Abstract
A simple model for the influence of host availability on vector bloodmeal choice is applied to estimate the relative availabilities of humans, cattle and other host populations to malaria vectors in African communities, using published human blood indices and ratios of cattle to humans. Cattle were bitten <0.01, 0.021 ± 0.11, 1.61 ± 0.16 and 1.61 ± 0.46 times as often as humans by Anopheles funestus, An. gambiae sensu stricto and An. arabiensis in Segera, Tanzania, and An. gambiae sensu lato in The Gambia, respectively. No significant feeding upon host species other than cattle or humans was detected. Even though An. gambiae s.l. in The Gambia were mostly An. gambiae s.s., they were 77 times more likely to choose cattle over humans than An. gambiae s.s. in Tanzania. The model accurately predicted cattle blood indices for the An. arabiensis population in Tanzania (predicted = 0.99 ± 0.21 × observed + 0.00 ± 0.10; r2 = 0.66). The potential effect of increased cattle abundance upon malaria transmission intensity was simulated using fitted relative availability parameters and assuming vector emergence rate, feeding cycle length and survivorship were unaffected. The model predicted that increased cattle populations would not affect malaria transmission in Tanzania but could drastically reduce transmission in The Gambia or where An. arabiensis is the dominant vector. We define the availability of a host as the rate at which a typical individual host-seeking vector encounters and feeds upon that host in a single feeding cycle. Mathematical models based on this definition also represent promising tools for quantifying the dependence of vector longevity, feeding cycle length and dispersal upon host availability.
Keywords: malaria, disease vectors, Anopheles gambiae, Anopheles arabiensis, Anopheles funestus, host feeding, bloodmeal choice, humans, cattle, mathematical modelling, Tanzania, The Gambia
Introduction
Vector species abundance, longevity, feeding cycle length and bloodmeal host choice are key predictors for the transmission intensity of insect-borne diseases such as malaria (Onori & Grab, 1980; Killeen et al., 2000b), dengue (Focks et al., 1995) and sleeping sickness (Rogers & Randolph, 1991). These parameters undoubtedly depend on the effort expended by the vector in the pursuit of bloodmeals and the relative availability of different species for bloodmeal acquisition. Here we focus on malaria because it is the world’s most important vector-borne disease and currently the target of a renewed global control campaign (Nabarro & Tayler, 1998). In particular, we focus on sub-Saharan Africa because this is where the bulk of the worldwide malaria burden occurs (Gallup & Sachs, 1999; Snow et al., 1999) and because factors which influence host choice by vectors in this region are poorly understood (Mutero et al., 1999).
The emergence rate, feeding cycle length, survival and bloodmeal host choice of malaria vectors are all influenced by the availability of exposed bloodmeal hosts to the vector (Garrett-Jones, 1964; Charlwood, 1986; Charlwood et al., 1986; Burkot et al., 1989; Service, 1991; Minakawa et al., 1999). Because the entomological inoculation rate (EIR) of malaria is proportional to the square of the proportion of bloodmeals taken from humans (Qh), it is very sensitive to changes in vector host choice (Koella, 1991; Killeen et al., 2000b). The practice of using domestic animals, which cannot act as reservoirs, as decoy hosts for pathogen-transmitting vector populations is referred to as zooprophylaxis (Service, 1991). Despite its long-standing anecdotal description and endorsement by the WHO, zooprophylaxis remains a poorly understood process, particularly for African malaria vector species (Mutero et al., 1999). Although relationships between human blood index and livestock abundance have been reported for Anopheles funestus and important vector species in the An. gambiae complex (White et al., 1972; Highton et al., 1979; Lindsay et al., 1993; Mbogo et al., 1993), these studies have been largely descriptive. Here we describe an availability-based model for mosquito host-seeking behaviour and apply it to the quantitative analysis of host choice by malaria vector populations in African communities.
Methods
Assumptions and definitions
The term host, as used in this paper, refers to that of the vector rather than the parasite and includes any animal from which a vector can take a bloodmeal. As previously, we assume that each malaria transmission focus is a discrete entity within which the vector, human and parasite populations interact with each other and we ignore exchanges with other nearby foci (Killeen et al., 2000b). For ease of reference and clarity, all symbols and definitions are listed in Table 1.
Table 1.
Symbols and definitions
| Symbol | Definition |
|---|---|
| A | Total availability of all hosts |
| Ac | Host species availability for cattle |
| Ah | Host species availability for humans |
| Ao | Host species availability for hosts other than cattle or humans |
| As | Host species availability for all hosts of a given species |
| as,j | Availability of any given host (j), of given species (s), defined as the mean rate at which a typical single host-seeking vector encounters and feeds upon that host in a single feeding cycle |
| āc | Mean availability of all cattle |
| āh | Mean availability of all humans |
| c | Cattle hosts |
| EIR | Entomological inoculation rate |
| EIR0 | Entomological inoculation rate where no cattle are available |
| EIRNc/Nh | Entomological inoculation rate at a given ratio of cattle to human population sizes |
| εs,j | Rate at which a single host-seeking mosquito encounters host j of species s, in a single feeding cycle |
| h | Human hosts |
| φs,j | Likelihood that, once a host-seeking vector encounters host j of species s, it will feed upon that host |
| η | Mean length of time spent by individual vectors seeking hosts and obtaining a bloodmeal in one feeding cycle |
| j | An integer representing one particular member of a host population of a given species |
| λ | Relative mean availability of the cattle population compared to that of humans |
| Nh | Number of humans within a discrete malaria transmission focus |
| Nc | Number of cattle within a discrete malaria transmission focus |
| Ns | Total population size of a given host species, s, within a discrete malaria transmission focus |
| o | Other hosts |
| Qc | Proportion of bloodmeals taken from cattle |
| Qh | Proportion of bloodmeals taken from humans |
| Qs | Proportion of bloodmeals taken from all hosts of a given species, s |
| ρ | Relative availability of all potential hosts other than cattle and humans, compared to that of the human population |
| s | An integer representing one species of potential bloodmeal hosts |
| S | Total number of potential bloodmeal host species present |
We define the availability of a given host to the vector population as the mean rate at which a typical single host-seeking vector encounters and feeds upon that host in a single feeding cycle. Thus the availability (a) of a given host (j) of a particular species (s) can be envisaged as the product of rate at which individual vectors encounter that host while host seeking (εs,j) and the likelihood that, once encountered, the vector will acquire a bloodmeal from that host (φs,j).
| [1] |
By this definition, the availability of a host is independent of the emergence rate and reflects both the access of individual hungry vectors to a host and their propensity to feed upon it. This definition encompasses the mean of all the variations in availability which occur over time-frames relevant to the transmission of vector-borne pathogens, typically years, months or days.
We also define the host species availability (As) as the sum of the availabilities of all hosts (j = 1, 2, . . . .Ns) of that species (s) as:
| [2] |
where Ns is the total number of hosts of species s within the transmission focus. Furthermore, the total host availability (A) is defined as the sum of the availabilities of all host species present which represent potential bloodmeal sources (s = 1, 2, ... .S):
| [3] |
Relating vector population feeding behaviours to host availability
Intuitively, these definitions lead directly to a relationship between total host availability and the mean length of time vectors spend seeking bloodmeals in a single feeding cycle. The mean host-seeking interval (η) is simply the inverse of the total availability of all hosts because this represents the rates at which individual vectors acquire bloodmeals from all potential bloodmeal sources:
| [4] |
Furthermore, these definitions lead directly to a relationship between the proportion of all bloodmeals taken by a vector population mat are acquired from any given host species as a function of availability. The proportions of bloodmeals acquired from any given host species (Qs) can be envisaged as the rate at which bloodmeals are acquired from that species relative to the rate at which they are acquired from all potential host species:
| [5] |
Bloodmeal host species choice as a function of relative population sizes
For the purposes of clarity to a non-specialist audience, here we convert numerical notation for host species, as used for rigorous mathematical notation, to more readily identified single letter initials for human (h), cattle (c) and other (o) potential bloodmeal hosts. Note that the use of the letters h and c is different to that of classical models, with which they should not be confused (Garrett-Jones & Shidrawi, 1969; Koella, 1991). Using this notation, equation [5] can be qualified to express the dependence of the human blood index (Qh) upon the availability of cattle (Ac), human (Ah) and other (Ao) hosts:
| [6] |
Where the sizes of the human (Nh) and cattle (Nc) populations are known, this can be further qualified in terms of the mean availability of hosts of these species (āh and āc, respectively):
| [7] |
which can be rearranged and expressed in terms of the relative availability of the cattle and other hosts populations compared to that of humans, using the following linear and non-linear equations:
| [8] |
| [9] |
where
| [10] |
and
| [11] |
We could identify only 2 publications for fitting the model, in which both the human blood index and the ratios or absolute sizes of human and cattle populations were available for large numbers (>10) of distinct communities in Africa. Host populations’ sizes and human blood indices in clusters of communities have been reported for An. funestus, An. gambiae sensu stricto and An. arabiensis in Segera, Tanzania (White et al., 1972) and for An. gambiae sensu lato in The Gambia (Lindsay et al., 1993). The dependence of the reported human blood indices for whole, individual communities upon the observed ratio of cattle to humans was quantified by fitting to the model described by equations [8]–[11] (Fig. 1, Table 2). The data for each vector population were fitted to both equations [8] and [9] by least squares linear regression and by least squares non-linear regression with sequential quadratic programming, constrained to ρ ⩾ 0, respectively (Ryan, 1997). Models presented in Table 2 and Figure 1 represent the best fit of the 2 methods. Logistic regression methods (Ryan, 1997) could not be applied because not all the human blood index data sets were available in explicit mosquito-by-mosquito binary format.
Fig. 1.
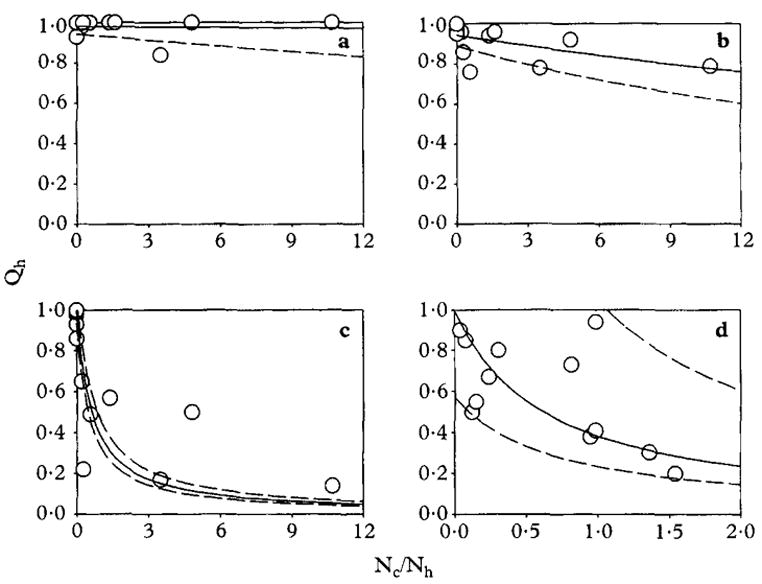
Regression analysis of the relationship between the human blood index (Qh) and the ratio of the number of cattle (Nc) and humans (Nh) in African villages. Each point represents a single village. The solid line represents the fitted models (Table 2), with dashed lines for their 95% confidence intervals, for (a) Anopheles funestus, (b) Anopheles gambiae s.s. and (c) Anopheles arabiensis at Segera, Tanzania (White et al., 1972) as well as (d) An. gambiae s.l. in The Gambia (Lindsay et al., 1993).
Table 2.
Results of regression analysis of human blood indices against the ratio of cattle to humans (see Figure 1)
| Parameters and variables | Segera, Tanzania(White et al., 1972) | The Gambia(Lindsays et al., 1993) | ||
|---|---|---|---|---|
| Vector species | An. funestus | An. gambiae s.s. | An. arabiensis | An. gambiae s.l. |
| Number of villages (n) | 12 | 13 | 12 | 12 |
| Degrees of freedom | 10 | 11 | 10 | 10 |
| Best-fit model equation | 9 | 8 | 8 | 9 |
| λa | 0.0011 ±0.0056 | 0.021 ±0.011 | 1.61 ±0.16 | 1.61 ±0.46 |
| ρb | 0.022 ±0.020 | 0.060 ±0.028 | 0.000 ±0.031 | 0.037 ±0.372 |
| r2 | 0.004 | 0.31 | 0.97 | 0.51 |
Testing the fitted model by predicting bovine blood indices
Similarly, to Qh (equation [7]), the cattle blood index (Qc) can be estimated as
| [12] |
So, by substituting equations [10] and [11] into equation [12] and rearranging, the cattle blood index can be estimated as:
| [13] |
In addition to Qh, Qc was also determined directly for the An. arabiensis populations in Tanzania and found to vary over quite a wide range (White et al., 1972). The cattle blood indices for An. arabiensis in 12 of these villages were predicted, using equation [13] and published values for Nc and Nh together with estimates of λ and ρ from fitting human blood indices. The predictive accuracy and precision as well as the mechanistic basis of the model were assessed by linear regression of predicted Qc values against those observed in the field.
Predicting possible impacts of cattle availability on malaria transmission intensity
The human blood index, Qh, is one of the key predictors of malaria EIR (Koella, 1991; Killeen et al., 2000b). Although it is clear that the sizes of the human and cattle populations are likely to influence other important predictors of EIR, little is known about these relationships for African malaria vectors (Mutero et al., 1999). We therefore apply the model to estimate the influence of the relative availabilities of humans, cattle and other hosts on EIR, assuming that emergence rate, feeding cycle length and survival per feeding cycle remain constant at any given site, irrespective of these demographic variations.
EIR is proportional to the square of Qh (Koella, 1991; Killeen et al., 2000b). Thus, assuming none of the other predictors change, the relative EIR transmitted by any vector species in the presence of cattle (EIRNc/Nh) compared to that in the absence of cattle (EIR0) can be calculated as a simple function of their respective human blood index:
| [14] |
Thus, the influence of cattle on EIR as a function of Qh, can be predicted using estimates of λ and ρ from Table 2 and assuming that other key predictors of EIR are constant. No major host species other than cattle or humans were identified for any of these vector populations by the preliminary model fitting or, in the case of the Tanzanian sites, by direct blood meal identification (White et al., 1972). We therefore assumed the availability of hosts other than cattle or humans to be negligible (ρ = 0) and predicted the response of EIR to increasing relative cattle abundance using only estimates of λ, and its 95% confidence intervals, as input parameters. For the 3 vector populations studied in Tanzania, this relationship was first studied individually for each species and then the impact of cattle abundance on overall EIR was estimated as an average, weighted according to the EIR transmitted by each species. The contribution of each vector species to overall EIR and its corresponding sensitivity was weighted according to reported sporozoite prevalence and relative abundance of these 3 vector species, for this site at the peak of the transmission season in April 1970 (White et al., 1972).
Results
Relative host species availabilities
Reported community-level human blood indices for An. funestus, An. gambiae s.s. and An. arabiensis in Segera, Tanzania (White et al., 1972) and for An. gambiae s. l. in The Gambia (Lindsay et al., 1993) were separately fitted to the model, equations [8] or [9], depending on which produced the best fit (Fig. 1, Table 2). The model failed to identify any dependence of the human blood index for An. funestus in Tanzania upon λ, the relative availability of cattle (Fig. 1 a, Table 2). The human blood index of An. gambiae s.s. in the same area was found to respond slightly to very high densities of cattle and this relationship approached significance (Fig. 1b. Table 2). In contrast, the more zoophilic An. arabiensis from the same part of Tanzania responds well to increasing cattle numbers and an excellent fit was obtained. No significant availability of hosts other than cattle and humans (ρ) was detected in any of these vector populations. Following this observation, log-transformed forms of equations [8] and [9], without the ρ parameter were explored but did not improve the fit of the model. The estimates for the relative availability parameters λ and ρ indicate, respectively, cattle will be bitten 1.61 ± 0.16 times as often as humans and that essentially no other animal species are fed upon by this vector population (Fig. 1c, Table 2). Although the model did not fit the data so well for An. gambiae s.l. in The Gambia, the relative availability parameters obtained for both cattle and other hosts, compared to humans, were essentially identical to those of An. arabiensis in Tanzania (Fig. 1d, Table 2). Thus, although the An. gambiae s.l. population in The Gambia is reported to be composed of 68% An. gambiae s.s. (Lindsay et al., 1993) it is, according to this analysis, 77 times more zoophilic than the An. gambiae s.s. population in Tanzania.
Model performance and validation
Although the best fits of the model to the data for An. arabiensis in Tanzania and An. gambiae s.l. in The Gambia had an approximately normal distribution of errors, this was not the case for both An. funestus and An. gambiae s.s. in Tanzania which had a clearly binomial distribution (Figs 1a and b). Furthermore, some heteroscedasticity (heterogeneity of variances) was apparent, with greater scatter of the observed human blood indices at moderate predicted values, between 0.8 and 0.2 (Figs 1c and d). Despite these shortcomings, the model was useful for quantifying the blood-feeding preferences of these vector populations and fitted remarkably well to the An. arabiensis data from Tanzania. Cattle blood indices for this An. arabiensis population were also reported and found to be distributed over a reasonably wide range (White et al., 1972). We therefore tested the validity of the model by predicting the cattle blood indices of each village, using the estimate for the relative availability of cattle (λ) and other hosts (ρ) obtained by fitting to the separately measured human blood index data. The model was found to be very accurate but not particularly precise (Fig. 2). Although considerable scatter was observed, the model predicted values for the cattle blood index which are, on average, almost exactly equal to those observed. This confirms that the effect of cattle on the human blood index is indeed caused by diversion of host-seeking mosquitoes to feeding on cattle and strongly supports the underlying principles of the model.
Fig. 2.
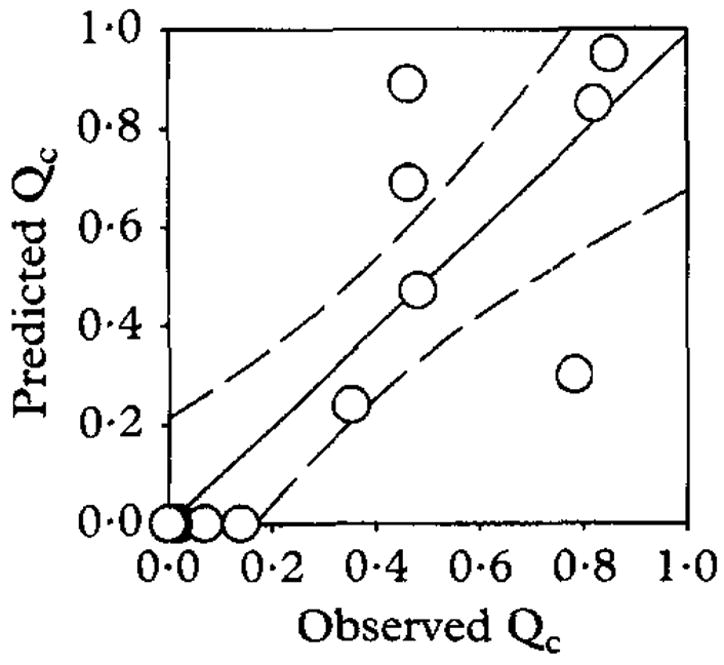
Cattle blood indices (Qc) for Anopheles arabiensis at Segera, Tanzania, predicted with equation [13], using model parameters fitted in Figure 1, compared to those observed in the field (White et al., 1972). Linear regression analysis: predicted Qc = 0.99 ± 0.21 × observed Qc + 0.00 ± 0.10, n = 12, d.f. = 10, r2 = 0.66.
Possible impacts of cattle abundance on malaria transmission intensity
Assuming that vector emergence rates, longevities and feeding cycle lengths were unchanged, the contribution of An. arabiensis to EIR in Tanzania was predicted to be very sensitive to the relative abundance of cattle (Fig. 3c). In contrast, those of sympatric An. funestus and An. gambiae s.s. were scarcely affected by cattle density as high as 10 per person (Figs 3a and b). We estimate, on the basis of reported species abundance and sporozoite prevalence (White et al., 1972), that these species were each responsible for approximately 0.3, 76.4 and 23.3% of overall EIR, respectively. The model therefore predicts that the overall malaria transmission intensity by the combined vector populations in Tanzania would be essentially unresponsive to the density of cattle in the area (Fig. 3d). Conversely, malaria transmission by the much more zoophilic An. gambiae s.l population in The Gambia was predicted to be extremely sensitive to the abundance of cattle (Fig. 3e). Assuming that they do not reduce the feeding cycle length of vector mosquitoes or increase their longevity or emergence rate, we estimate that a few extra cattle in every household of these villages could reduce their local community-level EIR 10-fold.
Fig. 3.
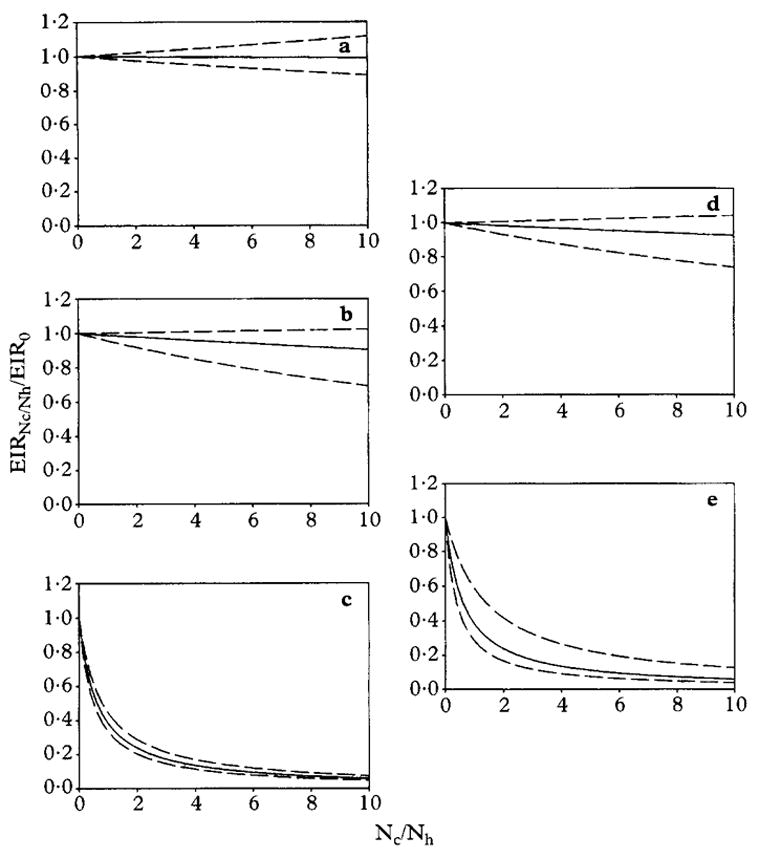
Predicted impact of increasing the ratio of cattle (Nc) to humans (Nh) on EIR transmitted by (a) Anopheles funestus, (b) Anopheles gambiae s.s., (c) Anopheles arabiensis and (d) the vector population as a whole at Segera, Tanzania (White et al., 1972) as well as (e) An. gambiae s.l. in The Gambia (Lindsay et al., 1993). Dashed lines represent the 95% confidence intervals.
Discussion
Overall, the model performed quite well with regards to fitting the available data for the relatively zoophilic An. arabiensis and An. gambiae s.l. vector populations from opposite sides of the African continent (Table 2, Fig. 1). Furthermore, the model managed to identify and quantify quite low levels of zoophily in An. gambiae s.s., and its failure to detect any significant non-human bloodmeal source for An. funestus is consistent with the extremely anthropophilic nature of this species and direct bloodmeal identifications of mosquitoes from the Tanzanian villages (White et al., 1972). It is also noteworthy that no quantitatively significant host species other than humans and cattle was identified for any of the 4 vector populations studied.
The striking similarity between the parameter estimates for the An. arabiensis in Tanzania and the An. gambiae s.l. population in The Gambia (Table 2) is somewhat surprising. This An. gambiae s.l. population was reported to consist of mostly An. gambiae s.s. (Lindsay et al., 1993). However, this estimate must be interpreted with some caution because the collection method used to determine species composition (CDC light traps in bedrooms) was quite different to those used to collect indoor-resting mosquitoes for bloodmeal identification (pyrethrum spray knockdown and bednet search). The much better fit of the model for data from Tanzania may be also explained by the rigorous identification of mosquitoes to species and the much greater range of relative cattle abundance in the Tanzanian villages.
Nevertheless, both the original data and this quantitative analysis indicate that An. gambiae s.s. is more zoophilic in West than East Africa and is consistent with the observation of human blood indices as low as 60% in Senegal (Lemasson et al., 1997) and 55% in The Gambia (Bøgh et al., 1999). These apparently large contrasts in host choice by An. gambiae in East and West Africa may result from between-site differences in innate behavioural traits of the vectors (Touré et al., 1994; Lanzaro et al., 1998; COETZEE et al., 2000), spatial relationships of hosts, vectors and larval habitats (Kitron & Spielman, 1989; Kitron, 1998), personal protection (Lindsay et al., 1989, 1993) or housing design (Gamage-Mendis et al., 1991; Ghebreyesus et al., 2000). Of these factors, the unusually longstanding and widespread use of bednets in The Gambia (Greenwood, 1993) deserves particular attention because such domestic personal protection measures can discourage feeding on humans and reduce human blood indices (Garrett-Jones, 1964; Charlwood & Graves, 1987; Lindsay et al., 1989; Githeko et al., 1996a; Bøgh et al., 1998).
Here we have modelled the impacts of zooprophylaxis, based solely upon reduction of the human blood index and hence the lifetime transmission potential of individual vectors (Killeen et al., 2000b). Taken at face value, these results (Fig. 3) would imply that zooprophylaxis could be an effective tool for malaria transmission control in The Gambia but not in Segera, Tanzania. This analysis also suggests that zooprophylaxis may be very useful in areas, such as East African rice irrigation schemes (Ijumba et al., 1990; Mukiama & Mwangi, 1990; Githeko et al., 1996b), where An. ambiensis predominates malaria transmission.
However, other important determinants of EIR such as emergence rate, feeding cycle length and survival per feeding cycle also depend on the availability of hosts (Charlwood, 1986; Charlwood et al., 1986; Charlwood & Graves, 1987; Burkot et al., 1989; Graves et al., 1990; Service, 1991). Some studies have indicated that increasing livestock numbers from low to moderate densities may increase malaria transmission by increasing the emergence rate of mosquitoes (Focks et al., 1988; Sota & Mogi, 1989). Furthermore, the proximity of livestock to humans can increase the rate at which zoophilic vectors encounter and feed upon humans (Schultz, 1989; Hewitt et al., 1994). Such effects may explain the direct correlation observed between livestock ownership and malaria burden in some communities (Adiamah et al., 1993; Bouma & Rowland, 1995; Mbogo et al., 1999; Ghebreyesus et al., 2000). The impacts of host availability on these other important predictors of EIR, and on its distribution, deserve more detailed study so that, among other things, the impact of livestock management on malaria transmission can be adequately understood and managed (Service, 1991; Mutero et al., 1999).
Interestingly, recent studies in The Gambia did not detect significant protection of individuals within villages by nearby cattle (Bøgh et al.,1999). This, however, does not exclude the possibility of protection of whole communities, as implied by our results, and the feeding preferences of house-resting An. arabiensis in The Gambia were found to depend on the proximity of cattle (Bøgh et al., 1999). We emphasize that although measures which modify personal availability, such as zooprophylaxis or bednets, may confer individual protection or even risk, they also influence the overall level of transmission at the community level (Hewitt et al., 1994; Bouma & Rowland, 1995; Killeen et al., 2000a) and that impacts at these 2 levels need to be resolved and quantified separately.
The observed non-normal distribution of errors and heteroscedasticity of some of the model fits can be explained in statistical terms by the fact that the data are constrained to values of between 0 and 1. This inevitably leads to asymmetric distribution and relatively limited range of the errors when the data approach either extreme (Ryan, 1997). Where data sets similar to those analysed here are available in the more desirable explicit binomial format, such models may be more appropriately fitted using logistic regression methods. Alternatively, non-normal error functions for continuous variables may also provide a relatively straightforward approach to fitting such models as these statistical constructs become accessible to non-specialist users through commercial software packages (Wilson & Grenfell, 1997). Such approaches should prevent anomalies associated with the current analysis including heteroscedasticity, skewed error distribution and estimated confidence intervals for proportions which exceed 1 or are less than 0 (Figs 1, 2 and 3).
Many of these anomalies can also be rationalized in terms of biological common sense by considering the roles of factors other than simple abundance upon relative host availability and the fact that each data point is a discrete village. Apart from the availability of hosts other than cattle and humans, villages may differ in terms of spatial relationships between hosts and larval habitats as well as each other. Similarly, shelter use, daily routine and personal protection may vary considerably from one community to another. Clearly such factors will be most influential where the vectors have reasonable opportunity to choose between at least 2 available host species. Thus wider variations of host choice can be expected in villages where both cattle and humans contribute substantially to the availability of bloodmeals for the vector.
The roles of such important individual- and community-level determinants of availability may be resolved using this model as a framework. Equation [1] resolves the availabilities of individual hosts into 2 major components relating to very different phases of mosquito blood-feeding behaviour: (a) foraging for hosts and (b) subsequent active choice of one of those hosts. The former, reflected by the encounter rate (εs,j), can be readily envisaged as a function of the spatial relationships between hosts and the larval habitats of vectors to which they are exposed. The latter, reflected by the bloodmeal acquisition likelihood (φs,j), can be envisaged as a function of the innate preference of the vector for that host species, variations in the attractiveness of individual hosts within a species and modifying factors in the host’s immediate surroundings such as other hosts, shelter and personal protection.
Further avenues for the extension of host availability models are also suggested by equation [4], which outlines a simple model describing the known restriction of host dispersal (Gillies, 1961; Trape et al., 1992; Manga et al., 1993; Thompson et al., 1997), feeding cycle length (Charlwood et al., 1986; Charlwood & Graves, 1987) and mortality rate (Charlwood, 1986) by host availability. This relationship is consistent with the observed dependence of adult dispersal upon the relative proximity of hosts and larval habitats (Edman et al., 1998) and the choice of oviposition sites in proximity to preferred hosts (Charlwood & Edoh, 1996; Minakawa et al., 1999). These relationships follow directly from the assumptions and definitions outlined here and constitute a promising framework for comprehensive quantification of the dependence of malaria transmission upon host availability.
Acknowledgments
We thank Adedapo Odulaja, Charles Mbogo and Alan Saul for excellent discussions and insightful comments on the manuscript. We are also thank 2 anonymous reviewers whose critiques were very constructive and helped improve the manuscript substantially. This work was supported by NIH-NIAID grants U19-AI-45511 (G.F.K., J.C.B.) and F32-1017 (F.E.M.) and Louisiana Educational Quality Scholarship Fund grant 1996-01-GF-23 (B.D.F.).
References
- Adiamah JH, Koram KA, Thomson MC, Lindsay SW, Todd SJ, Greenwood BM. Entomological risk factors for severe malaria in a peri-urban area of The Gambia. Annals of Tropical Medicine and Parasitology. 1993;87:491–500. doi: 10.1080/00034983.1993.11812801. [DOI] [PubMed] [Google Scholar]
- Bøgh C, Pedersen EM, Mukoko DA, Ouma JH. Permethrin-impregnated bed net effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Medical and Veterinary Entomology. 1998;12:52–59. doi: 10.1046/j.1365-2915.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- Bøgh C, Clarke SE, Lindsay SW. Variation in malaria transmission in rural Gambia. Bøgh, C. PhD thesis, University of Copenhagen; Copenhagen, Denmark: 1999. The effect of passive zooprophylaxis on malaria transmission in the Gambia, West Africa; pp. 56–71. [Google Scholar]
- Bouma M, Rowland M. Failure of passive zooprophylaxis: cattle ownership in Pakistan is associated with a higher malaria prevalence. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1995;89:351–353. doi: 10.1016/0035-9203(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Burkot TR, Dye C, Graves PM. An analysis of some factors determining the sporozoite rates, human blood indexes, and biting rates of members of the Anopheles punctulatus complex in Papua New Guinea. American Journal of Tropical Medicine and Hygiene. 1989;40:229–234. doi: 10.4269/ajtmh.1989.40.229. [DOI] [PubMed] [Google Scholar]
- Charlwood JD. Survival rate variation of Anopheles farauti (Diptera: Culicidae) between neighboring villages in coastal Papua New Guinea. Journal of Medical Entomology. 1986;23:361–365. doi: 10.1093/jmedent/23.4.361. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Edoh D. Polymerase chain reaction used to describe larval habitat use by Anopheles gambiae complex (Diptera: Culicidae) in the environs of Ifakara, Tanzania. Journal of Medical Entomology. 1996;33:202–204. doi: 10.1093/jmedent/33.2.202. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Graves PM. The effect of permethrin-impregnated bednets on a population of Anopheles farauti in coastal Papua New Guinea. Medical and Veterinary Entomology. 1987;1:319–327. doi: 10.1111/j.1365-2915.1987.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Graves PM, Birley MH. Capture-recapture studies with mosquitoes of the group Anopheles punctulatus Donitz (Diptera: Culicidae) from Papua New Guinea. Bulletin of Entomological Research. 1986;76:211–227. [Google Scholar]
- Coetzee M, Craig M, le Sueur D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitology Today. 2000;16:74–77. doi: 10.1016/s0169-4758(99)01563-x. [DOI] [PubMed] [Google Scholar]
- Edman JD, Scott TW, Costero A, Morrison AC, Harrington LC, Clark GG. Aedes aegypti (Diptera: Culicidae) movement influenced by availability of oviposition sites. Journal of Medical Entomology. 1998;35:578–583. doi: 10.1093/jmedent/35.4.578. [DOI] [PubMed] [Google Scholar]
- Focks D, McLaughlin RE, Smith BM. A dynamic life table model of Psorophora colombiae in the southern Louisiana rice agroecosystem with supporting hydrological submodel. Part 2. Model validation and population dynamics. Journal of the American Mosquito Control Association. 1988;4:282–299. [PubMed] [Google Scholar]
- Focks DA, Haile DG, Daniels E, Mount GA. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation and samples of simulation results. American Journal of Tropical Medicine and Hygiene. 1995;53:489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- Gallup JL, Sachs JD. Malaria, climate and poverty. Cambridge: Harvard Institute for International Development; 1999. Consulting Assistance on Economic Reform II, Discussion paper no. 48. [Google Scholar]
- Gamage-Mendis AC, Carter R, Mendis C, De Zoysa AP, Herath PR, Mendis KN. Clustering of malaria infections within an endemic population: risk of malaria associated with the type of housing construction. American Journal of Tropical Medicine and Hygiene. 1991;45:77–85. doi: 10.4269/ajtmh.1991.45.77. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C. The human blood index of malarial vectors in relationship to epidemiological assessment. Bulletin of the World Health Organization. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- Garrett-Jones C, Shidrawi GR. Malaria vectorial capacity of a population of Anopheles gambiae. Bulletin of the World Health Organization. 1969;40:531–545. [PMC free article] [PubMed] [Google Scholar]
- Ghebreyesus TA, Haile M, Witten KH, Getachew A, Yohannes M, Lindsay SW, Byass P. Household risk factors for malaria among children in the Ethiopian highlands. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94:17–21. doi: 10.1016/s0035-9203(00)90424-3. [DOI] [PubMed] [Google Scholar]
- Gillies MT. Studies on the dispersion and survival of Anopheles gambiae in East Africa, by means of marking and release experiments. Bulletin of Entomological Research. 1961;52:99–127. [Google Scholar]
- Githeko AK, Adungo NI, Karianja DM, Hawley WA, Vulule JM, Seroney IK, Ofulla AVO, Atieli FK, Ondijo SO, Genga IO, Odada PK, Situbi PA, Oloo JA. Some observations on the biting behaviour of Anopheles gambiae s.s., Anopheles arabiensis and Anopheles funestus and implications for malaria control. Experimental Parasitology. 1996a;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- Githeko AK, Service MW, Mbogo CM, Atieli FK. Resting behaviour, ecology and genetics of malaria vectors in large-scale agricultural areas of Western Kenya. Parassitologia. 1996b;38:481–489. [PubMed] [Google Scholar]
- Graves PM, Burkot TR, Saul AJ, Hayes RJ, Carter R. Estimation of anopheline survival rate, vectorial capacity and mosquito infection probability from malaria vector infection rates in villages near Madang, Papua New Guinea. Journal of Applied Ecology. 1990;27:134–146. [Google Scholar]
- Greenwood BM. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. 1. A review of the epidemiology and control of malaria in The Gambia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87(supplement 2):3–11. doi: 10.1016/0035-9203(93)90169-q. [DOI] [PubMed] [Google Scholar]
- Hewitt S, Kamal M, Muhammad N, Rowland M. An entomological investigation of the likely impact of cattle ownership on malaria in an Afghan refugee camp in the North West Frontier Province of Pakistan. Medical and Veterinary Entomology. 1994;8:160–164. doi: 10.1111/j.1365-2915.1994.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Highton RB, Bryan JH, Boreham PFL, Chandler JA. Studies on the sibling species Anopheles gambiae Giles and Anopheles arabiensis Patton (Diptera: Culicidae) in the Kisumu area, Kenya. Bulletin of Entomological Research. 1979;69:43–53. [Google Scholar]
- Ijumba JN, Mwangi RW, Beier JC. Malaria transmission potential of Anopheles mosquitoes in the Mwea-Tebere irrigation scheme, Kenya. Medical and Veterinary Entomology. 1990;4:425–432. doi: 10.1111/j.1365-2915.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. The potential impacts of integrated malaria transmission control on entomological inoculation rate in highly endemic areas. American Journal of Tropical Medicine and Hygiene. 2000a;62:545–551. doi: 10.4269/ajtmh.2000.62.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. American Journal of Tropical Medicine and Hygiene. 2000b;62:535–544. doi: 10.4269/ajtmh.2000.62.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitron U. Landscape ecology and epidemiology of vector-borne diseases: tools for spatial analysis. Journal of Medical Entomology. 1998;35:435–445. doi: 10.1093/jmedent/35.4.435. [DOI] [PubMed] [Google Scholar]
- Kitron U, Spielman A. Suppression of transmission of malaria through source reduction: antianopheline measures applied in Israel, the United States, and Italy. Reviews of Infectious Diseases. 1989;11:391–406. doi: 10.1093/clinids/11.3.391. [DOI] [PubMed] [Google Scholar]
- Koella JC. On the use of mathematical models of malaria transmission. Acta Tropica. 1991;49:1–25. doi: 10.1016/0001-706x(91)90026-g. [DOI] [PubMed] [Google Scholar]
- Lanzaro GC, Touré YT, Carnahan J, Zheng L, Dolo G, Traore S, Petrarca V, Vernick KD, Taylor CE. Complexities in the genetic structure of Anopheles gambiae populations in West Africa as revealed by microsatellite DNA analysis. Proceedings of the National Academy of Sciences of the USA. 1998;95:14260–14265. doi: 10.1073/pnas.95.24.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasson JJ, Fontenille D, Lochouarn L, Dia I, Simard F, Ba K, Diop A, Diatta M, Molez JF. Comparison of behavior and vector efficiency of Anopheles gambiae and An. arabiensis (Diptera: Culicidae) in Barkedji, a Sahelian area of Senegal. Journal of Medical Entomology. 1997;34:396–403. doi: 10.1093/jmedent/34.4.396. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Snow RW, Broomfield GL, Semega Janneh M, Wirtz RA, Greenwood BM. Impact of permethrin-treated bednets on malaria transmission by the Anopheles gambiae complex in The Gambia. Medical and Veterinary Entomology. 1989;3:263–271. doi: 10.1111/j.1365-2915.1989.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Alonso PL, Armstrong Schellenberg JRM, Hemingway J, Adiamah JH, Shenton FC, Jawa M, Greenwood BM. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. 7. Impact of permethrin-impregnated bed nets on malaria vectors. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87(Supplement 2):45–51. doi: 10.1016/0035-9203(93)90175-p. [DOI] [PubMed] [Google Scholar]
- Manga L, Fondjo E, Carnevale P, Robert V. Importance of low dispersion of Anopheles gambiae (Diptera: Culicidae) on malaria transmission in hilly towns in South Cameroon. Journal of Medical Entomology. 1993;30:936–938. doi: 10.1093/jmedent/30.5.936. [DOI] [PubMed] [Google Scholar]
- Mbogo CNM, Kabiru EW, Muiruri SK, Nzovu JMN, Ouma JH, Githure JI, Beier JC. Bloodfeeding behavior of Anopheles gambiae s. l. and Anopheles funestus in Kilifi District, Kenya. Journal of the American Mosquito Control Association. 1993;9:225–227. [PubMed] [Google Scholar]
- Mbogo CN, Kabiru E, Glass GE, Forster D, Snow RW, Khamala CPM, Ouma JH, Githure JI, Marsh K, Beier JC. Vector-related case-control study of severe malaria in Kilifi district, Kenya. American Journal of Tropical Medicine and Hygiene. 1999;60:781–785. doi: 10.4269/ajtmh.1999.60.781. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Mutero CM, Githure JI, Beier JC, Yan G. Spatial distribution and habitat characterization of anopheline mosquito larvae in western Kenya. American Journal of Tropical Medicine and Hygiene. 1999;61:1010–1016. doi: 10.4269/ajtmh.1999.61.1010. [DOI] [PubMed] [Google Scholar]
- Mukiama TK, Mwangi RW. Population and cytogenetic observations on Anopheles arabiensis Patton of Mwea Irrigation Scheme, Kenya. Insect Science and its Application. 1990;11:119–131. [Google Scholar]
- Mutero C, Mosha F, Odulaja A, Knols B, Bos R. Livestock management and malaria prevention in irrigation schemes. Parasitology Today. 1999;15:394–395. doi: 10.1016/s0169-4758(99)01522-7. [DOI] [PubMed] [Google Scholar]
- Nabarro DN, Tayler EM. The ‘roll back malaria’ campaign. Science. 1998;280:2067–2068. doi: 10.1126/science.280.5372.2067. [DOI] [PubMed] [Google Scholar]
- Onori E, Grab B. Indicators for the forecasting of malaria epidemics. Bulletin of the World Health Organization. 1980;58:91–98. [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. Mortality rates and population density of tsetse flies correlated with satellite imagery. Nature. 1991;351:739–741. doi: 10.1038/351739a0. [DOI] [PubMed] [Google Scholar]
- Ryan TP. Modern Regression Methods. New York: John Wiley & Sons; 1997. [Google Scholar]
- Schultz GW. Animal influence on man-biting rates at a malarious site in Palawan, Philippines. Southeast Asian Journal of Tropical Medicine and Public Health. 1989;20:49–53. [PubMed] [Google Scholar]
- Service MW. Agricultural development and arthropod-borne diseases: a review. Revista de Saude Publica (Sao Paulo) 1991;25:165–178. doi: 10.1590/s0034-89101991000300002. [DOI] [PubMed] [Google Scholar]
- Snow RW, Craig M, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant population. Buttetin of the World Health Organization. 1999;77:624–640. [PMC free article] [PubMed] [Google Scholar]
- Sota T, Mogi M. Effectiveness of zooprophylaxis in malaria control: a theoretical inquiry with a model for mosquito populations with two bloodmeal hosts. Medical and Veterinary Entomology. 1989;3:337–345. doi: 10.1111/j.1365-2915.1989.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Thompson R, Begtrup K, Cuamba N, Dgedge M, Mendis C, Gamage-Mendis A, Enosse SM, Barreto J, Sinden RE, Hogh B. The Matola malaria project: a temporal and spatial study of malaria transmission and disease in a suburban area of Maputo, Mozambique. American Journal of Tropical Medicine and Hygiene. 1997;57:550–559. doi: 10.4269/ajtmh.1997.57.550. [DOI] [PubMed] [Google Scholar]
- Touré YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M. Ecological genetic studies in the chromosomal form Mopti of Anopheles gambiae s. str. in Mali, West Africa. Genetica. 1994;94:213–223. doi: 10.1007/BF01443435. [DOI] [PubMed] [Google Scholar]
- Trape JF, Lefebvre-Zante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, Salem G. Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. American Journal of Tropical Medicine and Hygiene. 1992;47:181–189. doi: 10.4269/ajtmh.1992.47.181. [DOI] [PubMed] [Google Scholar]
- White GB, Magayuka SA, Boreham PFL. Comparative studies on sibling species of the Anopheles gambiae Giles complex (Dipt., Culicidae): bionomics and vectorial activity of species A and species B at Segera, Tanzania. Bulletin of Entomological Research. 1972;62:295–317. [Google Scholar]
- Wilson K, Grenfell BT. Generalized linear modeling for parasitologists. Parasitology Today. 1997;13:33–38. doi: 10.1016/s0169-4758(96)40009-6. [DOI] [PubMed] [Google Scholar]


