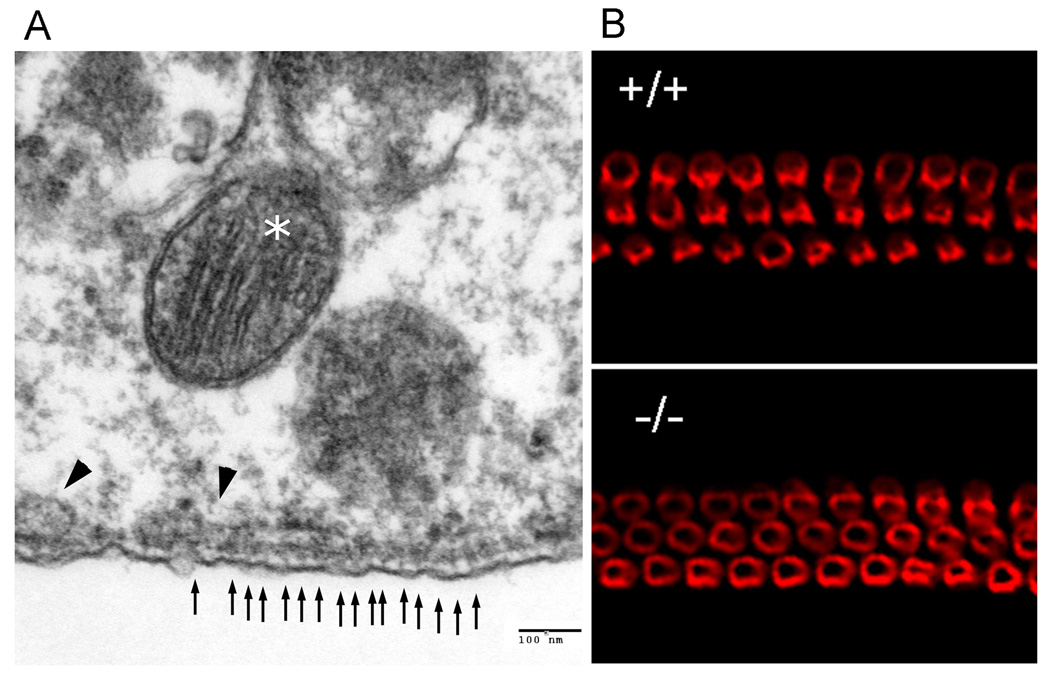
(A) Normal tri-laminar structure in the lateral walls of Glut5−/− mice. A row of evenly distributed dots (small arrows), most likely the pillars, is found along the lateral wall in OHCs of Glut5−/− mice. One layer of SSC (arrow heads) is located inside the wavy plasma membrane. In addition, many mitochondria (asterisk) are also positioned in the cytoplasm close to the lateral wall. (B) Prestin immunostaining in whole-mount preparations from the basal turns of wildtype and Glut5−/− mice. Deletion of Glut5 does not affect the targeting of prestin to the OHC lateral wall.
To determine if prestin was affected by the disruption of Glut5, immunostaining using a prestin C-terminus antibody was performed on the Glut5−/− cochlear sections and whole-mount basilar membrane specimens at P28 (Fig. 3B). The prestin signal appeared in the lateral wall of OHCs in Glut5−/− mice and no significant hair cell loss was observed in Glut5−/− cochleae (data not shown). In addition, Myosin7a and Myosin1c, the two hair-bundle related motor proteins, and synaptophysin, a synaptic marker of OHCs, were also present in Glut5−/− mice (immunostaining data not shown).
We also examined potential defects in the glycogen content of OHCs in Glut5−/− mice. However, no significant differences were observed between wildtype and Glut5−/− OHCs (data not shown) [15].
2.4. Electromotility and NLC of isolated Glut5−/− OHCs
To ascertain whether the electromotility and NLC of OHCs isolated from Glut5−/− mice were affected, we directly measured both electromotility and NLC in isolated OHCs from wildtype and Glut5−/− mice (Fig. 4). The cell lengths of wildtype and Glut5−/− OHCs at −70 mV holding potential for motility measurements were 27.4±1.5 µm (n=9) and 25.4±1.3 µm (n=7). We compared the magnitude of maximal motile response (saturated response) at the membrane potentials of −100 and +60 mV. The motility magnitude of wildtype and Glut5−/− OHCs was 955.7±284.6 nm (n=9) and 1150.8±291.9 nm (n=7). A Student’s t-test showed that no significant differences existed between wildtype and Glut5−/− OHC motility magnitudes (p=0.2). Moreover, Glut5−/− OHCs (n=7) displayed wildtype-like (n=9) NLC: for wildtype vs Glut5−/−, Qmax=968.9±118.5 vs 936.2±191.5 fC, alpha=30.9±3.7 vs 31.4±2.3 mV, Vpkcm=−54.2±11.5 vs −50.7±14.0 mV, charge density=127.9±10.6 vs 125.2±40.5 fC/pF. Student’s t-tests showed no significant differences between wildtype (n=9) and Glut5−/− (n=7) OHC (Qmax: p=0.65, alpha: p=0.72, Vpkcm: p=0.58, charge density: p=0.85).
Fig. 4.
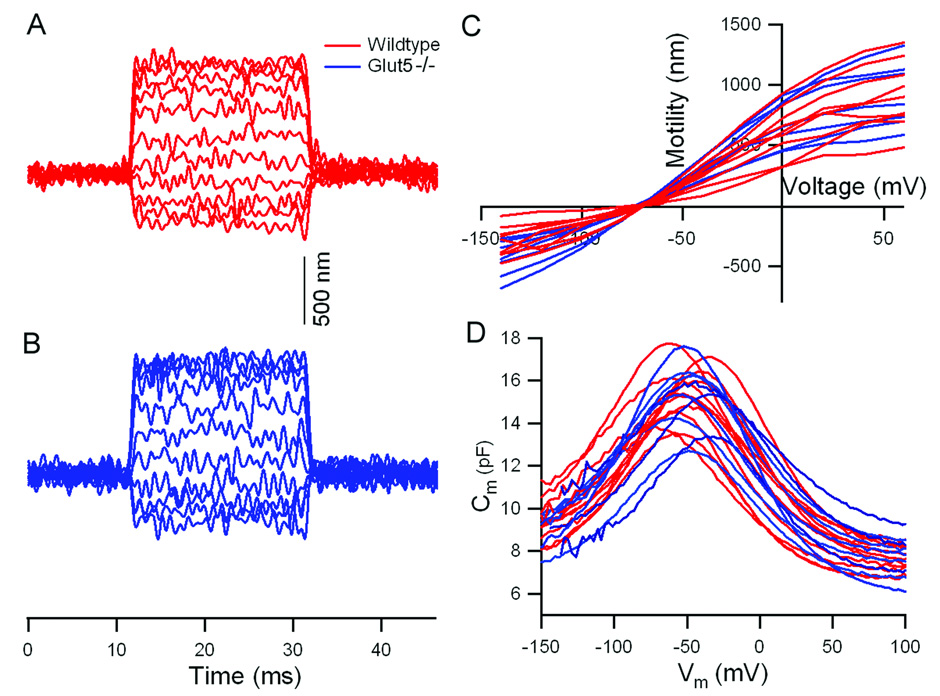
Motility and NLC measured from +/+ and Glut5−/− OHCs in vitro. (A, B) Representative examples of electromotility from +/+ (red) and Glut5−/− (blue) OHCs measured when the membrane potential was stepped from a holding potential of −70 mV to different values (from −140 to 60 mV) at 20-mV increments. Contraction is plotted upward. (C) Individual motility functions obtained from +/+ (red) and Glut5−/− (blue) OHCs. (D) Individual NLC curves (+/+ in red, Glut5−/− in blue) measured using the two-sine voltage stimulus protocol (10mV peak at 390.6 and 781.2Hz) with subsequent fast Fourier transform-based admittance analysis.
2.5. Cochlear physiology
We further characterized Glut5−/− mice between 1 and 2 months of age by measuring thresholds, input-output functions and tone-on-tone masking tuning curves for the compound action potential (CAP) (Fig. 5) [10,18]. No changes in sensitivity, tuning or high-level outputs were detected in Glut5−/− mice when compared to wildtype controls. Auditory brainstem response (ABR) thresholds with click, 4, 8, 16, and 32 kHz stimuli were also similar between Glut5−/− and wildtype mice at P28 (data not shown).
Fig. 5.
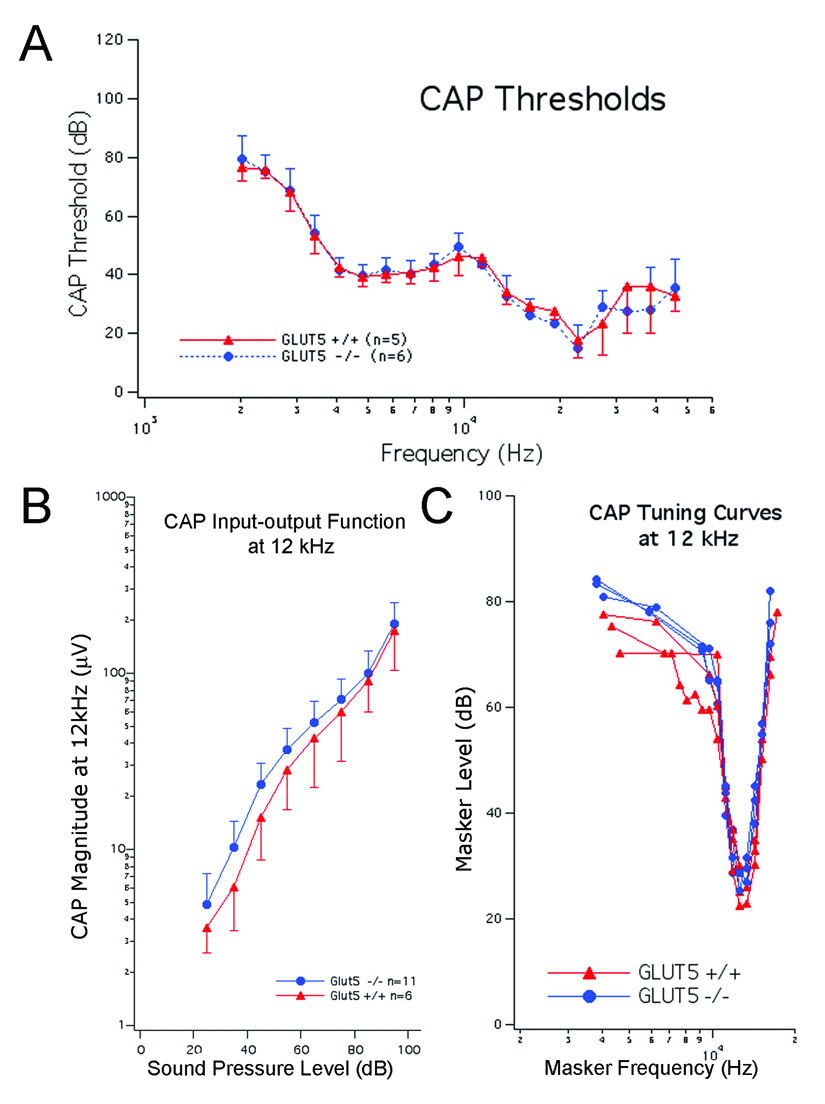
In vivo cochlear physiology of Glut5−/− mice. All measurements were obtained from +/+ (red) and Glut5−/− (blue) mice at 1–2 months of age. Bars represent standard deviations. (A) Mean CAP thresholds. (B) Mean CAP input-output functions at 12 kHz. (C) CAP tuning curves at 12 kHz obtained using simultaneous masking.
3. Discussion
In our characterization of Glut5−/− mice, we discovered that the lack of Glut5 did not alter cochlear morphology, OHC electromotility or NLC. After measuring various indices of peripheral and central auditory physiology (i.e., ABR and CAP), we also demonstrated that the lack of Glut5 did not change cochlear amplification or tuning. At the morphological level, no apparent abnormality was observed in the cochleae of Glut5−/− mice. Immunostaining showed normal expression of prestin, myosin1c, myosin7a, and synaptophysin. TEM analysis (Fig. 3) also displayed the regular tri-laminar structure in the lateral wall of OHCs.
Given that we could not definitively demonstrate, despite numerous attempts with various antibodies, that Glut5 is present in wildtype OHCs, and that no Glut5 mRNA was detected in our two sensitive OHC RT-PCR methods, we conclude that Glut5 is either not expressed, or expressed at undetectable levels. Immunostaining results in previous publications were obtained using a commercial antibody raised against a human GLUT5 C-terminal peptide. It is now known that this antibody does not stain sperm in mice, and does not react with rodent tissues. More importantly, the experiments lacked the most appropriate negative controls, i.e., Glut5−/− mice. Hence, it remains possible that the human antibody recognized a Glut5-like epitope as suggested initially [19] and that such an epitope was missing in prestin knockout mouse OHCs. Unfortunately, this original antibody is now unavailable, making further tests impossible.
In support, we found that Glut5 is absent in all accessible cDNA libraries constructed from various tissues (inner ear, cochlea, otocyst, organ of Corti, or hair cells) from several species including mouse and analyzed by various groups (dbEST ID: 18222; 9974; 11139; 10920; 4088; 14415; 16641) [6,23,27,31–34,41]. Based on cochlear RT-PCR results, however, Glut5 mRNA is present in other cochlear cells of wildtype mice, although the cochlear cDNA used could have been contaminated with blood cells. Hence, a functional Glut5 could still be present in OHCs with a long half-life and extremely low mRNA level. Therefore, it is possible that interactions between prestin and Glut5 in transfected 293 cells occurred predominantly in subcellular compartments during protein maturation and sorting, rather than at the plasma membrane [38]. In addition, it is possible that the interaction between Glut5 and prestin only exist when they are both over-expressed in a heterologous system. A possible role of Glut5 in the metabolism of OHCs requires further study. Regardless, our results in Glut5−/− mice demonstrate that this transporter is not required for normal OHC electromotility and cochlear amplification in vivo.
4. Experimental procedure
4.1. The floxed Glut5 targeting construct
Three BAC clones (C466F17, C87K12, and C83I21, Invitrogen) were identified containing the Glut5 gene in RPCI22 mouse BAC high-density membranes. A 14.4kb Glut5 genomic fragment was retrieved from the BAC clone to make the targeting vector as described previously [18]. The targeting vector for the floxed Glut5 was finalized after the FRT-neo-FRT-loxP cassette was inserted into the plasmid.
4.2. Embryonic stem (ES) cells
Linearized targeting vector was electroporated into 129/SvEv (129S6) ES cells. The ES cell clones were screened by Southern blot using an external probe and primers G5 EP 3903 (5’-GAG CAA GCT GGT CAT GCA TCT) and G5EP 4153 (5’-CCA GTG CTC ACC CAA CGT TT), which detected a 19 kb band for the wildtype allele and a 6 kb band for the floxed allele.
4.3. Germline transmission
High chimera mice generated from blastocyte injection were crossed with C57BL6/J and both lines (E7 and A11) went through germline. F1 mice carrying the floxed Glut5 allele were then either intercrossed or crossed with EIIA-Cre mice. To eliminate the EIIA-Cre mosaic effect, we chose germline transmitted knockout mice that were negative for Cre in the N1F3 and N1F4 generations. The primers used for genotyping are listed as follows: G5 Flox 931F (5’-GCT GTG CTC GAC GTT GTC AC) and G5 Flox 1431 R (5’-ACC GTA AAG CAC GAG GAA GC) for the floxed allele; G5 KO-69F (5’-GTT GGT CGC GTT GAA CTG C) and G5 KO-271R (5’-AGG GCA CAG ACC GAC AGA AC) for the deleted Glut5 allele; G5 geno F (5’-GGC AGT GTG TGG AGT CAT CG) and G5 geno R (5’-GGG AAC ATG GAC ACC GTC AG) for wildtype allele; Cre F (5’-TGC AAC GAG TGA TGA GGT TC) and Cre R (5’-ACG AAC CTG GTC GAA ATC AG) for the Cre allele.
4.4. RNA purification and real-time RT-PCR
Tissue RNA was prepared with Omniscript Reverse Transcriptase as described previously [25]. For real-time PCR, specific primers and probe covering exons 9~10 of the Glut5 cDNA (G5 taq 668F: 5’-TGC TGA TCC AGA AGA AAG ATG AAG; G5 taq 727R: 5’-CGT CTT TCC AGC CTC GGA; G5 taqman probe: 6FAM-AGC TGC TGA GAG AGC CCT CCA GAC C-BOH) were used. Real-time PCR was performed as previously described [25].
4.5. Laser capture microdissection
These procedures were described previously [2,17]. Laser capture microdissection was performed using the PixCell II system (Arcturus). We employed a method described previously [30] but with some modifications. Briefly, mice (8 weeks old) were perfused intracardially with 4% PFA in phosphate buffer. Cochleae were then post-fixed 8 minutes [2] or overnight, decalcified, embedded in paraffin, and sectioned at 12 µm. We used the Paradise Whole Transcript RT Reagent System (Arcturus, Mountain View, CA) to purify mRNA from both whole cells and laser captured cells from cochlear sections. The mRNA pool size range was 0.1 to 1.2 kb. For PCR, we designed 1 pair of primers to amplify the cDNA of Glut5 (G5-RT-PCR-F: 5’-AAT GGG CTG CAG CCA AAT TG; and G5-RT-PCR-R: 5’-GAA GGC CAA ACA GCT GGG C) and another pair for β-actin (β-actin-F: 5’-AAT TTC TGA ATG GCC CAG GT and β-actin-R: 5’-TGT GCA CTT TTA TTG GTC TCA A). We used cDNA from whole P9cochleae as a positive control, and mRNA from whole P9 cochleae without reverse transcription as a negative control.
4.6. ABR recording
The ABR assay was performed as described previously [18,39]. Briefly, click and tone pips at 4, 8, 16, and 32 kHz were generated using a Tucker Davis Technologies (TDT, Gainsville, FL) workstation (System III).
4.7. NLC and electromotility of OHCs
The OHC electromotility and NLC were measured as described previously [18,21].
4.8. CAP measurements
Cochlear in vivo physiology was studied in +/+ and Glut5−/− mice anaesthetized with Sodium Pentobarbital (80 mg/kg, IP) as described previously [10,13,18].
4.9. Immunohistochemistry
Mice were intracardiacally perfused and fixed with 4% PFA. The inner ear was isolated, post-fixed, decalcified, and embedded in paraffin. De-paraffinized sections were routinely treated with 10 mM sodium citrate buffer (pH=6.0) in 85°C for 15 min for unmasking antigen. Slides were blocked with blocking solution, incubated in primary antibody overnight at 4°C, rinsed with PBS five times. Slides were then incubated in either Alexa 488-conjugated secondary antibody raised in goat (Molecular Probes, Eugene, OR) or processed with Vectastain ABC kit according to the product manual (Vector). Fluorescence stained sections were mounted on the slide with the Vectashield mounting media with DAPI (Vector). DAB stained sections were counterstained shortly with Hematoxylin.
4.10. TEM
Tissues were briefly washed in 0.1 M PBS, post-fixed in 0.8% osmium tetroxide/ 3% ferrocyanide/ 0.1 M PBS for 2 hours, and washed with deionized, distilled water, then dehydrated through a series of ascending concentrations of ethanol and stained en bloc with 2% uranyl acetate/100% ETOH under a vacuum for 1 hour at 60°C, and embedded in Spurr's resin (Ted Pella), polymerized for 2 days at 60°C. Ultra-thin sections were cut with a diamond knife, counterstained, and observed by transmission electron microscopy.
4.11. Western blot
50 µg of sperm whole cell lysates were subjected to SDS-polyacrylamide gel electrophoresis, followed by blotting onto a polyvinyliden difluoride membrane (Millpore). Because heat may induce the formation of high-molecular-weight membrane protein aggregation, sperm whole cell lysates were not boiled. Primary antibodies used were custom-made anti-mouse Glut5-C terminal antibody (0.5 µg/ml) [38], and anti-GAPDH monoclonal antibody (1:1000 dilution, Chemicon). The membrane was then incubated for 1 h at room temperature with appropriate secondary antibody coupled with horseradish peroxidase. Immunoreactive bands were visualized by ECL detection reagents (GE Healthcare, Piscataway, NJ).
Acknowledgments
This work is supported in part by ALSAC, The Hugh Knowles Center and by NIH grants DC00089 (to P. D.), DC06471, DC05168, DC008800 and CA21765 (to J. Zuo), DC 004696 (to D.Z.Z.H.), DC006412 (to J. Zheng). J. Zuo is a recipient of a Hartwell Individual Biomedical Research Award.
Abbreviations
- Glut5
Glucose transporter 5
- OHCs
outer hair cells
- NLC
nonlinear capacitance
- TEM
transmission electron microscopy
- ABR
Auditory brainstem response
- LCM
laser-capture microscopy
- CAP
compound action potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler HJ, Belyantseva IA, Merritt RC, Jr., Frolenkov GI, Dougherty GW, Kachar B. Expression of prestin, a membrane motor protein, in the mammalian auditory and vestibular periphery. Hear Res. 2003;184:27–40. doi: 10.1016/s0378-5955(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CT, Zheng J. Isolation of outer hair cells from the cochlear sensory epithelium in whole-mount preparation using laser capture microdissection. J Neurosci Methods. 2007;162:229–236. doi: 10.1016/j.jneumeth.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angulo C, Rauch MC, Droppelmann A, Reyes AM, Slebe JC, Delgado-Lopez F, Guaiquil VH, Vera JC, Concha Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J Cell Biochem. 1998;71:189–203. [PubMed] [Google Scholar]
- 4.Ashmore JF. Transducer motor coupling in cochlear outer hair cells. In: Wilson JP, Kemp DT, editors. Cochlear Mechanisms. London: Plenum Press; 1989. pp. 107–116. [Google Scholar]
- 5.Ashmore JF, Geleoc GS, Harbott L. Molecular mechanisms of sound amplification in the mammalian cochlea. Proc Natl Acad Sci U S A. 2000;97:11759–11764. doi: 10.1073/pnas.97.22.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beisel KW, Shiraki T, Morris KA, Pompeia C, Kachar B, Arakawa T, Bono H, Kawai J, Hayashizaki Y, Carninci P. Identification of unique transcripts from a mouse full-length, subtracted inner ear cDNA library. Genomics. 2004;83:1012–1023. doi: 10.1016/j.ygeno.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Belyantseva IA, Adler HJ, Curi R, Frolenkov GI, Kachar B. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J Neurosci. 2000;20:RC116. doi: 10.1523/JNEUROSCI.20-24-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- 9.Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992;267:14523–14526. [PubMed] [Google Scholar]
- 10.Cheatham MA, Huynh KH, Gao J, Zuo J, Dallos P. Cochlear function in Prestin knockout mice. J Physiol. 2004;560:821–830. doi: 10.1113/jphysiol.2004.069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corpe CP, Bovelander FJ, Munoz CM, Hoekstra JH, Simpson IA, Kwon O, Levine M, Burant CF. Cloning and functional characterization of the mouse fructose transporter, GLUT5. Biochim Biophys Acta. 2002;1576:191–197. doi: 10.1016/s0167-4781(02)00284-1. [DOI] [PubMed] [Google Scholar]
- 12.Dallos P. The active cochlea. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dallos P, Cheatham MA. Compound action potential (AP) tuning curves. J Acoust Soc Am. 1976;59:591–597. doi: 10.1121/1.380903. [DOI] [PubMed] [Google Scholar]
- 14.Davis H. An active process in cochlear mechanics. Hear Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- 15.Ding DL, McFadden SL, Wang J, Hu BH, Salvi RJ. Age- and strain-related differences in dehydrogenase activity and glycogen levels in CBA and C57 mouse cochleas. Audiol Neurootol. 1999;4:55–63. doi: 10.1159/000013822. [DOI] [PubMed] [Google Scholar]
- 16.Forge A. Structural features of the lateral walls in mammalian cochlear outer hair cells. Cell Tissue Res. 1991;265:473–483. doi: 10.1007/BF00340870. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Maison SF, Wu X, Hirose K, Jones SM, Bayazitov I, Tian Y, Mittleman G, Matthews DB, Zakharenko SS, Liberman MC, Zuo J. Orphan glutamate receptor delta1 subunit required for high-frequency hearing. Mol Cell Biol. 2007;27:4500–4512. doi: 10.1128/MCB.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Wang X, Wu X, Aguinaga S, Huynh K, Jia S, Matsuda K, Patel M, Zheng J, Cheatham M, He DZ, Dallos P, Zuo J. Prestin-based outer hair cell electromotility in knockin mice does not appear to adjust the operating point of a cilia-based amplifier. Proc Natl Acad Sci U S A. 2007;104:12542–12547. doi: 10.1073/pnas.0700356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geleoc GS, Casalotti SO, Forge A, Ashmore JF. A sugar transporter as a candidate for the outer hair cell motor. Nat Neurosci. 1999;2:713–719. doi: 10.1038/11174. [DOI] [PubMed] [Google Scholar]
- 20.Gulley RL, Reese TS. Regional specialization of the hair cell plasmalemma in the organ of corti. Anat Rec. 1977;189:109–123. doi: 10.1002/ar.1091890108. [DOI] [PubMed] [Google Scholar]
- 21.Jia S, He DZ. Motility-associated hair-bundle motion in mammalian outer hair cells. Nat Neurosci. 2005;8:1028–1034. doi: 10.1038/nn1509. [DOI] [PubMed] [Google Scholar]
- 22.Kalinec F, Holley MC, Iwasa KH, Lim DJ, Kachar B. A membrane-based force generation mechanism in auditory sensory cells. Proc Natl Acad Sci U S A. 1992;89:8671–8675. doi: 10.1073/pnas.89.18.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klockars T, Perheentupa T, Dahl HH. In silico analyses of mouse inner-ear transcripts. J Assoc Res Otolaryngol. 2003;4:24–40. doi: 10.1007/s10162-002-2058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig J, Oliver D, Frank G, Klocker N, Gummer AW, Fakler B. Reciprocal electromechanical properties of rat prestin: the motor molecule from rat outer hair cells. Proc Natl Acad Sci U S A. 2001;98:4178–4183. doi: 10.1073/pnas.071613498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott BM, Jr., Baucom JM, Hudspeth AJ. Analysis and functional evaluation of the hair-cell transcriptome. Proc Natl Acad Sci U S A. 2007;104:11820–11825. doi: 10.1073/pnas.0704476104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto K, Tatsumi S, Morimoto A, Minami H, Yamamoto H, Sone K, Taketani Y, Nakabou Y, Oka T, Takeda E. Characterization of the rabbit intestinal fructose transporter (GLUT5) Biochem J. 1994;303(Pt 3):877–883. doi: 10.1042/bj3030877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakazawa K, Spicer SS, Schulte BA. Postnatal expression of the facilitated glucose transporter, GLUT 5, in gerbil outer hair cells. Hear Res. 1995;82:93–99. doi: 10.1016/0378-5955(94)00162-j. [DOI] [PubMed] [Google Scholar]
- 30.Pagedar NA, Wang W, Chen DH, Davis RR, Lopez I, Wright CG, Alagramam KN. Gene expression analysis of distinct populations of cells isolated from mouse and human inner ear FFPE tissue using laser capture microdissection--a technical report based on preliminary findings. Brain Res. 2006;1091:289–299. doi: 10.1016/j.brainres.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 31.Peters LM, Belyantseva IA, Lagziel A, Battey JF, Friedman TB, Morell RJ. Signatures from tissue-specific MPSS libraries identify transcripts preferentially expressed in the mouse inner ear. Genomics. 2007;89:197–206. doi: 10.1016/j.ygeno.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pompeia C, Hurle B, Belyantseva IA, Noben-Trauth K, Beisel K, Gao J, Buchoff P, Wistow G, Kachar B. Gene expression profile of the mouse organ of Corti at the onset of hearing. Genomics. 2004;83:1000–1011. doi: 10.1016/j.ygeno.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Robertson NG, Khetarpal U, Gutierrez-Espeleta GA, Bieber FR, Morton CC. Isolation of novel and known genes from a human fetal cochlear cDNA library using subtractive hybridization and differential screening. Genomics. 1994;23:42–50. doi: 10.1006/geno.1994.1457. [DOI] [PubMed] [Google Scholar]
- 34.Roche JP, Wackym PA, Cioffi JA, Kwitek AE, Erbe CB, Popper P. In silico analysis of 2085 clones from a normalized rat vestibular periphery 3' cDNA library. Audiol Neurootol. 2005;10:310–322. doi: 10.1159/000087348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos-Sacchi J, Navarrete E. Voltage-dependent changes in specific membrane capacitance caused by prestin, the outer hair cell lateral membrane motor. Pflugers Arch. 2002;444:99–106. doi: 10.1007/s00424-002-0804-2. [DOI] [PubMed] [Google Scholar]
- 37.Sugawara-Yokoo M, Suzuki T, Matsuzaki T, Naruse T, Takata K. Presence of fructose transporter GLUT5 in the S3 proximal tubules in the rat kidney. Kidney Int. 1999;56:1022–1028. doi: 10.1046/j.1523-1755.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Currall B, Yamashita T, Parker LL, Hallworth R, Zuo J. Prestin-prestin and prestin-GLUT5 interactions in HEK293T cells. Dev Neurobiol. 2007;67:483–497. doi: 10.1002/dneu.20357. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Gao J, Guo Y, Zuo J. Hearing threshold elevation precedes hair-cell loss in prestin knockout mice. Brain Res Mol Brain Res. 2004;126:30–37. doi: 10.1016/j.molbrainres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Gao J, Zuo J. Apoptotic cell death in the organ of Corti and absence of Glut5 in outer hair cells in prestin knock-out mice. Daytona, FL: ARO midwinter meeting; 2003. [Google Scholar]
- 41.Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]


