Abstract
Background
Emotion regulation strategies are thought to differ in when and how they influence the emotion-generative process. However, no study to date has directly probed the neural bases of two contrasting (e.g., cognitive versus behavioral) emotion regulation strategies. This study used functional magnetic resonance imaging (fMRI) to examine cognitive reappraisal (a cognitive strategy thought to have its impact early in the emotion-generative process) and expressive-suppression (a behavioral strategy thought to have its impact later in the emotion-generative process).
Methods
Seventeen women viewed 15s neutral and negative-emotion eliciting films under four conditions - watch neutral, watch negative, reappraise negative, and suppress negative - while providing emotion experience ratings and having their facial expressions videotaped.
Results
Reappraisal resulted in early (0−4.5s) prefrontal cortical (PFC) responses, decreased negative emotion experience, and decreased amygdala and insular responses. Suppression produced late (10.5−15s) PFC responses, decreased negative emotion behavior and experience, but increased amygdala and insula responses.
Conclusions
These findings demonstrate the differential efficacy of reappraisal and suppression on emotional experience, facial behavior, and neural response, and highlight intriguing differences in the temporal dynamics of these two emotion regulation strategies.
Keywords: Emotion, emotion regulation, cognitive control, insula, amygdala, fMRI
Introduction
From moment-to-moment, emotions influence attention (1), decision-making (2), memory (3), physiological responses (4; 5), and social interactions (6). However, even as they shape a wide range of intra- and inter-personal processes, emotions are themselves subject to modification. The ability to successfully regulate emotion is related to a number of important psychological, social and physical health outcomes (7-9). Conversely, difficulties with emotion regulation have been postulated as a core mechanism underlying mood and anxiety disorders (10). Understanding the differential impact of distinct types of emotion regulation on experience, behavior and neural dynamics may inform clinical practice and research.
To study emotion regulation (ER), we employed a theoretically-derived process model of emotion regulation that delineates when in the emotion-generative process different strategies have their primary impact (11). This model distinguishes between antecedent-focused strategies, which modulate emotional response tendencies early on, before they give rise to full-fledged responses, and response-focused strategies, which modulate the emotional responses themselves later on once they have arisen. Here we focus on one antecedent-focused (cognitive reappraisal) and one response-focused (expressive-suppression) strategy that differentially influence negative emotion experience, behavior, physiological responses (12).
Reappraisal is a cognitive-linguistic strategy that alters the trajectory of emotional responses by reformulating the meaning of a situation. Reappraisal can intervene relatively early in the emotion-generative process, recruiting executive cognitive control processes instantiated in a distributed brain network, including areas in medial, dorsolateral and ventrolateral PFC, and dorsal anterior cingulate cortex (ACC) (13-20). Reappraisal effectively down-regulates emotional experience and behavior, startle eye blink response (21) and emotion-related neural responses that together modulate ongoing emotion experience in emotion-appraisal brain systems, including the amygdala, subgenual ACC, ventromedial PFC, and insula (22). Over the long-term, frequent use of reappraisal leads to enhanced control of emotion, interpersonal functioning, and psychological and physical well-being (23).
Expressive-suppression is a strategy directed towards inhibiting behaviors associated with emotional responding (e.g., facial expressions, verbal utterances, gestures). Suppression is, by definition, implemented following emotion generation, produces decreased expressive behavior, typically with little or no change in ongoing emotion experience, and increased sympathetic activation of the cardiovascular system (7). No neuroimaging study has examined expressive suppression in response to emotional stimuli. Investigations of inhibitory control in human and non-human primates, however, suggest that the right ventrolateral PFC is associated with volitional response inhibition (24-31) and overriding prepotent imitative facial responses by producing an opposing expression (32). Because suppression has different effects on emotional expression, behavior, and physiology, it is unknown whether suppression produces increased, decreased or unaltered amygdala and insula activity. Over the long-term, frequent use of expressive-suppression results in diminished control of emotion, interpersonal functioning, memory, well-being, and greater depressive symptomatology (23).
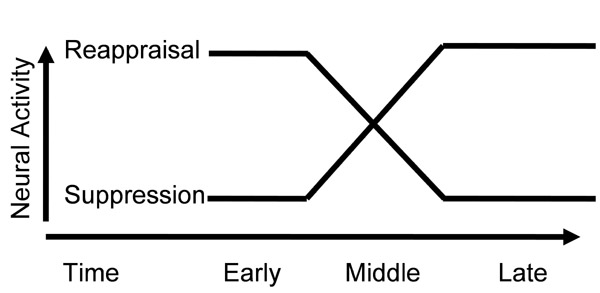
We have interpreted the differential consequences of reappraisal and suppression as arising from their differential temporal characteristics. In our model (Figure 1), reappraisal involves early selection and implementation of a cognitive strategy that diminishes emotion without the need for sustained effort over time. Suppression, in contrast, involves increasing efforts to actively inhibit prepotent facial emotion behaviors as they arise in response to emotion-inducing stimuli.
Figure 1.
Hypothesized temporal dynamics of prefrontal cortex emotion regulatory neural activity related to cognitive reappraisal and expressive suppression.
The aim of the present study was to test the temporal dynamics and consequences of reappraisal and suppression. We used films to generate a transient but powerful negative affective state. Disgust, an evolutionarily derived “aversive affective state evoked by repulsive stimuli” (33), p. 3) was chosen as the target emotion because it reliably induces robust emotional experience, emotion-expressive behavior (34), and increased insula, PFC, and amygdala activation (35; 36). We employed a within-subjects design to compare the effects of reappraisal and suppression on emotion experience, expressive behavior, and neural response in the context of disgust-eliciting films.
Methods and Materials
Participants
Seventeen right-handed females (mean age=22.7± 3.5 years), who reported no history of psychiatric or medical disorders or medication use, provided informed consent and were paid $20 per hour. Women were selected because they show stronger emotional responses than men in the context of disgust-inducing stimuli (37).
Film Stimuli
Forty 15s film clips, consisting of 10 non-affective neutral nature scenes and 30 disgust-inducing surgical procedures, vomiting and animal slaughter, were rated by a different group of 19 young females on a scale from 1=not negative to 5=extremely negative. This group reported significantly greater negative emotion experience for negative versus neutral films (negative mean=2.83 vs. neutral mean=1.09; t(18)=9.18, p<.001).
Procedure
Pre-MRI, participants were trained in specific reappraisal and suppression strategies while viewing 8 practice films. Reappraisal instructions encouraged thinking objectively to decrease emotional reactivity to films, for example, by assuming the perspective of a medical professional watching an instructional video or focusing on technical aspects of the film. Suppression instructions focused on training participants to keep their face still while viewing films so that someone watching their face would not be able to detect what was being experienced subjectively. During MRI, films were visually projected to a screen 6 inches from the participant's eyes inside the head-coil. Button responses were recorded using Eprime software. The film viewing task consisted of three 9-minute runs.
Experimental Task
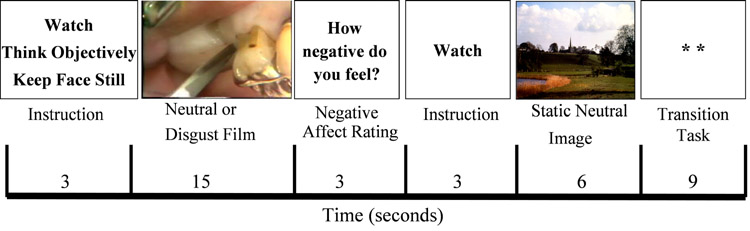
There were two counterbalanced pseudo-randomized orders of the 40 film stimuli that matched negative films with the different instructions to reappraise, suppress or watch. The task consisted of four conditions: 10 watch-neutral, 10 watch-negative, 10 reappraise-negative, and 10 suppress-negative trials. Each trial consisted of: 1) 3s instruction (“Watch”, “Think objectively,” “Keep face still”), 2) 15s film, (3) 3s How negative do you feel? rating (1=not at all to 3=moderately to 5=extremely), 4) 3s Watch instruction, and 5) 6s static landscape image (Figure 2). There were no order effects on negative emotion ratings, disgust expressive-behavior, and BOLD responses for watch-negative versus watch-neutral films (all p's>.22).
Figure 2.
Experimental design for a single trial. The experiment consisted of 40 trials with 40 unique film clips. There were 10 trials each of watch neutral, watch negative, reappraise negative, and suppress negative. A single trial consisted of a 3s instruction to either Watch, Think objectively, or Keep face still, 15s film clip, 3s How negative do you feel? rating (1=not at all to 3=moderately to 5=extremely), 3s Watch instruction, 6s static pleasant landscape image, and 9s counting the number of asterisks on the screen. A single trial lasted 29s.
Face Behavior Recording and Coding
A black and white pinhole video camera (SmartLabs, Inc.) was shielded and positioned on the MR head-coil to record continuous facial behavior from the forehead to the mouth. Participants' facial expressions during film viewing were independently rated by two female coders who were blind to the films and experimental conditions using a face behavior coding system developed in our laboratory (38) to measure disgust expressive-behavior on a scale of 0=none, 1=slight, 2=moderate, 3=strong. Due to technical problems, face behavior coding was unavailable for 2 participants.
Image Acquisition
Imaging was performed on a GE 3 Tesla Signa magnet with a T2*-weighted gradient echo spiral-in/out pulse sequence (39) and a custom-built quadrature “dome” elliptical bird cage head-coil. Head movement was minimized using a bite-bar. 908 functional volumes were obtained across three functional runs from 22 sequential axial slices [TR=1500 ms, TE=30 ms, flip angle=60 degrees, FOV=22 cm, matrix=64×64, single-shot, in-plane resolution=3.438 mm2 and slice thickness=5 mm]. 3D high-resolution anatomical scans were acquired using fast spin-echo SPGR (.85942 × 1.5 mm; FOV=22 cm, frequency encoding=256).
fMRI Data Preprocessing
Each functional run was subjected to preprocessing steps using AFNI (40) software: co-registration, motion correction, 4 mm3 isotropic Gaussian spatial-smoothing, high-pass filtering (.011 Hz), and linear detrending. No volumes demonstrated motion in the x, y, or z directions in excess of ±1.0 mm. There was no evidence of stimulus-correlated motion when conducting correlations of condition-specific reference functions and x, y, z motion correction parameters (all ps>.05).
fMRI Statistical Analysis
A multiple-regression model implemented with AFNI 3dDeconvolve included baseline parameters to remove mean, linear, and quadratic trends, and motion-related variance. For the purposes of the present analysis, we focused on neural response while actively implementing reappraisal and suppression during the 15s films. Separate reference functions for the 15s film in each conditions, and for the early (0−4.5s), middle (4.5−10.5s) and late (10.5−15s) components of each 15s film were convolved with a gamma variate model (41) of the hemodynamic response function. Statistical maps were resampled to 3.438 mm3, converted to Talairach atlas space (42) and second-level statistical parametric maps were produced according to a random-effects analysis to enhance the generalizability of the results.
To correct for multiple comparisons, AlphaSim, a Monte Carlo simulation bootstrapping program in the AFNI library, was employed to identify a joint-probability consisting of a voxel-wise threshold and a minimum cluster-volume threshold to establish a cluster-wise p-value that protects against false positive detection of activation clusters (43). For 15s film analyses (e.g., watch-negative versus watch-neutral contrast) a voxel-wise threshold of p<0.0025 (t>3.572) resulted in a minimum cluster-volume threshold of 203 mm3 (5 voxels × 3.438 mm3) to protect against false positive detection at p<0.001. For the early, middle and late component analyses, a voxel-wise threshold of p<0.005 (t>3.248) resulted in a minimum cluster-volume threshold of 163 mm3 (4 voxels × 3.438 mm3) to protect against false positive detection at p <0.005. Because more time points (10) per 15s block contributed to the contrast of watch-negative versus watch-neutral conditions compared to the 3 or 4 time points included in early (3 time points), middle (4), and late (3) component analyses, there was lesser power in the component compared to the block analyses. For this reason, we used a slightly less stringent joint-probability cluster-threshold for the component analyses (p <0.005) than for the block contrast analyses (p <0.001).
BOLD signal intensity was represented as percent signal change of watch-negative, reappraise-negative and suppressive-negative conditions from the mean of the watch-neutral condition. To control for cluster size differences, time series was extracted using a spherical mask (radius=5 mm, volume=524 mm3) centered at the peak BOLD signal voxel within a cluster. For presentation purposes only, the time series was temporally-smoothed such that each time point represents the average of time points x-1, x, x+1.
Results
Negative Emotion Induction Manipulation Check
To assess whether the negative films elicited negative emotion, we contrasted watch-negative versus watch-neutral conditions on three indices of negative emotion: experience, expressive behavior and neural response.
Experience
The watch-negative versus watch-neutral contrast was significant (t=7.46, p<.000005, η2=.78), confirming the induction of negative emotion experience (Figure 3).
Figure 3.
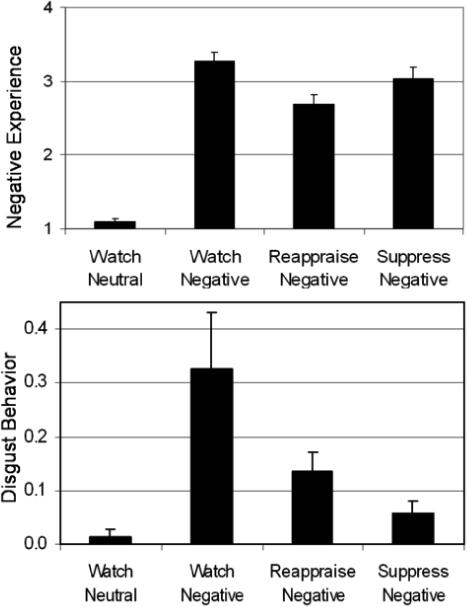
Effect of reappraisal and suppression on negative emotion ratings and disgust facial expression intensity during the four film viewing conditions. Error bars = SEM.
Expressive behavior
Two raters coded participants' facial expressions during MR scanning for disgust expressions. Inter-rater reliability during the watch-negative condition was adequate (kappa=.76). The watch-negative versus watch-neutral contrast was significant (t(14)=2.96, p<.05, η2=.40), confirming that watching negative films induced emotion-expressive behavior (Figure 3).
Neural responses
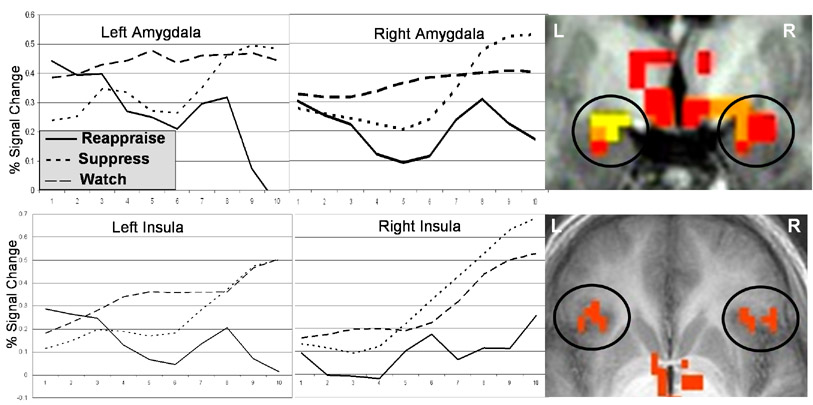
The watch-negative versus watch-neutral contrast resulted in enhanced responses in bilateral dorsal amygdala and anterior insula (Figure 4), frontal cortex (medial, dorsomedial, dorsolateral, ventrolateral), temporal cortex (inferior, superior), occipital (lingual gyrus), parietal cortex (superior parietal lobule), and subcortical regions (caudate, thalamus, hypothalamus) (Table 1). There were no significant brain responses for watch-neutral versus watch-negative.
Figure 4.
fMRI BOLD signal time-series in percent signal change relative to the watch-neutral condition across 15s (10 time points × 1.5s each) for reappraisal, suppression, and watch-negative conditions in bilateral amygdala and insula.
Table 1.
Watch-Negative versus Watch-Neutral Film BOLD Response
| Brain Regions | BA | x y z | % Signal Change | Vol (mm3) | t-value |
|---|---|---|---|---|---|
| Watch-Negative > Watch-Neutral | |||||
| Frontal Lobes | |||||
| L Inferior Frontal Gyrus / | 47 | −41 24 −19 | .27 | 3,820 | 3.61 |
| L Insula | 13 | −35 23 1 | .17 | 3.93 | |
| Medial PFC | 9 | 0 55 25 | .21 | 3,129 | 4.47 |
| R Middle Frontal Gyrus / DLPFC | 6/8 | 52 11 43 | .27 | 2,113 | 4.05 |
| Dorsomedial PFC | 6 | 3 21 67 | .32 | 2,072 | 3.91 |
| L Inferior Frontal Gyrus / DLPFC | 9 | −52 7 32 | .18 | 772 | 4.29 |
| R Middle Frontal Gyrus / DLPFC | 46 | 49 28 22 | .13 | 569 | 3.99 |
| L Inferior Frontal Gyrus | 45 | −48 18 5 | .13 | 406 | 3.73 |
| R Inferior Frontal Gyrus | 45 | 52 18 5 | .14 | 366 | 4.01 |
| L Precentral Gyrus | 6 | −41 −3 60 | .18 | 325 | 3.85 |
| L Superior Frontal Gyrus / medial PFC | 9 | −14 59 36 | .25 | 284 | 4.62 |
| L Middle Frontal Gyrus / DLPFC | 46 | −52 28 22 | .14 | 203 | 5.18 |
| R dorsomedial PFC | 6 | 7 38 60 | .19 | 203 | 3.99 |
| Temporal Lobes | |||||
| L Inferior Temporal Gyrus | 18/19 | −48 −75 −2 | .62 | 15,151 | 5.71 |
| R Superior Temporal Gyrus / | 38 | 41 24 −23 | .23 | 3,170 | 3.75 |
| R Insula | 13 | 35 20 −1 | .14 | 3.89 | |
| Parietal Lobes | |||||
| R Superior Parietal Lobule | 7 | 34 −44 63 | .38 | 8,859 | 4.01 |
| L Postcentral Gyrus | 2 | −45 −24 39 | .12 | 203 | 4.35 |
| Occipital Lobes | |||||
| L Lingual Gyrus | 18 | −7 −86 −16 | .37 | 366 | 4.18 |
| Subcortical Regions | |||||
| R Amygdala | 17 −6 −9 | .21 | 2,682 | 4.68 | |
| L Amygdala | −17 −3 −9 | .28 | 731 | 4.52 | |
| L Caudate Body | −10 4 12 | .14 | 1,829 | 3.81 | |
| R Caudate Body | 10 1 15 | .13 | 528 | 3.70 | |
| Pulvinar Thalamus | 0 −30 5 | .27 | 3,901 | 4.19 | |
| L Hypothalamus | −4 −6 −9 | .14 | 610 | 3.99 | |
| R Pulvinar Thalamus | 17 −24 8 | .10 | 284 | 4.25 |
Note. A voxel-wise t-test on the full block contrast of Watch-Negative versus Watch-Neutral films was used to generate these results. Talairach coordinates, % signal change and t-statistic are reported for the voxel within each cluster with the maximum BOLD response. Statistical thresholding was based on the following parameters: voxel threshold t-value ≥ 3.572, p<0.0025, cluster volume threshold ≥ 203 mm3 (5 voxels × 3.438 mm3), and joint-probability cluster threshold p<0.001. BA = Brodmann Area, DLPFC = dorsolateral prefrontal cortex, L = left, PFC = prefrontal cortex, R = right
Emotion Regulation Analyses
We examined the effects of reappraisal and suppression on (a) emotion experience and behavior, (b) emotion-related brain regions (amygdala and insula regions of interest identified in the contrast of watch-negative versus watch-neutral films), and (c) regulation-related brain regions (identified in the reappraise versus watch-negative and suppress versus watch-negative film contrasts. Based on our model of emotion regulation, we expected differential temporal effects of (antecedent-focused) reappraisal and (response-focused) suppression on emotion-generative and emotion regulatory brain regions. To test for hypothesized differential ER effects on neural temporal dynamics, analyses focused on neural responses during the early (0−4.5s), middle (4.5−10.5s), and late (10.5−15s) periods of each 15s film. We first examined each ER strategy separately, and then directly compared reappraisal versus suppression.
Cognitive Reappraisal
Reappraisal (versus watch-negative) reduced negative emotion experience (t=4.70, p<.0005, η2=.58), approached significant reduction of disgust facial behavior (t=1.82, p=.09, η2=.20), and reduced emotion-related neural signal during the late (but not early and middle) components in right amygdala (reappraisal vs. watch-negative, t=2.20, p<.05, η2=.21), left insula (t=2.22, p<.05, η2=.24) and marginally in left amygdala (t=1.89, p<.08, η2=.18). These results show that reappraisal effectively down-regulated negative emotion experience with a concomitant reduction of emotion-related neural signal by the end of the 15s films.
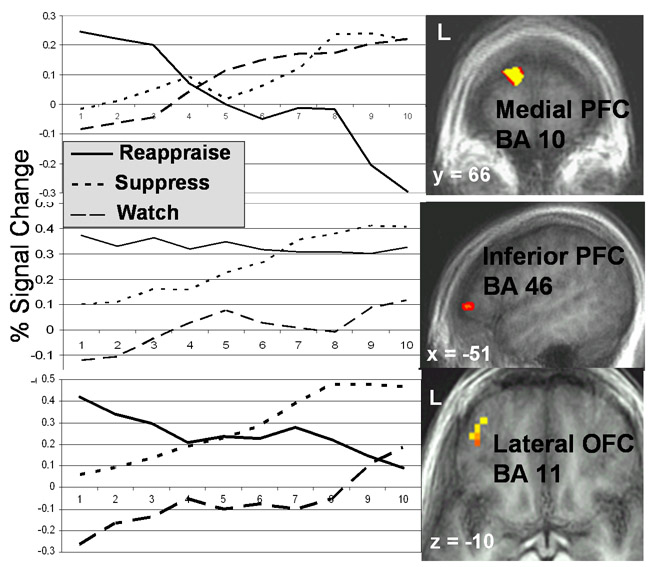
During the early (but not middle and late) period, reappraisal produced enhanced responses in PFC-related cognitive control of emotion (medial, dorsolateral, ventrolateral PFC, and lateral OFC), linguistic processing (left inferior frontal gyrus, left posterior superior temporal gyrus), visual attention (precuneus, lingual and angular gyri), and feature detection (middle and superior temporal gyri) (Table 2). Three representative early reappraisal-related neural responses, including medial PFC, left inferior PFC and left OFC, are shown in Figure 5. There were no areas of greater response for watch-negative versus reappraise-negative.
Table 2.
Reappraise versus Watch-Negative and Suppress versus Watch-Negative Film BOLD Response for Early (0−4.5s), Middle (4.5−10.5s), and Late (10.5−15s) Components
| Brain Regions | BA | x y z | % Signal Change | Vol (mm3) | t-value |
|---|---|---|---|---|---|
| Reappraise > Watch-Negative | |||||
| Early | |||||
| Frontal Lobes | |||||
| Medial PFC | 10 | −11 67 18 | .30 | 163 | 3.20 |
| R Inferior Frontal Gyrus / DLPFC | 10,46 | 48 42 1 | .18 | 163 | 3.28 |
| L Inferior Frontal Gyrus | 46 | −51 41 2 | .15 | 163 | 4.65 |
| L Middle Frontal Gyrus | 6 | −37 7 41 | .09 | 244 | 3.47 |
| L Lateral Orbitofrontal Cortex | 11 | −28 42 −6 | .14 | 163 | 3.68 |
| L Lateral Orbitofrontal Cortex | 11 | −38 45 −10 | .30 | 203 | 4.08 |
| L Ventrolateral PFC | 47 | −35 38 −6 | .12 | 163 | 3.42 |
| Temporal Lobes | |||||
| L Anterior Superior Temporal Gyrus | 38 | −38 11 −33 | .11 | 528 | 4.26 |
| L Anterior Superior Temporal Gyrus | 38 | −58 7 −6 | .14 | 447 | 3.39 |
| L Anterior Superior Temporal Gyrus | 38 | −52 21 −19 | .16 | 325 | 3.67 |
| L Inferior Temporal Gyrus | 21 | −62 −3 −16 | .14 | 284 | 7.24 |
| L Posterior Middle Temporal Gyrus | 22 | −58 −34 5 | .14 | 894 | 3.29 |
| L Posterior Middle Temporal Gyrus | 21 | −58 −37 −2 | .16 | 244 | 3.42 |
| L Inferior Temporal Gyrus | 21 | −45 1 −30 | .13 | 203 | 4.53 |
| L Posterior Middle Temporal Gyrus | 39 | −30 −62 22 | .06 | 203 | 3.39 |
| L Posterior Superior Temporal Gyrus | 39 | −41 −54 22 | .11 | 163 | 3.54 |
| Parietal Lobes | |||||
| L Angular Gyrus | 39 | −48 −68 36 | .15 | 894 | 3.78 |
| Occipital Lobes | |||||
| R Lingual Gyrus | 19 | 7 −65 −4 | .14 | 325 | 5.52 |
| Middle and Late | None | ||||
| Watch-Negative > Reappraise | None | ||||
| Suppress > Watch-Negative | |||||
| Early and Middle | None | ||||
| Late | |||||
| Frontal Lobes | |||||
| Dorsomedial PFC | 8,6 | 0 18 54 | .12 | 203 | 4.46 |
| R Dorsolateral PFC | 9 | 39 11 32 | .14 | 406 | 5.12 |
| R Dorsolateral PFC | 46 | 48 21 22 | .16 | 244 | 4.86 |
| R Dorsolateral PFC | 46 | 50 38 15 | .21 | 1,463 | 5.25 |
| R Inferior Frontal Gyrus | 45 | 53 20 11 | .16 | 163 | 4.85 |
| R Ventrolateral PFC | 10 | 30 48 −2 | .22 | 203 | 4.41 |
| L Ventrolateral PFC | 10 | −34 56 1 | .24 | 447 | 4.05 |
| L Ventrolateral PFC | 10,46 | −45 45 5 | .17 | 366 | 4.19 |
| L Anterior Insula | 13 | −34 18 7 | .10 | 284 | 4.53 |
| Temporal Lobes | |||||
| R Posterior Middle Temporal Gyrus | 39,19 | 52 −65 15 | .17 | 528 | 4.70 |
| R Posterior Middle Temporal Gyrus | 21 | 62 −54 5 | .21 | 731 | 4.97 |
| R Posterior Inferior Temporal Gyrus | 37,19 | 52 −72 1 | .18 | 244 | 4.04 |
| L Posterior Middle Temporal Gyrus | 37,19 | −58 −68 8 | .21 | 203 | 5.71 |
| L Fuisform Gyrus | 37 | −45 −58 −15 | .19 | 163 | 4.66 |
| Parietal Lobes | |||||
| L Inferior Parietal Lobule | 39 | −38 −65 39 | .10 | 163 | 4.17 |
| Occipital Lobes | |||||
| L Lateral Precuneus | 19 | −31 −78 42 | .16 | 244 | 4.28 |
| R Superior Occipital Gyrus / Angular Gyrus | 39,19 | 35 −72 29 | .12 | 203 | 5.20 |
| Watch-Negative > Suppress | |||||
| Early and Middle | None | ||||
| Late | |||||
| Occipital Lobes | |||||
| Medial Cuneus | 19 | −3 −92 24 | .29 | 1,463 | 4.56 |
| Lingual Gyrus | 18 | −1 −74 −2 | .27 | 2,845 | 4.94 |
| Lingual Gyrus | 18 | −7 −82 −2 | .20 | 244 | 4.61 |
Note. These results are derived from separate voxel-wise t-tests for the early, middle and late components of each block for the contrasts of Reappraise versus Watch-Negative and Suppress versus Watch-Negative. Talairach coordinates, % signal change and t-statistic are reported for the voxel within each cluster with the maximum BOLD response. Statistical thresholding was based on the following parameters: voxel threshold t-value ≥ 3.248, p<0.005, cluster volume threshold ≥ 163 mm3 (4 voxels × 3.438 mm3), and joint-probability cluster threshold p<0.005. BA = Brodmann Areas, DL = dorsolateral, L = left, PFC = prefrontal cortex, R = right.
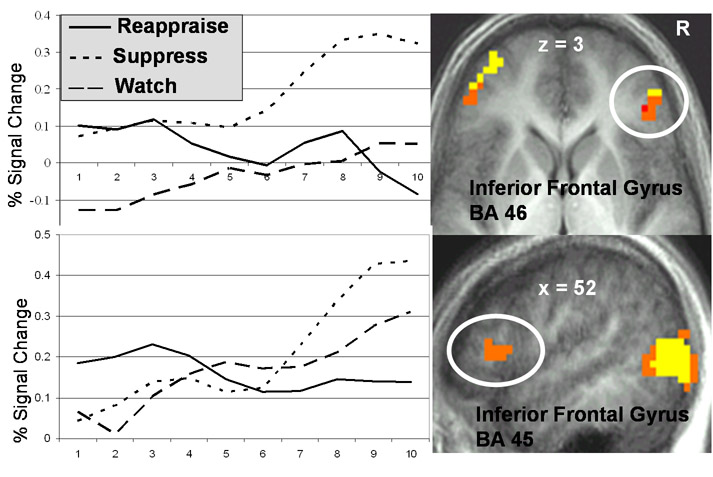
Figure 5.
fMRI BOLD signal time-series in percent signal change relative to the watch-neutral condition for reappraisal, suppression, and watch-negative conditions in medial prefrontal cortex BA 10 (−11 67 18), left inferior prefrontal cortex BA 46 (−51 41 2), and left lateral orbitofrontal cortex BA 11 (−38 45 −10).
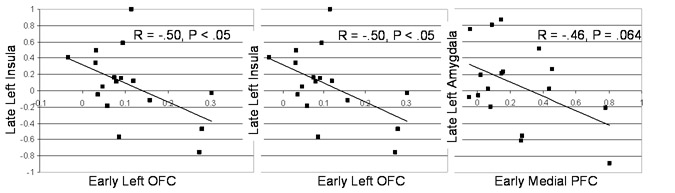
Correlation analyses demonstrated that increased early (0−4.5s) reappraisal-related medial PFC and left OFC responses were associated with significantly decreased late (10.5−15s) left amygdala and left insula responses (Figure 6). These findings indicate that implementation of reappraisal strategies may initiate interactions of regulatory and regulated brain systems that evolve over time and influence subsequent neural, experiential and behavioral indices of emotion.
Figure 6.
Association of early (0−4.5s) enhanced cognitive reappraisal-related BOLD responses in orbitofrontal cortex and medial prefrontal cortex with reduced late (10.5−15s) insula and amygdala responses.
Expressive-Suppression
Suppression (versus watch-negative) reduced negative emotion experience (t=3.30, p<.005, η2=.41) and facial behavior (t=2.49, p<.05, η2=.32). In limbic regions previously identified in the watch-negative versus watch-neutral contrast, responses in the late component were greater for suppression in right insula (t=2.42, p<.05, η2=.27) and marginally greater in right amygdala (t=1.86, p=.08, η2=.18), but similar in left amygdala (p>.57) and left insula (p>.90). These findings demonstrate no reduction in emotion-related neural activity in amygdala, and indeed suggest enhanced responding for suppression in right insula.
Suppression (versus watch-negative) produced greater responses only during the late (not early and middle) period in regions implicated in inhibitory control (right ventrolateral PFC, Figure 7), cognitive regulation (dorsomedial, dorsolateral PFC), visual-sensory multimodal association (posterior occipito-temporal lobes) and visual-spatial processing (precuneus and occipital areas) (Table 2). Greater responses for watch-negative (versus suppression) occurred only during the late period in visual processing areas (cuneus and lingual gyrus).
Figure 7.
fMRI BOLD signal time-series in percent signal change relative to the watch-neutral condition for reappraisal, suppression, and watch-negative conditions in right inferior prefrontal cortex BA 46 (50 42 3) and right inferior prefrontal cortex BA 45 (52 20 11).
Comparison of Reappraisal and Suppression
Direct contrast of ER strategies revealed that reappraisal produced greater down-regulation of negative emotion experience (t=3.29, p<.005, η2=.40), while suppression produced greater reduction in disgust facial behavior (t=2.94, p<.05, η2=.40). These findings highlight the differential impact of reappraisal and suppression on negative emotion experience and behavior.
Neurally, in the previously identified watch-negative emotion-related regions, a 2 (ER: reappraisal, suppression) × 2 (time: early, late) repeated-measures ANOVA of BOLD signal revealed an ER × time interaction in bilateral amygdala (left, F(2,15)=4.31, p=.054, η2=.21; right, F(2,15)=4.74, p<.05, η2=.23) and insula (left, F(2,15)=5.03, p<.05, η2=.24; right, F(2,15)=4.04, p=.06, η2=.20). Follow-up t-tests showed that reappraisal compared to suppression resulted in reduction at the late period in amygdala (left: t=2.72, p<.05, η2=.32; right: t=2.82, p<.05, η2=.33) and insula (left: t=2.04, p=.059, η2=.21; right: t=2.27, p<.05, η2=.24). These results show that reappraisal was more effective than suppression in down-regulation of BOLD responses in emotion-related limbic regions.
In the 3 reappraisal and 2 suppression related activation clusters displayed in Figures 7 & 9, a 2 (ER: reappraisal, suppression) × 2 (time: early, late) repeated-measures ANOVA of BOLD signal resulted in a significant interaction of ER × time in left IFG BA46, F(2,15)=8.38, P>.05, η2=.34, mPFC BA10, F(2,15)=7.89, P>.05, η2=.33, right IFG BA46, F(2,15)=5.38, P>.05, η2=.25, right IFG BA45, F(2,15)=13.31, P>.005, η2=.45, but only a trend towards significance in left OFC BA11, F(2,15)=3.69, P=.07, η2=.19.
Direct reappraisal versus suppression contrasts during the early, middle, and late film components produced neural activity very similar to the results reported above. Reappraisal generated significant responses only during the early period in PFC regions (medial, dorsomedial, left inferior frontal gyrus), insula, superior and middle temporal lobes, and cuneus. Suppression produced significant responses only during the late period in PFC regions (ventromedial, ventrolateral, dorsolateral, rostral and dorsal ACC), left fusiform gyrus, inferior parietal lobule, middle and superior occipital gyrus, and thalamus (Table 3). There were no differential BOLD responses during the middle period. These direct contrasts re-affirmed the pattern of results described above, namely, differentially greater PFC BOLD responses for reappraisal during the early period only and for suppression during the late period only.
Table 3.
Reappraise versus Suppress BOLD Response for Early (0−4.5s), Middle (4.5−10.5s) and Late (10.5−15s) Components
| Brain Regions | BA | x y z | % Signal Change | Vol (mm3) | t-value |
|---|---|---|---|---|---|
| Reappraise > Suppress | |||||
| Early | |||||
| Frontal Lobes | |||||
| L Medial PFC | 10 | −14 72 15 | .24 | 203 | 3.60 |
| Dorsomedial PFC | 9 | −7 59 43 | .28 | 203 | 3.60 |
| L Inferior Frontal Gyrus | 45 | −48 25 8 | .11 | 203 | 3.38 |
| L Insula | 13 | −38 −17 19 | .10 | 203 | 3.99 |
| Temporal Lobes | |||||
| L Anterior Superior Temporal Gyrus | 38 | −38 28 −23 | .31 | 1,178 | 4.21 |
| L Posterior Superior Temporal Gyrus | 22 | −55 −41 8 | .09 | 406 | 3.41 |
| L Middle Temporal Gyrus | 20 | −41 −6 −19 | .09 | 244 | 4.60 |
| L Anterior Middle Temporal Gyrus | 21 | −55 7 −30 | .13 | 163 | 3.74 |
| R Anterior Superior Temporal Gyrus | 38 | 38 18 −19 | .13 | 163 | 3.49 |
| Occipital Lobes | |||||
| Cuneus Posterior Cingulate | 18, 23, 29 | 3 −72 15 | .30 | 26,495 | 4.12 |
| L Cuneus | 18 | −17 −95 8 | .13 | 447 | 4.00 |
| R Cuneus | 19 | 17 −99 15 | .19 | 284 | 4.04 |
| Middle and Late | None | ||||
| Suppress > Reappraise | |||||
| Early and Middle | None | ||||
| Late | |||||
| Frontal Lobes | |||||
| Dorsal Anterior Cingulate | 24 | 0 −10 43 | .18 | 1,422 | 4.06 |
| Dorsal Anterior Cingulate | 24 | 0 0 39 | .19 | 813 | 4.35 |
| Dorsal Anterior Cingulate | 32 | 0 14 43 | .16 | 244 | 4.88 |
| Rostral Anterior Cingulate | 32 | −3 45 1 | .23 | 203 | 4.86 |
| R Inferior Frontal Gyrus | 46 | 48 31 15 | .14 | 528 | 4.04 |
| R Inferior Frontal Gyrus | 9, 44 | 48 11 22 | .13 | 203 | 4.31 |
| Ventromedial PFC | 10 | −7 59 −9 | .24 | 244 | 4.02 |
| L ventrolateral PFC | 10 | −34 49 8 | .13 | 244 | 4.79 |
| R dorsolateral PFC | 46 | 45 21 22 | .15 | 244 | 5.55 |
| R ventrolateral PFC | 44 | 52 7 12 | .12 | 203 | 4.05 |
| Temporal Lobes | |||||
| L Fusiform Gyrus | 37 | −45 −58 −12 | .26 | 284 | 4.35 |
| Parietal Lobes | |||||
| L Inferior Parietal Lobule | 40 | −62 −24 26 | .14 | 203 | 4.16 |
| Occipital Lobes | |||||
| R Middle Occipital Gyrus | 19, 37 | 45 −72 1 | .23 | 894 | 4.38 |
| L Superior Occipital Gyrus | 19 | −38 −79 32 | .16 | 203 | 4.52 |
| Subcortical | |||||
| Thalamus | 0 −20 1 | .14 | 203 | 4.66 |
Note. Voxel-wise t-tests for the early, middle, and late components of each block for the contrast of Reappraise versus Suppress provided these results. Talairach coordinates, % signal change and t-statistic are reported for the voxel within each cluster with the maximum BOLD response. Statistical thresholding was based on the following parameters: voxel threshold t-value ≥ 3.248, p<0.005, cluster volume threshold ≥ 163 mm3 (4 voxels × 3.438 mm3), and joint-probability cluster threshold p<0.005. BA = Brodmann Area, L = left, PFC = prefrontal cortex, R = right.
Discussion
The goal of this study was to test a process model of emotion regulation that predicted differential effects of two distinct regulation strategies – cognitive reappraisal (an antecedent-focused strategy) and emotion-expression suppression (a response-focused strategy). These two ER strategies were evaluated in the context of 15s films which elicited negative emotion experience, disgust facial behavior, and emotion-related neural responses in dorsal amygdala and anterior insular cortex.
Reappraisal
The implementation of cognitive reappraisal reduced negative emotion experience in accordance with previous studies of cognitive reappraisal of negative emotion (7). Consistent with these findings, reappraisal increased activity in cognitive control PFC regions and decreased amygdala and insula responses that were functionally identified during the watch-negative condition. These findings align well with results of prior studies which reported cognitive reappraisal down-regulated amygdala and insula responses to negative emotional stimuli (13-16). In our study, reappraisal also enhanced signal in medial, dorsolateral and ventrolateral PFC regions, regions previously identified in cognitive regulation of negative emotion (14; 15; 17; 18).
In prior studies, these PFC regions have been implicated in cognitive control, strategy selection, implementation and monitoring (20; 22) and appear to function in conjunction with left ventrolateral PFC, superior temporal and posterior parietal lobe regions involved in linguistic processing (44). The coordination of this distributed cortical network and its associated cognitive functions appears to modulate neural processing of emotional intensity and salience in limbic brain regions.
The current study advances our understanding of the interaction of regulatory PFC regions and regulated limbic areas by showing that early (0−4.5s) PFC responses, specifically in the medial and left ventrolateral PFC, are associated with subsequent late (10.5−15s) reduction in amygdala and insula activity. These findings suggest that initial implementation of reappraisal strategies may influence neural, experiential and behavioral indices of emotion over time. The demonstration of reappraisal-related PFC influence on subsequent measures of emotion highlights the importance of incorporating dynamic temporal features of neural signal, emotion experience and behavior as components of models of emotion reactivity and regulation. The effectiveness and timing of PFC modulation of limbic-mediated emotional reactivity may have important health consequences, including the ability to regulate neuroendocrine stress hormones through cognitive reappraisal (8; 19).
Suppression
Suppression of emotion-expressive behavior involved volitional control of facial motor muscles in the presence of emotionally-evocative film clips. Suppression reduced negative emotion experience and behavior, but sustained elevated responses in amygdala and insula. A pattern of reduced emotion facial behavior with elevated physiological activation during suppression has been noted in previous studies (12). For example, previous studies examining the acute effects of volitional inhibition of expressive-behavior have reported suppression of emotional facial expressions along with increased sympathetic activation of the cardiovascular system while watching emotionally evocative films (45) and reacting to acoustic startle (46). This suggests that successful expressive-suppression may be achieved in emotionally challenging situations but at a cost, namely, sustained activation that may be physiologically taxing and lead to disturbances in psychological and physical functioning (7; 8; 47). The unexpected decrease in negative emotion may be due to the redirection of attention to control of facial behavior and away from the emotional experience of the negative films.
Suppression produced significant responses only at the late (10.5−15s) period in an extensive network of brain regions implicated in cognitive control (PFC), visual-sensory multimodal association (posterior occipito-temporal lobes) and visual-spatial (precuneus and occipital areas) processing. In particular, suppression produced significant responses in areas of right ventrolateral PFC previously related to inhibitory motor control (24-31). The enhanced PFC inhibitory control signal only at the late component may reflect increasing efforts to sustain inhibition of disgust facial expression and/or efforts to exert more cognitive control to counter neural input from amygdala and insula conveying ongoing emotional salience of the negative films.
Reappraisal versus Suppression
The direct contrast of reappraisal versus suppression demonstrated greater reduction of negative emotion experience for reappraisal and of disgust facial behavior for suppression in concordance with results from previous studies (5; 7). In the neural domain, specifically in the late period of the negative films, reappraisal reduced while suppression enhanced or maintained elevated signal in bilateral dorsal amygdala and anterior insula. While reappraisal has been shown to reduce both behavioral and neural indices of emotional reactivity (22), there are no functional neuroimaging studies of control of disgust facial behavior.
The divergent effects of reappraisal and suppression on limbic response converge with previous findings of differential effects on autonomic physiological responses, specifically, increased cardiovascular activation during suppression, and reduced or unchanged sympathetic physiological responses during reappraisal (7). These results demonstrate that reappraisal and suppression produce differential effects on emotion experience and behavior, and opposite effects on neural response in two important components of the limbic emotion processing system.
Reappraisal and suppression produced both common and unique areas of BOLD response in PFC regulatory areas. Brain regions common to both ER strategies included medial and bilateral dorsolateral PFC and lateral OFC areas previously implicated in cognitive control of negative emotion (14; 15; 17; 18) suggesting that there might be some shared cognitive processes. While reappraisal was primarily associated with left PFC responses, suppression was associated with a more bilateral pattern of PFC responses. Extensive neuroanatomical connections between medial and lateral PFC and limbic regions, including amygdala and insula, have been identified (48; 49). In particular, lesion studies have found that the medial PFC plays an essential role in inhibitory control of amygdala output (50). However, it is likely that the specific function of the medial PFC during emotion regulation will strongly depend on the neural context (i.e., the profile of neural activity in co-active connected areas) (51).
An important implication of these results is that ER strategies require time for their impact on brain-behavioral-experiential indices of emotion to emerge. In this study, early BOLD response in medial and left ventrolateral PFC during cognitive reappraisal was associated with later reduction of left amygdala and insula responses, and subsequent reduction in negative emotion experience ratings. This finding supports the process model of ER, in that the effects of reappraisal and suppression have varying temporal trajectories that impact that strategy's effect on emotion experience, behavior and neural systems.
Implications for Psychopathology and Treatment
There is increasing recognition that most psychiatric conditions involve emotion dysregulation and that clinical interventions benefit when they are informed by empirical understanding of emotion processes (10). More recent formulations of cognitive-behavioral interventions have explicitly incorporated modules that address emotion reactivity, affect tolerance, and skills training in emotion regulation (52). Despite empirical evidence of the emotional consequences of cognitive reappraisal and suppression, far less is understood about the brain-behavioral mechanisms underlying psychopathology and modulated by clinical interventions.
The current study elucidates the differential impact of two distinct emotion regulation strategies on brain-behavioral processes. While hyperreactivity of limbic systems that detect and experience emotion has been reliably observed in many psychiatric conditions (e.g., anxiety disorders), our findings are a first step in characterizing the temporal features of bottom-up (emotional reactivity) and top-down (regulatory) brain-behavioral mechanisms that are common targets of pharmacological, psychotherapeutic and direct brain stimulation interventions. Specifically, it may be informative to characterize brain-behavior relationships during emotion reactivity and regulation (i.e., reappraisal and inhibitory cognitive control functioning) in clients with histories of mood and anxiety disorders. This may enhance our ability to match clients to specific treatment modalities that more directly address their emotion dysregulation profile, and to determine empirically how much and for how long different interventions modulate brain-behavioral systems.
Limitations
To maximize disgust reactivity, the study sample included only women and thus the results cannot be generalized to male participants. Additionally, we used disgust-eliciting films to examine the temporal dynamics of ER, and it is not known whether results will generalize to the regulation of other types of emotional states or stimuli. Although we observed significant differences in facial expression behavior between conditions, the head coil structure and bite-bar (as well as the knowledge that responses were being videotaped) may have dampened facial emotion-expressive behaviors during MR scanning. Unfortunately, emotion experience ratings were collected during fMRI only after each 15s film clip. Continuous measurement of emotion experience, expressive behavior and autonomic responding may help to fully understand emotion reactivity and regulation. Finally, our relatively small sample size precluded the examination of individual differences. One important direction for future research clearly is to consider the role of personality and psychopathology in emotional reactivity and emotion regulation.
Acknowledgments
This research was supported by NIMH Grants MH58147 and MH66957. We would like to thank Kevin Ochsner, Ph.D. for his help with this project. We wish to thank Julie Hall and Cynthia R. Cox for their help with coding facial behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors of this manuscript do not have any direct or indirect conflicts of interest to disclose.
References
- 1.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 4.Cacioppo JT, Gernston GG, Larsen JT, Poehlmann KM, Ito TA. The psychophysiology of emotion. In: Lewis M, Haviland-Jones JM, editors. Handbook of emotions. Vol. 2. Guilford Press; New York: 2000. pp. 173–191. [Google Scholar]
- 5.Levenson RW. Autonomic specificity and emotion. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. Oxford University Press; New York: 2003. pp. 212–224. [Google Scholar]
- 6.Keltner D, Kring A. Emotion, social function, and psychopathology. Review of General Psychology. 1998;2:320–342. [Google Scholar]
- 7.Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 8.Abelson JL, Liberzon I, Young EA, Khan S. Cognitive modulation of the endocrine stress response to a pharmacological challenge in normal and panic disorder subjects. Arch Gen Psychiatry. 2005;62:668–75. doi: 10.1001/archpsyc.62.6.668. [DOI] [PubMed] [Google Scholar]
- 9.Gross JJ. The Handbook of Emotion Regulation. Guilford Press; New York: 2007. [Google Scholar]
- 10.Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross JJ, editor. Handbook of emotion regulation. Guilford; New York: 2007. pp. 542–559. [Google Scholar]
- 11.Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. Guilford; New York: 2007. pp. 3–24. [Google Scholar]
- 12.Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- 13.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. J Cogn Neurosci. 2002;14:913–21. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- 17.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levesque J, Eugene F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- 19.Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 21.Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–22. [PubMed] [Google Scholar]
- 22.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 24.Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006 doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott R, Deakin B. Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: evidence from functional magnetic resonance imaging studies in healthy human subjects. Int Rev Neurobiol. 2005;65:89–116. doi: 10.1016/S0074-7742(04)65004-5. [DOI] [PubMed] [Google Scholar]
- 27.Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–92. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vollm B, Richardson P, McKie S, Elliott R, Deakin JF, Anderson IM. Serotonergic modulation of neuronal responses to behavioural inhibition and reinforcing stimuli: an fMRI study in healthy volunteers. Eur J Neurosci. 2006;23:552–60. doi: 10.1111/j.1460-9568.2005.04571.x. [DOI] [PubMed] [Google Scholar]
- 29.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–8. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 30.Brass M, Derrfuss J, von Cramon DY. The inhibition of imitative and overlearned responses: a functional double dissociation. Neuropsychologia. 2005;43:89–98. doi: 10.1016/j.neuropsychologia.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur J Neurosci. 2004;19:3105–12. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee TW, Dolan RJ, Critchley HD. Controlling Emotional Expression: Behavioral and Neural Correlates of Nonimitative Emotional Responses. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaitl D, Schienle A, Stark R. Neurobiology of fear and disgust. Int J Psychophysiol. 2005;57:1–4. doi: 10.1016/j.ijpsycho.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Rozin P, Fallon AE. A perspective on disgust. Psychol Rev. 1987;94:23–41. [PubMed] [Google Scholar]
- 35.Schienle A, Schafer A, Stark R, Walter B, Vaitl D. Relationship between disgust sensitivity, trait anxiety and brain activity during disgust induction. Neuropsychobiology. 2005;51:86–92. doi: 10.1159/000084165. Epub 2005 Feb 28. [DOI] [PubMed] [Google Scholar]
- 36.Calder AJ. Disgust discussed. Ann Neurol. 2003;53:427–8. doi: 10.1002/ana.10565. [DOI] [PubMed] [Google Scholar]
- 37.Schienle A, Schafer A, Stark R, Walter B, Vaitl D. Gender differences in the processing of disgust- and fear-inducing pictures: an fMRI study. Neuroreport. 2005;16:277–80. doi: 10.1097/00001756-200502280-00015. [DOI] [PubMed] [Google Scholar]
- 38.Gross JJ, Levenson RW. Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993:970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- 39.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 40.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 41.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 42.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- 43.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 44.Iacoboni M, Wilson SM. Beyond a single area: motor control and language within a neural architecture encompassing Broca's area. Cortex. 2006;42:503–6. doi: 10.1016/s0010-9452(08)70387-3. [DOI] [PubMed] [Google Scholar]
- 45.Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. J Abnorm Psychol. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- 46.Hagemann T, Levenson RW, Gross JJ. Expressive suppression during an acoustic startle. Psychophysiology. 2006;43:104–12. doi: 10.1111/j.1469-8986.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 47.Gross JJ. The Handbook of Emotion Regulation. Guilford Press; in press. [Google Scholar]
- 48.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 49.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 50.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McIntosh AR. Contexts and catalysts: a resolution of the localization and integration of function in the brain. Neuroinformatics. 2004;2:175–82. doi: 10.1385/NI:2:2:175. [DOI] [PubMed] [Google Scholar]
- 52.Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. Guilford Press; New York: 1993. [Google Scholar]