Abstract
Striatal projection neurons use GABA as their neurotransmitter and express the rate-limiting synthesizing enzyme glutamic acid decarboxylase (GAD) and the vesicular GABA transporter vGAT. The chronic systemic administration of an agonist of dopamine D1/D5-preferring receptors is known to alter GAD mRNA levels in striatonigral neurons in intact and dopamine-depleted rats. In the present study, the effects of a single or subchronic systemic administration of the dopamine D1/D5-preferring receptor agonist SKF-81297 on GAD65, GAD67, PPD and vGAT mRNA levels in the striatum and GABAA receptor α1 subunit mRNA levels in the substantia nigra, pars reticulata, were measured in rats with a unilateral 6-OHDA lesion. After a single injection of SKF-81297, striatal GAD65 mRNA levels were significantly increased at 3 but not 72 hours. In contrast, striatal GAD67 mRNA levels were increased and nigral α1 mRNA levels were decreased at 72 but not 3 hours. Single cell analysis on double-labeled sections indicated that increased GAD or vGAT mRNA levels after acute SKF-81297 occurred in striatonigral neurons identified by their lack of preproenkephalin expression. Subchronic SKF-81297 induced significant increases in striatal GAD67, GAD65, preprodynorphin and vGAT mRNA levels and decreases in nigral α1 mRNA levels. In the striatum contralateral to the 6-OHDA lesion, subchronic but not acute SKF-81297 induced a significant increase in GAD65 mRNA levels. The other mRNA levels were not significantly altered. Finally, striatal GAD67 mRNA levels were negatively correlated with nigral α1 mRNA levels in the dopamine-depleted but not dopamine-intact side. The results suggest that different signaling pathways are involved in the modulation by dopamine D1/D5 receptors of GAD65 and GAD67 mRNA levels in striatonigral neurons. They also suggest that the down-regulation of nigral GABAA receptors is linked to the increase in striatal GAD67 mRNA levels in the dopamine-depleted striatum.
Introduction
The striatum controls motor activity by processing information from the cortex and channeling it to the basal ganglia output nuclei, the substantia nigra, pars reticulata (SNr) and the entopeduncular nucleus (EP) via a striatonigral and striatopallidal GABAergic projection. Striatonigral and striatopallidal projection neurons express the two isoforms of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD67 and GAD65) and the vesicular GABA transporter vGAT (Mercugliano et al., 1992; Wang et al., 2007). Most striatonigral neurons also co-express the peptides dynorphin and substance P and dopamine D1 receptors whereas most striatopallidal neurons co-express the peptide enkephalin and dopamine D2 receptors (Gerfen and Young, 1988; Le Moine et al., 1995; Surmeier et al., 1998). It is well documented that dopamine D1 receptors stimulate GABA neurotransmission in striatonigral neurons. For instance, in intact rats, the intrastriatal injection of an agonist of dopamine D1/D5 receptors increases GABA release in the SNr and EP (You et al., 1994; Ferre et al., 1996) and the systemic administration of the dopamine D1/D5-preferring receptor agonist SKF-38393 increases GAD65 mRNA levels in striatonigral neurons (Laprade and Soghomonian, 1995; 1997).
In rats with a 6-hydroxydopamine (6-OHDA) lesion of dopamine neurons, the systemic chronic or subchronic administration of the metabolic precursor of dopamine l-DOPA or the agonist SKF-38393 increases above normal GAD65, GAD67 and vGAT mRNA levels in striatonigral neurons (Laprade and Soghomonian, 1999; Consolo et al., 1999; Carta et al., 2001; 2002; Nielsen and Soghomonian, 2004; Wang et al., 2007). The effects of l-DOPA are paralleled by enhanced increases in GABA release in the SNr (Yamamoto et al., 2006) and decreases in GABAA receptor binding (Gnanalingham and Robertson, 1993; Nielsen and Soghomonian, 2004) and mRNA levels encoding for GABAA receptor subunits (Katz et al., 2005) in the SNr and EP. Altogether, these findings suggest that the loss of dopamine input to the striatum results in a sensitization of dopamine receptor-stimulated GABA synthesis and vesicular GABA release by striatonigral neurons and a compensatory down-regulation of GABAA receptors in the SNr/EP.
It has been documented that a single administration of the D1/D2 receptor agonist apomorphine to 6-OHDA-lesioned rats induces maximal sensitization of locomotor behavior to a subsequent injection of SKF-38393 at 72 but not 3 hours (Morelli et al., 1989). Activation of the direct striatonigral GABA projection could be involved in these effects but it is unclear if a single exposure to a D1/D5-preferring agonist can induce long-lasting effects on mRNA levels encoding for molecules involved in GABA-mediated signaling. In intact rats, a chronic administration of SKF-38393 increases GAD65 but not GAD67 mRNA levels in striatonigral neurons (Laprade and Soghomonian, 1997). In contrast, in rats with a neonatal 6-OHDA lesion, such treatment induces an increase in both GAD65 and GAD67 mRNA levels in striatonigral neurons (Laprade and Soghomonian, 1999). This suggests that the two GAD isoforms are under control of different regulatory factors in striatonigral neurons and that increased GAD67 expression in these neurons could be involved in the behavioral responses induced by agonists of dopamine receptors in dopamine-depleted animals. In light of these previous findings, the objective of this study was to quantify GAD65, GAD67 and vGAT mRNA levels in the striatum and GABAA receptor α1 subunit mRNA levels in the SNr at 3 or 72 hours after a single acute administration or 3 hours after a subchronic administration of the full agonist of dopamine D1/D5-preferring receptors, SKF-81297 to 6-OHDA-lesioned rats. The results show that acute SKF-81297 increases GAD67 and GAD65 mRNA levels with a different time-course in the dopamine-depleted striatum while subchronic SKF-81297 increases GAD65, GAD67 and vGAT mRNA levels. In addition, the results show that the increase in GAD67 mRNA levels in striatonigral neurons induced by a single injection of SKF-81297 is paralleled by a decrease in α1 subunit mRNA levels in the SNr.
Methods
Subjects and drug treatment
A total of 27 adult male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing 250-300g were used for this experiment. Rats were maintained under a 12-h light-dark cycle with constant temperature and humidity and free access to food and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine and performed in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and potentially stressful procedures.
All rats were anesthetized with a mixture of ketamine (80 mg/kg) and zylazine (10 mg/kg) intraperitoneally and placed in a stereotaxic apparatus. Rats were unilaterally depleted of dopamine by intracerebral injections of 6-OHDA (8.0 μg of free base in 2 μl of saline with 1.0% ascorbic acid; Sigma Chemical Co., St-Louis, MO) into the left rostal substantia nigra, pars compacta (anterior/posterior=3.4mm, lateral=2.0mm, height=2.8mm) and the left median forebrain bundle (anterior/posterior=4.0mm, lateral=1.1mm, height=1.99) with the incisor bar at 0mm. Manual injections of 6-OHDA were carried out with a Hamilton syringe over 2 min, and the syringe was kept in place for an additional 5 min to allow for drug diffusion before removal. Thirty minutes before 6-OHDA, rats received an s.c. injection of desipramine (25mg/kg) to prevent uptake into noradrenergic neurons. After the end of the surgery and following recovery from the anesthesia, the rats were returned to their cage.
Three weeks following the surgery, the rats were randomly divided into four groups: vehicle, acute-3 hours, acute-72 hours, and subchronic. Rats in the vehicle or acute-3 hours group received a single injection of vehicle or SKF-81297 (5mg/kg; Sigma), respectively and were killed three hours after the injection. Rats in the acute-72 hours group received a single injection of SKF-81297 (5mg/kg) and were killed 72 hours after the injection. The subchronic group received one injection a day of SKF-81297 (5mg/kg) for three days and killed three hours after the last injection. All rats were deeply sedated with CO2 before being killed by decapitation. Brains were rapidly removed, frozen on powdered dry ice, and maintained at −80°C. Coronal 10 μm-thick sections were cut on a Microm HM-505 cryostat at striatal (interaural 10.0-10.6 mm) or nigral (interaural 3.50-3.80mm) level according to the atlas of Paxinos and Watson (1986) and thaw-mounted on chromalum gelatin-coated glass slides. Tissue sections were stored at −80°C until further processing.
3H-mazindol binding radioautography
To assess the extent of the lesion of dopamine neurons, levels of 3H-mazindol binding were measured in the striatum as previously described (Soghomonian et al., 1994). Fresh-frozen tissue sections were dried under a flow of air. Sections were rinsed for 5 min at 4 °C in 50 mM Tris buffer with 120 mM NaCl and 5 mM KCl to wash off endogenous ligand. Two sections were then incubated for 40 min at 4 °C in 15 nM 3H-mazindol (PerkinElmer Life Sciences, Boston, MA, USA, specific activity 21.0 Ci/mmol) in 50 mM Tris buffer with 300 mM NaCl and 5 mM KCl. Desipramine (0.3 mM; Sigma) was added to block binding to norepinephrine transporters. Nonspecific binding was determined in the presence of 30 μM unlabeled benztropine (Sigma) Sections were then quickly rinsed in ice-cold buffer, distilled water and air-dried. All sections were apposed to Kodak Biomax MR X-ray films (Eastman Kodak, Rochester, NY, USA) at room temperature for 35-45 days. The films were developed in Kodak D-19 for 3.5 min at 14 °C.
In situ hybridization histochemistry
Brain sections were processed for in situ hybridization histochemistry as previously described (Nielsen and Soghomonian, 2004). Briefly, coronal brain sections were quickly dried under a flow of air at room temperature and immediately fixed for 5 min in 3% paraformaldehyde in a phosphate buffer (pH 7.2). Sections were then sequentially rinsed in 2×SSC, phosphate buffer saline (0.1 M), 0.25% acetic anhydride with triethanolamine for 10 min, Tris-glycine for 30 min and dehydrated in ethanol. Sections were hybridized overnight at 42 °C with 3×105 cpm in 50 μl of [35S]-labeled oligonucleotide probe for the GABAA receptor α1 subunit or for 4 h at 52 °C with 4.0 ng in 20 μl of [35S]-labeled cRNA probe for GAD67, GAD65, PPD, or vGAT diluted in hybridization solution (40% formamide, 10% dextran sulfate, 4× SSC, 10 mM dithiothreitol, 1.0% sheared salmon sperm DNA, 1.0% yeast tRNA, 1× Denhardt’s solution). Synthesis of the [35S]-labeled cRNA probes was performed by in vitro transcription from cDNAs encoding for GAD65, GAD67, PPD or vGAT in the presence of [35S]-labeled UTP (PerkinElmer). For post-hybridization washes, the sections were washed in 1 × SSC for 1 h and 0.5 × SSC for 30 min at 42 °C for the oligonucleotide probe or for the cRNA probes in 50% formamide at 52 °C for 5 and 20 min and RNAse A (100 μg/ml; Sigma) for 30 min at 37 °C. Sections were then dehydrated in ethanol and defatted in xylene. The slides that were hybridized with the oligonucleotide probe were dipped in Kodak NTB3 nuclear emulsion (Kodak Chemical Co. Rochester, NY) diluted 1:1 with water containing ammonium acetate, dried for 3 h and stored in light-tight boxes for 10 days. Slides were developed in Kodak 19 developer, stained with hematoxylin and eosin and mounted with Eukitt™ (Sigma) mounting medium. The slides that were hybridized with the cRNA probes were apposed to Kodak BioMax MR X-ray films for 10–15 days.
For the double-labeling experiments, sections were processed with a combination of a 35S-labeled GAD67 or vGAT riboprobe and a digoxigenin-labeled (DIG-labeled) preproenkephalin (PPE) ribroprobe. Synthesis of the DIG-labeled PPE probe was performed by in vitro transcription from a cDNA encoding for PPE (Yoshikawa et al., 1984; inserted into pSP64) in the presence of digoxigenin-labeled UTP (Roche Applied Science, Indianapolis, IN, USA). Sections were hybridized at 52 °C for 4 h with 20 μl of the DIG-labeled PPE probe (200-400 ng) and the radioactive GAD67 or vGAT probe as described above. Pre-hybridization and post-hybridization washes were as described above. Following post-hybridization washes, sections were covered with 90 μl of an anti-digoxigenin Fab fragment conjugated with alkaline phosphatase (Roche Applied Science) and incubated overnight at 4 °C. Sections were then incubated in the dark for 1.5–3 h in 75 mg/ml Nitroblue tetrazolium chloride, 50 mg/ml X-phosphate (Roche Applied Science), and 0.24 mg/ml levamisol (Sigma). The reaction was terminated by rinsing the slides in 10 mM Tris buffer with 1 mM EDTA (pH 8.0) and 2× SSC for 15 min. Following a brief dehydration in 70% ethanol, sections were coated with Amersham LM-1 nuclear emulsion (Amersham Biosciences, Piscataway, NJ), air dried for 4 hours and stored at room temperature in light-tight boxes with desiccant for 10 days. Sections were developed in Kodak D-19 developer for 3.5 min at 14 °C, and mounted with crystal mount™ (Biomedia, Foster City, CA, USA).
Quantification of X-ray film autoradiographs
The levels of 3H-mazindol and GAD67, GAD65, PPD, and vGAT mRNA labeling were quantified on X-ray films in a dorsal sector of the striatum at rostro-caudal level A=10 (Paxinos and Watson, 1986) as shown on Figure 2A. The films were viewed on a Macintosh computer connected to a Sony CCD video camera. The analog signal was converted to a digital image of 640×480 pixels using NIH image 1.61 software. Relative mRNA levels were expressed as relative optical density (OD), which was calculated after standardization against Kodak gelatin filters and subtraction of the optical density of the film. Bilateral measurements were obtained from three consecutive slides per rat for GAD67, GAD65, and vGAT, or two adjacent slides per rat for PPD with n=6 or 7 rats for each group. The value for each rat was calculated by averaging the values from three or two sections. Differences in mRNA labeling between ipsi-and contralateral sides were analyzed with a Student’s paired t-test. Differences between groups were compared by a one-way ANOVA followed by a Bonferroni post hoc analysis. A level of p< 0.05 for a two-tailed test was considered critical for statistical significance.
Figure 2.
Negative images of x-ray films from striatal sections processed for in situ hybridization with a 35S-radioactive GAD67 (A-C) or GAD65 (D-F) cRNA probe. Sections are from 6-OHDA-lesioned rats injected with once with vehicle (A and D) or SKF-81297 and examined 3 (B and E) or 72 (C and F) hours later. The stars indicate the ipsilateral side. The black line on Figure 1A indicates the striatal dorso-lateral area used in our quantitative analyses.
Quantification of emulsion autoradiographs
GAD67 or vGAT mRNA levels in PPE-negative or -positive neurons were quantified in a dorsolateral sector of the striatum as indicated above and GABAA receptor α1 subunit mRNA levels were quantified in the SNr (rostro-caudal level A=3.7 according to Paxinos and Watson, 1986) at the single cell level on emulsion radioautographs. Labeling was quantified using the threshold function of NIH Image 1.61 software and a Nikon Eclipse E600 microscope connected to a Sony CCD video camera. Each neuron was placed in a circle of fixed diameter (d=94 pixels). The area covered by silver grains in the circle was measured using a 60× objective under dark-field illumination in order to measure GAD67 mRNA levels in PPE-positive neurons or bright-field illumination to measure GAD67 or vGAT mRNA levels in PPE-negative neurons in the striatum or GABAA mRNA levels in the SNr. The investigator who carried out the analysis of silver grains was blind to the experimental groups. The measured values were expressed as a number of pixels per neuron. The validity of the system has previously been established by a +0.90 correlation coefficient between numbers of pixels and numbers of silver grains per cell (Weiss and Chesselet, 1989). This linear relationship was tested on a small sample of sections and neurons before the beginning of the quantification. Because the criterion for selection of silver grains using the threshold function can vary from one investigator to another and between different experimental conditions, the quantitative analysis of silver grains was carried out by the same investigator and independently for PPE-negative and PPE-positive profiles. The background distribution of silver grains was assessed for each experiment by placing the selection circle over fifty different and randomly sampled regions of the neuropil and the average number of pixels calculated. Only the cell profiles that had a value higher than background were selected for the analysis. Forty to fifty such neuronal profiles from each brain side and each section were randomly sampled and analyzed in a dorso-lateral striatal sector (as shown on figure 2A) or in the substantia nigra, pars reticulata, and at least five rats from each group and two slides per rat were analyzed. The value for each rat was calculated as the average number of pixels per cell from two sections. Differences in mRNA labeling between ipsi-and contralateral sides were analyzed with a paired Student’s t-test. Differences between groups were analyzed by a one-way ANOVA followed by a Bonferroni’s post hoc analysis. A level of p<0.05 for a two-tailed test was considered critical for statistical significance.
Results
Effect of 6-OHDA lesions on 3H-mazindol binding
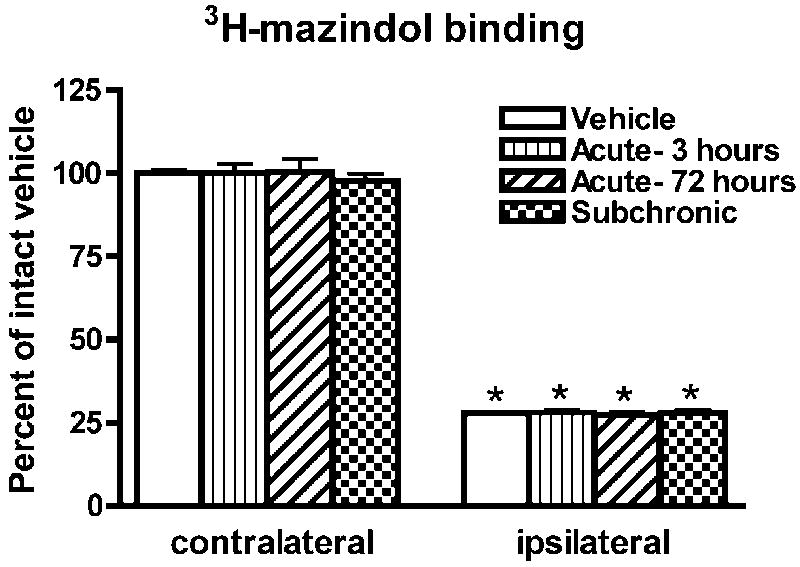
There was no significant difference between groups in the levels of 3H-mazindol binding in the striatum contralateral to the 6-OHDA-lesion (F(3,23)=0.16; p=0.92). On the side ipsilateral to the 6-OHDA lesion, a marked loss of 3H-mazindol labeling was measured when compared to the contralateral striatum (Figure 1). The difference in labeling intensity between the ipsi- and contralateral striatum was highly significant in all groups (Figure 1) but there were no significant differences in labeling between groups in the ipsilateral striatum.
Figure 1.
3H-mazindol binding was measured by computerized densitometry on x-ray films from sections of the striatum of rats with a unilateral 6-OHDA lesion. Data are mean±S.E.M. of relative o.d. values expressed as a percent of the contralateral vehicle. *p<0.0001 with contralateral side (paired t test).
GAD, vGAT, and PPD mRNA labeling in the striatum
Quantitative analysis of GAD, vGAT, and PPD mRNA labeling was performed on X-ray film radioautographs in the dorsal striatum (Figure 2). In all experimental groups, GAD67 mRNA labeling was significantly higher in the ipsi- compared to contralateral striatum (Figure 2A-C and 3A). Comparison between groups indicated that GAD67 mRNA levels were significantly different in the striatum ipsilateral to the 6-OHDA lesion (ANOVA: F(3,22)=3.58; p<0.05). Further post-hoc analysis showed that GAD67 mRNA levels were comparable in the vehicle and acute-3 hours group. However, GAD67 mRNA levels were significantly increased in the acute-72 hours and subchronic groups when compared to the vehicle or acute-3 hours group (Figure 3A). One-way ANOVA showed no significant differences between groups on the side contralateral to the 6-OHDA lesion (F(3,22)=1.00; p=0.41).
Figure 3.
Levels of GAD67 (A), GAD65 (B), and vGAT (C) mRNA labeling were quantified by computerized densitometry on X-ray films in the striatum of 6-OHDA-lesioned rats injected with vehicle or SKF-81297 (acute-3 hours, acute-72 hours, and subchronic). Three sections per animal were analyzed with n=6 in the vehicle group and n=7 in the acute-3 hours, acute-72 hours or subchronic group. Data are mean±S.E.M. expressed as a percent of the contralateral vehicle. Differences between ipsi- and contralateral side were analyzed with a paired t-test (*p<0.01). Differences between groups are analyzed with a one-way ANOVA followed by a Bonferroni post hoc test (#p<0.05 compared to vehicle; @p<0.05 compared to acute-3 hours group; ¶p<0.05 compared to acute-72 hours group).
In all experimental groups, GAD65 mRNA labeling was significantly higher in the ipsi-compared to contralateral striatum (Figure 2D-F and 3B). One-way ANOVAs showed that GAD65 mRNA levels in the ipsilateral or contralateral striatum to the lesion were significantly different between groups (F(3,23)=3.89; p<0.05 for the ipsilateral striatum; F(3,23)=6.44; p<0.01 for the contralateral striatum). Post-hoc analysis on the side ipsilateral to the 6-OHDA-lesion showed that GAD65 mRNA labeling was significantly increased in the acute-3hours and subchronic group when compared to the vehicle group (Figure 3B). GAD65 mRNA levels in the subchronic group were also significantly higher when compared to the acute-72 hours group (Figure 3B). In the striatum contralateral to the 6-OHDA-lesion, GAD65 mRNA levels were significantly higher in the subchronic group when compared to all other groups (Figure 3B).
Consistent with a previous study (Wang et al., 2007), vGAT mRNA levels in 6-OHDA-lesioned rats treated with vehicle were significantly higher in the ipsi- compared to contralateral striatum (Figure 3C). In the other groups, vGAT mRNA levels were also higher in the ipsi-compared to contralateral striatum (Figure 3C). A one-way ANOVA showed that vGAT mRNA levels in the ipsilateral striatum were significantly different between groups (F(3,21)=3.64; p<0.05). Post-hoc analysis showed that vGAT mRNA levels were significantly higher in the subchronic compared to the vehicle group (Figure 3C). No difference was found in vGAT mRNA levels between groups in the contralateral striatum.
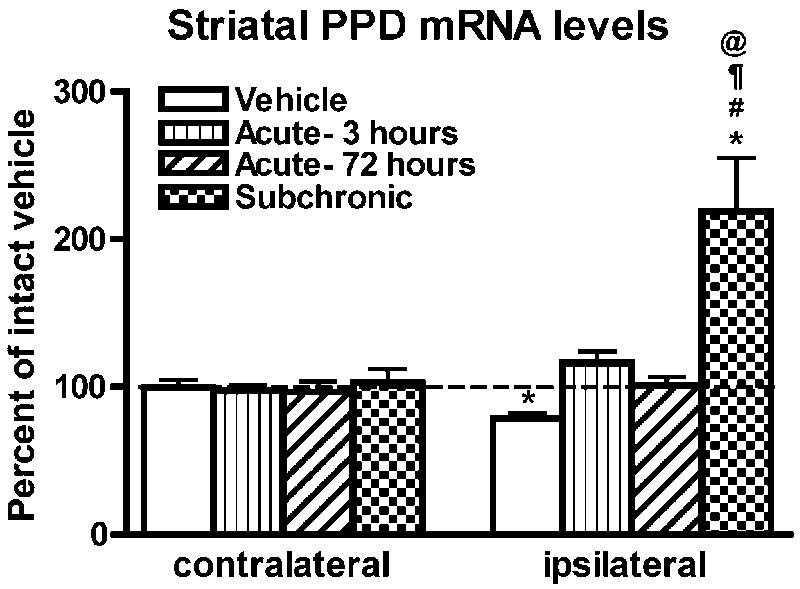
In agreement with previous findings (i.e. Gerfen et al., 1990; Andersson et al., 1999), in the vehicle group, PPD mRNA levels were significantly decreased in the ipsi- compared to contralateral striatum (Figure 4). In 6-OHDA-lesioned rats treated with subchronic SKF-81297, PPD mRNA levels were significantly higher on the ipsi- compared to contralateral side (Figure 4) but no difference was found between sides in the acute-3 or -72 hours group. One-way ANOVA of PPD mRNA labeling in the striatum ipsilateral to the 6-OHDA lesion indicated a significant difference between groups (F(3,23)=10.24, p<0.001). Post hoc comparisons indicated that PPD mRNA levels were significantly higher in the subchronic when compared to all other groups (Figure 4). No significant differences in PPD mRNA levels between groups were observed on the side contralateral to the 6-OHDA lesion (F(3,23)=0.21, p=0.90).
Figure 4.
Level of PPD mRNA labeling in the striatum of 6-OHDA-lesioned rats injected with vehicle or SKF-81297 (acute-3 hours, acute-72 hours, and subchronic). Data are the mean±S.E.M. expressed as a percent of contralateral vehicle. *p<0.01 compared to contralateral side. Differences between groups were analyzed with a one-way ANOVA followed by followed by a Bonferonni post hoc test (#p<0.05 compared to vehicle; ¶p<0.05 compared to acute-3 hours; @p<0.05 compared to acute-72 hours).
GAD and vGAT mRNA levels in PPE-negative neurons
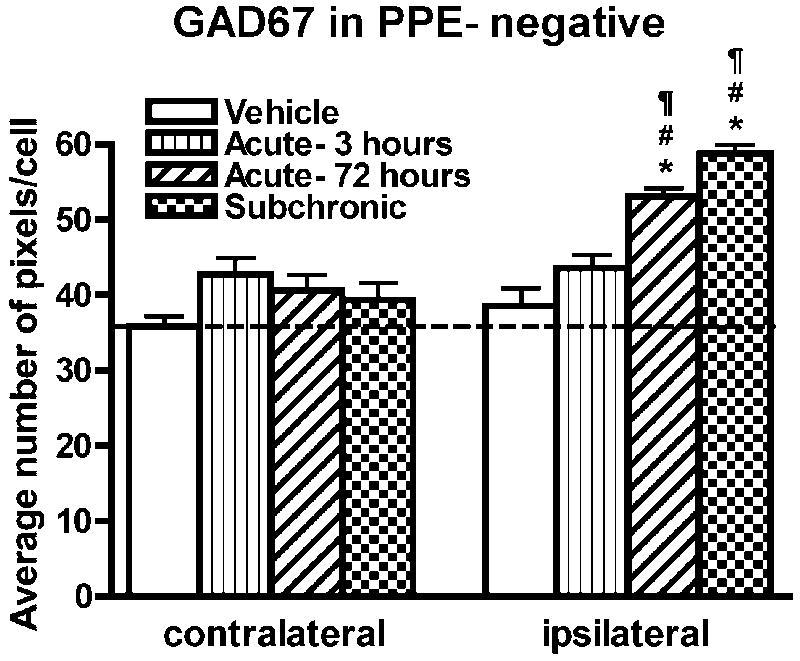
Previous studies have shown that 6-OHDA lesions result in increased GAD and vGAT mRNA levels in PPE-positive striatal neurons whereas the administration of a D1/D5-preferring agonist of dopamine receptors to unlesioned or 6-OHDA-lesioned rats or the indirect agonist l-DOPA to 6-OHDA-lesioned rats primarily increases these mRNAs in striatal neurons that do not express PPE (Laprade and Soghomonian, 1997; 1999; Carta et al., 2003; Nielsen and Soghomonian, 2004; Wang et al., 2007). In light of these previous results, we assumed that the effects of SKF-81297 also occurred in PPE-negative neurons. This possibility was further tested in our study following the quantitative analysis of sections double-labeled with a combination of radioactive GAD67 or vGAT and digoxigenin PPE cRNA probes. First, the ratio of PPE-positive and PPE-negative neurons on striatal sections was measured to assess the sensitivity of the method to detect PPE in striatal neurons. On average, 46% of all detectable neurons in the dorsolateral striatum were found to be PPE-positive. In PPE-negative neurons visualized with the eosin-hematoxylin counterstain under bright-field microscopy, GAD67 mRNA levels were significantly higher in the ipsi- compared to contralateral striatum in the subchronic and acute-72 hours groups whereas no differences between sides were observed in the vehicle or acute-3 hours group (Figure 5E and F and 6). Comparison between groups further indicated that GAD67 mRNA levels in PPE-negative neurons were significantly higher in the striatum ipsilateral to the 6-OHDA lesion in the subchronic and acute-72 hours group when compared to the vehicle or acute-3hours group (one-way ANOVA; F(3,17)=27.15; p<0.0001) (Figure 5E and F and 6). In accordance with results gathered on X-ray films, there were no statistical differences in GAD67 mRNA labeling between groups in PPE-negative neurons in the contralateral striatum. Quantitative analysis of vGAT mRNA levels in PPE-negative neurons was carried out only in the striatum ipsilateral to the 6-OHDA lesion and in the vehicle, 3 hours and subchronic group, which showed significant differences on x-ray films. In PPE-negative neurons, significant differences between groups (ANOVA: F(2,13)=11.52; p<0.005) were found. Post-hoc analysis indicated that vGAT mRNA levels in PPE-negative neurons were significantly higher in the subchronic (55.4±4.87 pixels per cell) compared to the vehicle (35.6±1.9 pixels per cell; p<0.01) or acute 3 hours (40.6±1.6 pixels per cell; p<0.05) group. GAD67 and vGAT mRNA levels in PPE-positive cells were also quantified in the ipsilateral striatum in the vehicle, 3 hours and subchronic group. No significant differences in GAD67 (one-way ANOVA: F(2,16)=0.14; p=0.87) or vGAT (one-way ANOVA: F(2,16)=0.46; p=0.64) mRNA labeling were seen between groups.
Figure 5.

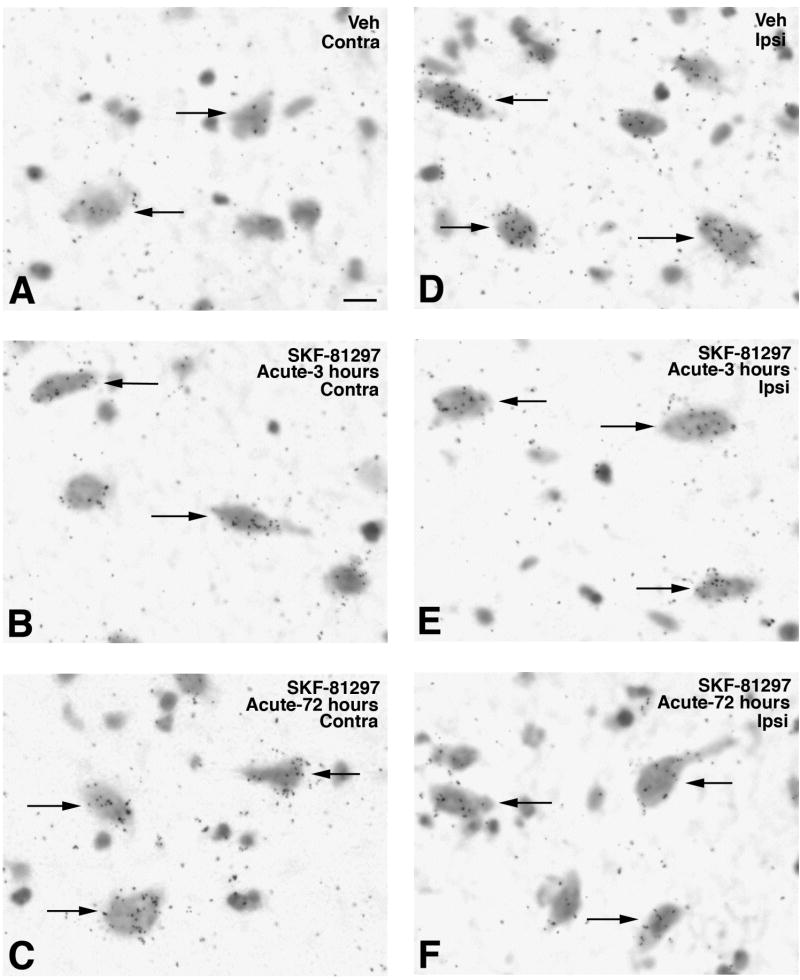
Bright-field photomicrographs illustrating GAD67 (A and B) or vGAT (C and D) mRNA labeling in striatal neurons. Sections are from the ipsilateral striatum in a 6-OHDA-lesioned rat injected with vehicle (A and C) or SKF-81297 (B and D). Arrows show examples of PPE-positive profiles and arrowheads show examples of PPE-negative profiles. Scale bar=15μm.
Figure 6.
GAD67 mRNA labeling in PPE-negative striatal neurons was quantified by computerized image analysis of emulsion autoradiographs in 6-OHDA-lesioned rats injected with vehicle or SKF-81297 (acute-3 hours, acute-72 hours or subchronic). Data are mean±S.E.M. expressed as an average number of pixels per labeled neuron. Two slides per animals were analyzed with n=5 per group. *p<0.05 compared to the respective contralateral side. Differences between groups are analyzed with a one-way ANOVA followed by a Bonferroni post hoc test. #p<0.05 compared to vehicle; ¶p<0.05 compared to acute-3 hours.
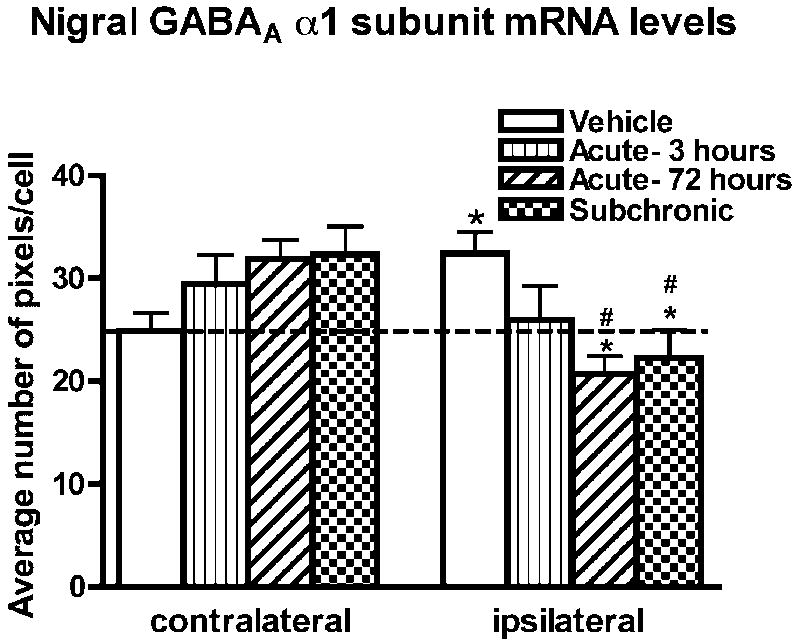
GABAA α1 receptor subunit mRNA labeling in the SNr
In 6-OHDA-lesioned rats treated with vehicle, GABAA α1 receptor subunit mRNA levels in the SNr were significantly higher on the ipsi- compared to contralateral side (Figure 7A, 7D and 8). In contrast, GABAA α1 receptor subunit mRNA levels were significantly lower in the ipsi-compared to contralateral SNr in the acute-72 hours (Figure 7C, 7F and 8) or subchronic group (Figure 8). No difference was observed between sides in the acute-3 hours group (Figure 7C, 7F and 8). A one-way ANOVA showed that GABAA α1 receptor subunit mRNA levels in the ipsilateral SNr were significantly different between groups (F(3,15)=4.49; p<0.05). Post-hoc comparisons indicated that GABAA α1 receptor subunit mRNA levels were significantly lower in the acute-72 hours and subchronic group compared to the vehicle group. Although a trend for an increase was seen, no significant differences in GABAA α1 receptor subunit mRNA levels were found in the contralateral SNr between groups (F(3,15)=2.11; p=0.14). The observation that acute SKF-81297 significantly decreased nigral α1 mRNA levels at 72 hours when striatal GAD67 mRNA levels were increased in striatonigral neurons suggested a possible link between the two effects. This possibility was further supported by the negative and significant correlation between α1 mRNA levels in the SNr and striatal GAD67 mRNA levels on the side ipsi- but not contralateral to 6-OHDA (Figure 9).
Figure 7.
Bright-field photomicrographs illustrating GABAA receptor α1 subunit mRNA labeling in the contralateral (Contra) and ipsilateral (Ipsi) SNr of 6-OHDA-lesioned rats injected with vehicle (A and D) or SKF-81297 (B, C and E, F) and killed 3 (B and E) or 72 (C and F) hours after SKF-81297. Arrows illustrate labeled neurons. Scale bar=10μm.
Figure 8.
Quantification of GABAA receptor α1 subunit mRNA levels was carried out at the single cell level by computerized image analysis on emulsion radioautographs in the contralateral and ipsilateral SNr of rats injected with vehicle or SKF-81297 (acute-3 hours, acute-72 hours, or subchronic). The data were obtained from two slides per animal with n=5 animals per condition and are the mean±S.E.M. expressed as an average number of pixels per neuron. *p<0.05 compared with respective contralateral side to 6-OHDA-lesion (paired t-test). Differences between groups are analyzed with a one-way ANOVA followed by a Bonferroni post hoc test. #p<0.05 compared to vehicle.
Figure 9.
Correlation between mRNA labeling for the α1 subunit in the SNr and GAD67 in striatonigral neurons on the side ipsi (A) or contralateral (B) to the 6-OHDA lesion. mRNA labeling was quantified at the single cell level as described in the methods section. r=Pearson correlation coefficient.
Discussion
Results in the present study show that a single injection of SKF-81297 increases striatal GAD65 mRNA levels at 3 but not 72 hours. In contrast, striatal GAD67 mRNA levels are increased and nigral GABAA receptor α1 mRNA levels are decreased at 72 but not 3 hours. On sections double-labeled with PPE, the SKF-91297-induced increases in GAD67 and vGAT mRNA levels are detected in striatonigral neurons. Furthermore, subchronic SKF-81297 induces significant increases in striatal GAD67, GAD65, PPD and vGAT mRNA levels and decreases in nigral α1 mRNA levels. Finally, in the striatum contralateral to the 6-OHDA lesion, subchronic SKF-81297 induces a significant increase in GAD65 mRNA levels only. Altogether, the results indicate that the acute or subchronic administration of a D1/D5-preferring agonist increases with a different time-course GAD67, GAD65 and vGAT mRNA levels in striatonigral neurons. In addition, we show that striatal GAD67 mRNA levels are negatively correlated with nigral α1 mRNA levels in the dopamine-depleted side.
Effects of unilateral 6-OHDA lesions on striatal GABA neurons
In accordance with previous studies, we found that a unilateral 6-OHDA lesion induces an increase in GAD67, GAD65 and vGAT mRNA levels and a decrease in PPD mRNA levels in the dopamine-depleted striatum (Lindefors et al., 1989; Gerfen et al., 1990; Soghomonian et al., 1992; Bacci et al., 2002; Carta et al., 2002; Nielsen and Soghomonian, 2004; Wang et al., 2007). The 6-OHDA-induced increases in GAD65, GAD67 and vGAT mRNA levels were primarily detected in striatal neurons that co-express PPE (Carta et al., 2003; Nielsen and Soghomonian, 2004; Wang et al., 2007) although a small increase was also reported in striatal PPE-negative neurons (Carta et al., 2003). Because these effects have been thoroughly documented, we did not compare mRNA labeling in PPE-positive neurons between the lesioned and unlesioned striatum. However, these previous findings are consistent with our double-labeling analysis since GAD67 and vGAT mRNA levels were respectively not altered or were decreased in PPE-negative striatal neurons. Most PPE-positive neurons in the rat striatum are retrogradely labeled by tracer injections in the globus pallidus and are therefore considered to be striatopallidal (Gerfen and Young, 1998). However, a small proportion of PPE-positive neurons can also be labeled following tracer injections in the substantia nigra (Gerfen and Young, 1988). Furthermore, single cell tracing studies indicate that most striatal efferent neurons that project to the globus pallidus can also send axon collaterals to the substantia nigra and/or to the entopeduncular nucleus (i.e. Wu et al., 2000). It is therefore possible that PPE-positive striatal neurons project to other basal ganglia regions than the globus pallidus. However, based on studies that have shown decreased GABAA receptor expression in the globus pallidus of 6-OHDA-lesioned rats (Pan et al., 1985; Gnanalingham and Robertson, 1993; Nielsen and Soghomonian 2004), we speculate that the increases in GAD and vGAT gene expression are restricted to striatal neurons that send the bulk of their axonal projections to the globus pallidus. Thus, altogether, these results add more evidence to the idea that the 6-OHDA-induced loss of dopamine input results in an increased expression of proteins involved in the modulation of GABA-mediated signaling in the so-called “striatopallidal” pathway.
Our study also confirms previous findings showing that mRNA levels of the α1 subunit of the GABAA receptor are increased in the SNr ipsilateral to the 6-OHDA lesion (Chadha et al., 2000; Katz et al., 2005). Because striatonigral neurons provide the most prominent GABA input to the SNr/EP (Smith et al., 1998) and that vGAT gene expression is decreased in these neurons (Wang et al., 2007), it is possible that the 6-OHDA-induced up-regulation of GABAA receptors in the SNr/EP is secondary to a decrease in GABA release from striatonigral neurons. However, because the SNr also receives a GABAergic input from the globus pallidus (Smith et al., 1998) and from recurrent axon collaterals of SNr neurons (Lantin-Le-Boulch et al., 1991), it cannot be ruled out that the changes in GABAA receptor expression in the SNr are linked to an altered activity of these other afferent systems. On the other hand, the 6-OHDA-induced increases in GAD and vGAT gene expression detected in striatopallidal neurons (Soghomonian et al., 1992; Carta et al., 2003; Wang et al., 2007) are paralleled by an increase in GABA tissue content and extracellular GABA levels (Tanaka et al., 1986; Tossman et al., 1986; Lindefors et al., 1989) and a decreased binding or expression of GABAA receptors in the globus pallidus (Pan et al., 1985; Gnanalingham and Robertson, 1993; Nielsen and Soghomonian, 2004). These findings are therefore consistent with the notion that the altered expression of GABAA receptors in the globus pallidus or SNr of 6-OHDA-lesioned rats is secondary to an altered activity of striatal GABA afferent inputs.
Effects of dopamine D1/D5 receptor agonists on striatonigral GABA neurons
The present study using the full D1/D5-preferring agonist SKF-81297 confirms earlier results showing that the chronic or subchronic administration of the partial D1/D5-preferring agonist, SKF-38393, to adult rats with a 6-OHDA-lesion increases GAD67 mRNA levels in PPE-negative striatonigral neurons (Laprade and Soghomonian, 1999; Carta et al., 2003). We now show that a full agonist of dopamine D1/D5 receptor also increases vGAT mRNA levels in PPE-negative neurons. Although we did not quantify GAD65 in PPE-negative neurons, based on previous studies in both intact and 6-OHDA-lesioned rats (Laprade and Soghomonian, 1997; 1999; Nielsen and Soghomonian, 2004), we also consider that the agonist-induced increases in GAD65 mRNA levels are selective for PPE-negative neurons. The proportion of PPE-positive neurons in our study was slightly lower (46% vs. 58-65%) than that previously reported following a combined in situ hybridization and retrograde labeling study (Gerfen and Young, 1988). Thus, it is possible that we did not detect all PPE-positive neurons and that some false-negative neurons were included in our analysis of PPE-negative neurons. In addition, striatal GABA interneurons also express GAD and possibly vGAT and the identification of these neurons was not attempted in our study. However, because no effects were detected in PPE-positive neurons and that the proportion of striatal interneurons compared to striatal projection neurons is very small, it is highly unlikely that the possible inclusion of few false-negative neurons or striatal interneurons had a significant impact on the outcome of our quantitative analyzes in PPE-negative neurons. Previous studies have shown that most PPE-negative striatal neurons project to the substantia nigra (Gerfen and Young, 1998) but a small proportion projects to the globus pallidus (Gerfen and Young, 1988). In addition, it has been reported that rat striatonigral neurons can send axon collaterals to the entopeduncular nucleus and the globus pallidus (i.e. Wu et al., 2000). Altogether, these findings indicate that PPE-negative neurons in the striatum can project to the substantia nigra, pars reticulata, as well as to other basal ganglia regions. However, as shown in this as well as previous studies, the systemic administration of agonists of dopamine receptors decreases GABAA receptor expression in the substantia nigra, pars reticulata, or entopeduncular nucleus but not the globus pallidus (Gnanalingham and Robertson, 1993; Katz et al., 2005; Nielsen and Soghomonian, 2004). Thus, we hypothesize that the increases in GAD or vGAT mRNA levels in PPE-negative neurons occur in the population of so-called striatonigral neurons that primarily sends a GABA projection to the substantia nigra, pars reticulata, and entopeduncular nucleus.
Previous studies have shown that the effects of SKF-38393 on GAD67 and GAD65 mRNA levels in striatonigral neurons are blocked by the co-administration of the antagonist SCH-23390 (Laprade and Soghomonian, 1999), supporting a selectivity for D1/D5 receptors. Because most striatal GABA neurons that do not co-express enkephalin express the dopamine D1 receptor but a small subset also expresses D5 (formerly D1b) receptors (Surmeier et al., 1996), it is likely that the effects of SKF-81297 on PPE-negative neurons involve activation of dopamine D1 rather than D5 receptors. Our results now provide original evidence that a single administration of SKF-81297 to 6-OHDA-lesioned rats is sufficient to increase GAD65 and GAD67 mRNA levels. However, we found that these effects occur with a different time course since GAD65 and GAD67 mRNAs were respectively and significantly increased 3 or 72 hours later. Another notable difference was that GAD65 mRNA levels were increased in the contralateral dopamine-unlesioned striatum following the subchronic administration of SKF-81297 whereas GAD67 mRNA levels were not changed in either experimental group. This differential effect on the GAD67 and GAD65 mRNA is consistent with previous studies showing that subchronic systemic SKF-38393 increases GAD65 but not GAD67 mRNA levels in striatonigral neurons of intact unlesioned rats (Laprade and Soghomonian, 1995; 1997). A similar dissociation has been found in unlesioned rats injected with SKF-81297 (unpublished observations). Altogether, these data suggest that dopamine D1/D5 receptors control GAD67 and GAD65 gene expression in striatonigral neurons via different signaling pathways. The expression of a number of transcription factors including ΔFosB or c-fos is upregulated in striatonigral neurons after systemic chronic l-DOPA or SKF-38393 administration to 6-OHDA-lesioned rats (Robertson et al., 1992; Doucet et al., 1996; Soghomonian et al., 1998; Carta et al., 2005; Pavon et al., 2006; Valastro et al., 2007). Other recent studies have shown that the systemic administration of agonists of dopamine D1/D5 receptors is able to activate an extracellular-regulated kinase (ERK) pathway in striatonigral neurons in dopamine-depleted but not in intact unlesioned rats (Gerfen et al., 2002). It is therefore possible that the effects of SKF-81297 on GAD67 gene expression in dopamine-depleted rats involve the activation of a signaling pathway in striatonigral neurons that is normally not under the control of dopamine D1 receptors. On the other hand, because the subchronic administration of SKF-81297 increased GAD65 mRNA levels in the dopamine-intact and dopamine-depleted striatum but that the effects were higher in the dopamine-depleted striatum, it is possible that dopamine D1/D5 receptors modulate GAD65 gene expression via a signaling pathway in striatonigral neurons that operates in normal physiological conditions but that becomes hyper-responsive following the loss of dopamine input to the striatum. In any case, the different time-course of increased GAD67 and GAD65 mRNA levels evidenced following acute SKF-81297 reinforces the notion that expression of the two genes is under the control of two different signaling pathways in striatonigral neurons.
The finding that the systemic subchronic administration of SKF-81297 increases vGAT mRNA levels in PPE-negative neurons provides new evidence that this mRNA can be regulated following a stimulation of dopamine D1/D5 receptors. A similar effect has recently been described following a subchronic systemic administration of l-DOPA to 6-OHDA-lesioned rats (Wang et al., 2007). In this previous study, increased vGAT mRNA levels induced by l-DOPA were paralleled by an increase in vGAT protein levels in the the SNr (Wang et al., 2007), suggesting that stimulation of dopamine receptors up-regulates vGAT gene expression and protein synthesis in striatonigral neurons leading to an increased axonal transport and vGAT accumulation in axon terminals in the SNr. In this context, it is of interest that subchronic SKF-81297 increased both GAD65 and vGAT mRNA levels in striatonigral neurons. GAD65 is preferentially distributed in axonal terminals and participates in the biosynthesis of a pool of GABA that is preferentially taken up by synaptic vesicles (Kaufman et al., 1991; Erlander et al., 1991; Martin and Rimwall, 1993; Soghomonian and Martin, 1998; Jin et al., 2003; Wu et al., 2007). Furthermore, the synaptic vesicle proton gradient is essential to the activation of GAD65 by ATP (Hsu et al., 1999). Thus, the increase in vGAT and GAD65 mRNA levels induced by subchronic SKF-81297 could lead to an increased expression and activity of the two proteins and a coordinated increase in the biosynthesis and vesicular storage of GABA in striatonigral neurons.
Another new finding in this study is that acute or subchronic SKF-81297 induced a down-regulation of post-synaptic GABAA receptors in the SNr on the side ipsilateral to the 6-OHDA lesion. Previous studies have shown that similar effects can be elicited by the chronic or subchronic systemic administration of l-DOPA (Gnanalingham and Robertson, 1993; Katz et al., 2005). In our study, we show that the effects can be detected as early as 3 hours after a single administration of SKF-81297 although they reached statistical significance only at 72 hours. This time-course was comparable to that of increased GAD67 mRNA levels in striatonigral neurons, which reached a maximum 72 hours after SKF-81297. On the other hand, the increase in GAD65 mRNA levels in the striatum contralateral to the 6-OHDA lesion was not paralleled by a decrease in α1 mRNA levels in the contralateral SNr. Therefore, although it cannot be ruled out that the mechanisms involved in the SKF-81297-induced increase in GAD67 mRNA levels in striatonigral neurons and decreased GABAA receptor mRNA levels in the SNr are completely independent, our results suggest that these two effects are linked. This possibility is further supported by the negative correlation between striatal GAD67 and nigral α1 mRNA levels in the dopamine-depleted but not dopamine-intact side. There is some evidence that the GAD67 isoform contributes to the biosynthesis of a pool of GABA that could be released via a carrier-mediated rather than a Ca++-dependent vesicular pathway (Soghomonian and Martin, 1998; Liu et al., 2006). In addition, the activation of dopamine D1 receptors can induce a carrier-mediated increase in GABA release in the striatum (Bernath et al., 1989; Schoffelmeer et al., 2000). Based on these data, it is possible that the down-regulation of GABAA receptors in SNr neurons is secondary to an alteration of a carrier-mediated release of GABA by the axon terminals of striatonigral neurons, a hypothesis that remains to be tested.
Previous studies have shown that a single administration of the mixed agonist of dopamine D1/D2 receptors apomorphine or of l-DOPA sensitizes the contralateral rotational behavior induced by a subsequent administration of a dopamine D1 receptor agonist (Morelli and Di Chiara, 1987; Rouillard et al., 1988; Carey, 1991). The effects of dopamine receptor agonists on priming of contralateral rotational behavior is time-dependent since it can be detected at 3 hours but peaks at 72 hours post-administration (Morelli et al, 1987). Our results show that GAD67 mRNA levels are significantly increased 72 but not 3 hours after a single administration of SKF-81297 and these effects are only detected in the dopamine-depleted striatum. This provides further support for the notion that GAD67 in striatonigral neurons is involved in the abnormal behavioral responses induced by the systemic administration of agonists of dopamine receptors in the dopamine-depleted rat.
Acknowledgments
This work was sponsored by the National Institutes of Health NS40783
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- Bacci JJ, Salin P, Kerkerian-Le Goff L. Systemic administration of dizocilpine maleate (MK-801) or L-dopa reverses the increases in GAD65 and GAD67 mRNA expression in the globus pallidus in a rat hemiparkinsonian model. Synapse. 2002;46:224–234. doi: 10.1002/syn.10117. [DOI] [PubMed] [Google Scholar]
- Bernath S, Zigmond MJ. Dopamine may influence striatal GABA release via three separate mechanisms. Brain Res. 1989;476:373–376. doi: 10.1016/0006-8993(89)91262-6. [DOI] [PubMed] [Google Scholar]
- Carey RJ. Chronic L-dopa treatment in the unilateral 6-OHDA rat: evidence for behavioral sensitization and biochemical tolerance. Brain Res. 1991;568:205–214. doi: 10.1016/0006-8993(91)91399-l. [DOI] [PubMed] [Google Scholar]
- Carta A, Fenu S, Morelli M. Alterations in GAD67, dynorphin and enkephalin mRNA in striatal output neurons following priming in the 6-OHDA model of Parkinson’s disease. Neurol Sci. 2001;22:59–60. doi: 10.1007/s100720170046. [DOI] [PubMed] [Google Scholar]
- Carta AR, Pinna A, Cauli O, Morelli M. Differential regulation of GAD67, enkephalin and dynorphin mRNAs by chronic-intermittent L-dopa and A2A receptor blockade plus L-dopa in dopamine-denervated rats. Synapse. 2002;44:166–174. doi: 10.1002/syn.10066. [DOI] [PubMed] [Google Scholar]
- Carta AR, Fenu S, Pala P, Tronci E, Morelli M. Selective modifications in GAD67 mRNA levels in striatonigral and striatopallidal pathways correlate to dopamine agonist priming in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 2003;18:2563–2572. doi: 10.1046/j.1460-9568.2003.02983.x. [DOI] [PubMed] [Google Scholar]
- Carta AR, Tronci E, Pinna A, Morelli M. Different responsiveness of striatonigral and striatopallidal neurons to L-DOPA after a subchronic intermittent L-DOPA treatment. Eur J Neurosci. 2005;21:1196–1204. doi: 10.1111/j.1460-9568.2005.03944.x. [DOI] [PubMed] [Google Scholar]
- Chadha A, Dawson LG, Jenner PG, Duty S. Effect of unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway on GABA(A) receptor subunit gene expression in the rodent basal ganglia and thalamus. Neuroscience. 2000;95:119–126. doi: 10.1016/s0306-4522(99)00413-3. [DOI] [PubMed] [Google Scholar]
- Consolo S, Morelli M, Rimoldi M, Giorgi S, Di Chiara G. Increased striatal expression of glutamate decarboxylase 67 after priming of 6-hydroxydopamine-lesioned rats. Neuroscience. 1999;89:1183–1187. doi: 10.1016/s0306-4522(98)00390-x. [DOI] [PubMed] [Google Scholar]
- Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, Chen JS, Iadarola MJ, St-Jean M, Wigle N, Blanchet P, Grondin R, Robertson GS. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum. Eur J Neurosci. 1996;8:365–381. doi: 10.1111/j.1460-9568.1996.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Ferre S, O’Connor WT, Svenningsson P, Bjorklund L, Lindberg J, Tinner B, Stromberg I, Goldstein M, Ogren SO, Ungerstedt U, Fredholm BB, Fuxe K. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci. 1996;8:1545–1553. doi: 10.1111/j.1460-9568.1996.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanalingham KK, Robertson RG. Chronic continuous and intermittent L-3,4-dihydroxyphenylalanine treatments differentially affect basal ganglia function in 6-hydroxydopamine lesioned rats--an autoradiographic study using [3H]flunitrazepam. Neuroscience. 1993;57:673–681. doi: 10.1016/0306-4522(93)90014-7. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Thomas C, Chen W, Davis KM, Foos T, Chen JL, Wu E, Floor E, Schloss JV, Wu JY. Role of synaptic vesicle proton gradient and protein phosphorylation on ATP-mediated activation of membrane-associated brain glutamate decarboxylase. J Biol Chem. 1999;274:24366–24371. doi: 10.1074/jbc.274.34.24366. [DOI] [PubMed] [Google Scholar]
- Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu CC, Kopke RD, Wu JY. Demonstration of functional coupling between gamma -aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100:4293–4298. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J, Nielsen KM, Soghomonian JJ. Comparative effects of acute or chronic administration of levodopa to 6-hydroxydopamine-lesioned rats on the expression of glutamic acid decarboxylase in the neostriatum and GABAA receptors subunits in the substantia nigra, pars reticulata. Neuroscience. 2005;132:833–842. doi: 10.1016/j.neuroscience.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantin le Boulch N, Truong-Ngoc NA, Gauchy C, Besson MJ. In vivo release of newly synthesized [3H]GABA in the substantia nigra of the rat: relative contribution of GABA striato-pallido-nigral afferents and nigral GABA neurons. Brain Res. 1991;559:200–210. doi: 10.1016/0006-8993(91)90003-e. [DOI] [PubMed] [Google Scholar]
- Laprade N, Soghomonian JJ. Differential regulation of mRNA levels encoding for the two isoforms of glutamate decarboxylase (GAD65 and GAD67) by dopamine receptors in the rat striatum. Brain Res Mol Brain Res. 1995;34:65–74. doi: 10.1016/0169-328x(95)00139-j. [DOI] [PubMed] [Google Scholar]
- Laprade N, Soghomonian JJ. Glutamate decarboxylase (GAD65) gene expression is increased by dopamine receptor agonists in a subpopulation of rat striatal neurons. Brain Res Mol Brain Res. 1997;48:333–345. doi: 10.1016/s0169-328x(97)00112-5. [DOI] [PubMed] [Google Scholar]
- Laprade N, Soghomonian JJ. Gene expression of the GAD67 and GAD65 isoforms of glutamate decarboxylase is differentially altered in subpopulations of striatal neurons in adult rats lesioned with 6-OHDA as neonates. Synapse. 1999;33:36–48. doi: 10.1002/(SICI)1098-2396(199907)33:1<36::AID-SYN4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Brene S, Herrera-Marschitz M, Persson H. Region specific regulation of glutamic acid decarboxylase mRNA expression by dopamine neurons in rat brain. Exp Brain Res. 1989;77:611–620. doi: 10.1007/BF00249614. [DOI] [PubMed] [Google Scholar]
- Liu J, Tai C, de Groat WC, Peng XM, Mata M, Fink DJ. Release of GABA from sensory neurons transduced with a GAD67-expressing vector occurs by non-vesicular mechanisms. Brain Res. 2006:1073–1074. 297–304. doi: 10.1016/j.brainres.2005.12.091. [DOI] [PubMed] [Google Scholar]
- Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem. 1993;60:395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- Mercugliano M, Soghomonian JJ, Qin Y, Nguyen HQ, Feldblum S, Erlander MG, Tobin AJ, Chesselet MF. Comparative distribution of messenger RNAs encoding glutamic acid decarboxylases (Mr 65,000 and Mr 67,000) in the basal ganglia of the rat. J Comp Neurol. 1992;318:245–254. doi: 10.1002/cne.903180302. [DOI] [PubMed] [Google Scholar]
- Morelli M, Di Chiara G. Agonist-induced homologous and heterologous sensitization to D-1- and D-2-dependent contraversive turning. Eur J Pharmacol. 1987;141:101–107. doi: 10.1016/0014-2999(87)90415-8. [DOI] [PubMed] [Google Scholar]
- Morelli M, Fenu S, Garau L, Di Chiara G. Time and dose dependence of the ‘priming’ of the expression of dopamine receptor supersensitivity. Eur J Pharmacol. 1989;162:329–335. doi: 10.1016/0014-2999(89)90296-3. [DOI] [PubMed] [Google Scholar]
- Nielsen KM, Soghomonian JJ. Normalization of glutamate decarboxylase gene expression in the entopeduncular nucleus of rats with a unilateral 6-hydroxydopamine lesion correlates with increased GABAergic input following intermittent but not continuous levodopa. Neuroscience. 2004;123:31–42. doi: 10.1016/j.neuroscience.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Pan HS, Penney JB, Young AB. Gamma-aminobutyric acid and benzodiazepine receptor changes induced by unilateral 6-hydroxydopamine lesions of the medial forebrain bundle. J Neurochem. 1985;45:1396–1404. doi: 10.1111/j.1471-4159.1985.tb07205.x. [DOI] [PubMed] [Google Scholar]
- Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Rouillard C, Bedard P, Falardeau P, Di Paolo T. Repeated stimulation of D-1 dopamine receptors increases the circling response to bromocriptine in rats with a 6-OHDA lesion. Eur J Pharmacol. 1988;157:125–133. doi: 10.1016/0014-2999(88)90375-5. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, Vanderschuren LJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH. Synergistically interacting dopamine D1 and NMDA receptors mediate nonvesicular transporter-dependent GABA release from rat striatal medium spiny neurons. J Neurosci. 2000;20:3496–3503. doi: 10.1523/JNEUROSCI.20-09-03496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Gonzales C, Chesselet MF. Messenger RNAs encoding glutamate-decarboxylases are differentially affected by nigrostriatal lesions in subpopulations of striatal neurons. Brain Res. 1992;576:68–79. doi: 10.1016/0006-8993(92)90610-l. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Pedneault S, Audet G, Parent A. Increased glutamate decarboxylase mRNA levels in the striatum and pallidum of MPTP-treated primates. J Neurosci. 1994;14:6256–6265. doi: 10.1523/JNEUROSCI.14-10-06256.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Laprade N, Sandstrom M, Bruno JP. c-fos gene expression is induced in a subpopulation of striatal neurons following a single administration of a dopamine D1-receptor agonist in adult rats lesioned with 6-OHDA as neonates. Brain Res Mol Brain Res. 1998;57:155–160. doi: 10.1016/s0169-328x(98)00071-0. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Yan Z, Song WJ. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. Adv Pharmacol. 1998;42:1020–1023. doi: 10.1016/s1054-3589(08)60921-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Niijima K, Mizuno Y, Yoshida M. Changes in gamma-aminobutyrate, glutamate, aspartate, glycine, and taurine contents in the striatum after unilateral nigrostriatal lesions in rats. Exp Neurol. 1986;91:259–268. doi: 10.1016/0014-4886(86)90066-x. [DOI] [PubMed] [Google Scholar]
- Tossman U, Segovia J, Ungerstedt U. Extracellular levels of amino acids in striatum and globus pallidus of 6-hydroxydopamine-lesioned rats measured with microdialysis. Acta Physiol Scand. 1986;127:547–551. doi: 10.1111/j.1748-1716.1986.tb07939.x. [DOI] [PubMed] [Google Scholar]
- Valastro B, Andersson M, Lindgren HS, Cenci MA. Expression pattern of JunD after acute or chronic L-DOPA treatment: comparison with deltaFosB. Neuroscience. 2007;144:198–207. doi: 10.1016/j.neuroscience.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Wang H, Katz J, Dagostino P, Soghomonian JJ. Unilateral 6-hydroxydopamine lesion of dopamine neurons and subchronic L-DOPA administration in the adult rat alters the expression of the vesicular GABA transporter in different subsets of striatal neurons and in the substantia nigra, pars reticulata. Neuroscience. 2007;145:727–737. doi: 10.1016/j.neuroscience.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LT, Chesselet MF. Regional distribution and regulation of preprosomatostatin messenger RNA in the striatum, as revealed by in situ hybridization histochemistry. Mol Brain Res. 1989;5:121–130. doi: 10.1016/0169-328x(89)90003-x. [DOI] [PubMed] [Google Scholar]
- Wu H, Jin Y, Buddhala C, Osterhaus G, Cohen E, Jin H, Wei J, Davis K, Obata K, Wu JY. Role of glutamate decarboxylase (GAD) isoform, GAD65, in GABA synthesis and transport into synaptic vesicles-Evidence from GAD65-knockout mice studies. Brain Res. 2007;1154:80–83. doi: 10.1016/j.brainres.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Wu Y, Richard S, Parent A. The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neurosci Res. 2000;38:49–62. doi: 10.1016/s0168-0102(00)00140-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Pierce RC, Soghomonian JJ. Subchronic administration of L-DOPA to adult rats with a unilateral 6-hydroxydopamine lesion of dopamine neurons results in a sensitization of enhanced GABA release in the substantia nigra, pars reticulata. Brain Res. 2006;1123:196–200. doi: 10.1016/j.brainres.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Williams C, Sabol SL. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984;259:14301–14308. [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Nylander I, Goiny M, O’Connor WT, Ungerstedt U, Terenius L. The striatonigral dynorphin pathway of the rat studied with in vivo microdialysis--II. Effects of dopamine D1 and D2 receptor agonists. Neuroscience. 1994;63:427–434. doi: 10.1016/0306-4522(94)90540-1. [DOI] [PubMed] [Google Scholar]