Summary points
Adjuvant systemic therapy has substantially reduced breast cancer mortality
For oestrogen receptor positive cancers, aromatase inhibitors are more effective than tamoxifen in postmenopausal women
Chemotherapy substantially improves the survival of selected patients
Commercially available molecular tests may further refine selection of patients for chemotherapy, and validation studies are under way
Breast cancer comprises a spectrum of related but different cancer subtypes, which have different causal genetic changes, may follow different clinical courses, and require different treatments tailored to the phenotype (fig 1). Here, in this second part of our review on breast cancer, we discuss the recent advances in the systemic therapy of breast cancer, and the current drive to individualise treatment according to patient and tumour factors.
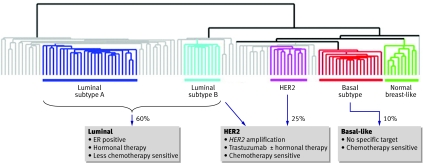
Fig 1 Diagram showing how breast cancer subtypes influence treatment. Breast tumours at the molecular level cluster into three main subtypes of cancer: luminal-type (with subtypes A and B), HER2, and basal-like. Luminal-type breast cancers express the oestrogen receptor (ER) and related genes and can be targeted with hormonal therapies; the less aggressive (subtype A) cancers may be less sensitive to chemotherapy than the more aggressive (type B) cancers. HER2 breast cancers, characterised by overexpression of the growth factor receptor HER2 with amplification of the HER2 gene, are aggressive with a poor prognosis. HER2 positive cancers can be targeted with the monoclonal antibody trastuzumab and also may be highly chemotherapy sensitive. About half of HER2 positive cancers express the oestrogen receptor. Basal-like cancers do not usually express either of the hormone receptors (oestrogen or progesterone) or overexpress the growth factor receptor HER2, a phenotype named “triple negative.” Basal-like cancers are highly proliferative and have a poor prognosis, although many are highly sensitive to chemotherapy. There is no subtype specific targeted therapy. (The clustering of genome-wide gene expression analysis is adapted from Sorlie et al1)
Although at diagnosis over 95% of women with breast cancer have no overt metastatic disease, half of these women would eventually die from breast cancer in the absence of systemic therapy. Adjuvant therapy is thought to eradicate micrometastates, preventing the emergence of clinical, evident disease that is incurable, and has been the most substantial advance in improving survival.
Adjuvant hormonal therapy
The first biological distinction directing therapy relates to the expression of the steroid hormone receptors (oestrogen receptor positive and/or progesterone receptor positive) on breast cancer cells. Overall, 70% of breast cancers are oestrogen receptor positive, with a frequency that rises with age, and for these cancers tamoxifen taken for five years reduces the risk of recurrence by 40% and breast cancer specific mortality by 31%.2 Hormonal therapies have no influence on the relapse rates of cancers that are oestrogen and progesterone receptor negative.
Improvements on tamoxifen
In postmenopausal women circulating androgens are converted to oestrogens by the aromatase enzyme. Aromatase inhibitors block this enzyme, thereby reducing circulating oestrogen to very low levels, but they cannot affect ovarian production of oestrogens and are therefore ineffective in premenopausal and perimenopausal women.
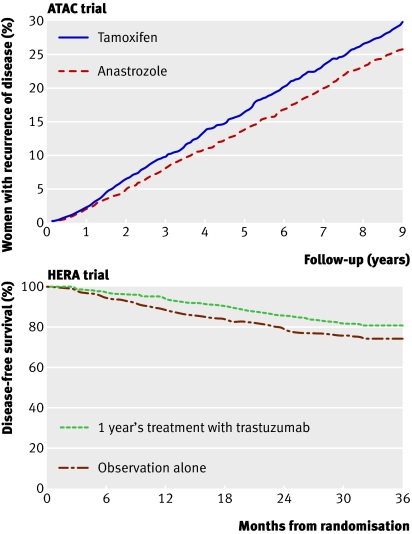
In postmenopausal women the use of adjuvant aromatase inhibitors compared with tamoxifen results in an incremental relative improvement in disease-free survival of 13-40%.3 Aromatase inhibitors have been found to be superior to tamoxifen irrespective of whether they are given straightaway (instead of tamoxifen for five years) (fig 2) or in a planned sequence (two to three years of tamoxifen followed by two to three years of aromatase inhibitors). Despite improvements in disease-free survival there has been no consistent effect on survival. This may reflect the low rates of recurrence in some studies and the late relapsing nature of breast cancers that are oestrogen receptor positive, which means that improvements in survival take many years to appear.
Fig 2 Top panel: The ATAC trial4 compared five years of adjuvant anastrozole with tamoxifen in postmenopausal women; the Kaplan-Meier curves show women with recurrence. After the five years’ treatment there was ongoing benefit of anastrozole (hazard ratio 0.85, P=0.003), with absolute reduction in recurrence of 4.1% after nine years’ follow-up. Bottom panel: The HERA trial5 compared one year of trastuzumab versus no treatment after adjuvant chemotherapy. Trastuzumab reduced the absolute risk of recurrence by 6.3% at three years. Adapted from Forbes et al4 and Smith et al5
Who should have an aromatase inhibitor?
All postmenopausal women should be considered for an aromatase inhibitor unless contraindicated. No consensus on the optimal schedule has yet been reached. The fact that some cancers relapse within the first two years suggests that aromatase inhibitors should be the preferred initial therapy. For cancers at lower risk of relapse (in which the absolute benefit of aromatase inhibitors compared with tamoxifen is small), many clinicians still prefer to use tamoxifen followed by aromatase inhibitors. The balance of different side effect profiles and comorbidities is also important in drug choice.
What are the side effects of hormone therapies?
Aromatase inhibitors and tamoxifen have a different spectrum of side effects (table).6 Tamoxifen causes more vasomotor symptoms, thromboembolism, gynaecological intervention, and strokes, whereas aromatase inhibitors are associated with more arthralgias, bone thinning, and fracture. For some patients the arthralgia associated with an aromatase inhibitor, which is caused by oestrogen suppression, may lead to discontinuation of therapy. Initial concerns that aromatase inhibitors might be associated with cardiovascular side effects have now been discounted.
Selected side effects from the ATAC randomised study of five years of adjuvant anastrozole versus tamoxifen. Incidence, odds ratio (anastrozole versus tamoxifen), and P value. Adapted from Howell et al6
| Side effect | Anastrozole (% of women) | Tamoxifen (% of women) | Odds ratio* | P value |
|---|---|---|---|---|
| Hot flushes | 35.7 | 40.9 | 0.80 | <0.0001 |
| Arthralgia | 35.6 | 29.4 | 1.32 | <0.0001 |
| Vaginal bleeding | 5.4 | 10.2 | 0.50 | <0.0001 |
| Vaginal discharge | 3.5 | 13.2 | 0.24 | <0.0001 |
| Endometrial cancer | 0.2 | 0.8 | 0.29 | 0.02 |
| Fractures | 11.0 | 7.7 | 1.49 | <0.0001 |
| Ischaemic cardiovascular disease | 4.1 | 3.4 | 1.23 | 0.1 |
| Ischaemic cerebrovascular events | 2.0 | 2.8 | 0.70 | 0.03 |
| Venous thromboembolic events | 2.8 | 4.5 | 0.61 | 0.0004 |
| Cataracts | 5.9 | 6.9 | 0.85 | 0.1 |
*Odds ratio of <1 favours anastrozole.
Patients taking aromatase inhibitors should have bone mineral density measured at baseline. If density is normal the risk of developing osteoporosis is minimal and requires no further monitoring.7 8 Patients with a low baseline require an interventional approach, with regular bone density assessment, calcium and vitamin D supplementation, and consideration of bisphosphonates to maintain bone density.9 Reassuringly, risk of fracture may return to baseline after stopping aromatase inhibitors.4
Can we individualise hormonal therapy?
Several patient factors are important in selecting the optimal hormone therapy strategy, although no tumour characteristics have yet been conclusively identified that alter the relative benefit of an aromatase inhibitor compared with tamoxifen.10
Premenopausal patients who develop amenorrhoea after chemotherapy often restart ovarian function once they start taking aromatase inhibitors.11 Unless highly sensitive oestrogen assays are available, it is advisable to start treatment with tamoxifen and switch to aromatase inhibitors once menopause is confirmed.
Pharmacogenomics may be important in deciding choice of drug. The liver P450 system metabolises tamoxifen to active metabolites, and polymorphisms in CYP2D6 that impair metabolism are associated with inferior outcomes with tamoxifen,12 with higher relapse rates.13 Conversely, a polymorphism in CYP2C19 that increases activity is associated with good outcome.13 Tests for these enzymes are not yet routinely available. Selective serotonin reuptake inhibitor antidepressants may also interfere with the metabolism of tamoxifen through inhibiting CYP2D614 and should be avoided in patients who are taking tamoxifen. Before this finding, selective serotonin reuptake inhibitors were used to manage hot flushes in patients taking tamoxifen, but this is no longer considered safe. Interestingly, hot flushes may be a surrogate for benefit for both tamoxifen and aromatase inhibitors, associated with a better outcome.15
Can we improve on tamoxifen in premenopausal patients?
For premenopausal women the suppression of ovarian hormone production with gonadotrophin releasing hormone agonists, or oophorectomy, presents a further hormonal therapy. However, whether gonadotrophin releasing hormone agonists add benefit to tamoxifen is still not clear,16 with results from further studies awaited. It is also not yet clear whether suppression of ovarian function with gonadotrophin releasing hormone agonists combined with an aromatase inhibitor is more effective than tamoxifen in premenopausal patients.
What is the optimal duration of therapy?
Women with oestrogen receptor positive cancer have as many relapses after five years’ follow-up as in the first five years,17 and extended adjuvant therapy trials have examined the duration of treatment. Two trials in postmenopausal women of an aromatase inhibitor given after five years of tamoxifen showed a 40% decrease in recurrence rates compared with placebo,18 19 with an improvement in survival in node positive patients. Recently the ATLAS study also found that 10 years of tamoxifen may be superior to five years, although further follow-up is required.20
Adjuvant chemotherapy
How beneficial is chemotherapy?
Since trials conducted in the 1980s, anthracycline (doxorubicin or epirubicin) based chemotherapy has been the standard adjuvant chemotherapy for breast cancer and reduces mortality by 38% for women younger than 50 and 20% for women aged 50-69.2 Several studies have shown that the addition of a taxane (docetaxel or paclitaxel) to anthracycline based chemotherapy reduces the relative risk of death further by about 15%.21 In oestrogen receptor positive breast cancer the benefits of chemotherapy accrue in addition to the benefits of hormonal therapy.2 Few women older than 69 have been included in chemotherapy studies, and the UK ACTION (adjuvant chemotherapy in older women)study is examining the role of chemotherapy specifically in this age group.
Can we improve selection of patients for chemotherapy?
Many women receive chemotherapy for only small absolute gains in survival (fig 3); most would be cured by local treatment and hormonal therapy alone and do not need chemotherapy. A small proportion are cured by the addition of chemotherapy, and some relapse despite chemotherapy (fig 3). The challenge is to identify those women who do benefit from chemotherapy, reduce the use of chemotherapy in those who do not derive benefit, and identify the optimal chemotherapy regimen for different cancers.
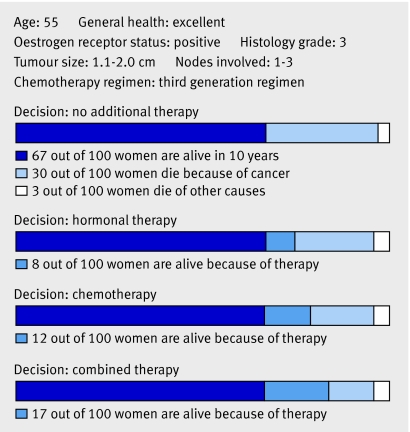
Fig 3 Prediction of adjuvant therapy benefit. The web based program Adjuvant! Online (www.adjuvantonline.com) estimates the benefit of adjuvant therapy. Graphically displayed are the benefits of adjuvant therapy for a woman with a 10 year breast cancer specific mortality of 30%, that can be reduced by 8% with hormonal therapy and a further 9% with chemotherapy. For every 100 similar women treated with chemotherapy, 91 might receive chemotherapy unnecessarily, although 9 will be cured. Adapted with permission from Adjuvant! Online version 8 (www.adjuvantonline.com).
Gene expression profiling, and an understanding of the molecular biology of breast cancer, has allowed the development of molecular tests to better select patients for chemotherapy. Prognostic tests use the pattern of gene expression to estimate the risk of a cancer relapsing and to identify patients who have a good prognosis and do not need chemotherapy. Predictive tests determine chemotherapy sensitivity of a tumour to distinguish the patients who would be cured by chemotherapy from those who would relapse despite chemotherapy. Ultimately a predictive test might be used to select the optimal chemotherapy regimen. The most useful test would be one that combines both prognostic and predictive power.
So far, most molecular tests developed have been prognostic tests, and finding true predictive tests of chemotherapy sensitivity has proved more difficult. Currently three molecular prognostic tests are commercially available:
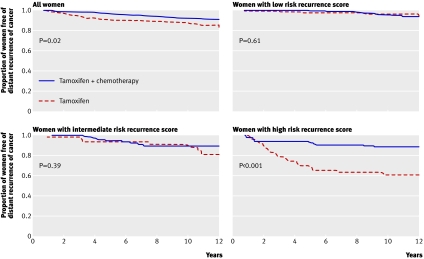
Oncotype DX (Genomic Health, USA)—The assay analyses the expression of a panel of 21 genes, with the results provided as a recurrence score. The test identifies women with oestrogen receptive positive and node negative cancers who do well on tamoxifen alone and will derive no extra benefit from chemotherapy22 23 (fig 4). This is the most extensively validated test.
MammaPrint (Agendia, Netherlands)—This test measures the expression of 70 genes in the cancer for estimating the prognosis of node negative cancers (whether oestrogen receptor positive or negative).24 A validation study (MINDACT) is currently recruiting.
H/I test (AviaraDx, USA)—This test is based on a two gene expression ratio (HOXB13-IL17BR) and is for patients taking tamoxifen.25
Fig 4 Kaplan-Meier plots of distant recurrence (a recurrence score for expression of a panel of 21 genes (Oncotype DX) predicts benefit from chemotherapy in oestrogen receptor (ER) positive, lymph node negative patients taking tamoxifen). These show modest benefit from chemotherapy in all women, substantial benefit in women with a high risk recurrence score, and probably no benefit in women with a low score. The potential benefit for women with an intermediate risk score is the subject of the ongoing TAILORx randomised trial. Adapted from Paik et al22
This first generation of molecular tests predominantly assay tumour proliferation and expression and activity of the oestrogen receptor. Their utility is limited because few patients with clinically high risk disease can be identified as having a sufficiently good prognosis to avoid chemotherapy.26 These molecular tests have not entered routine practice in the United Kingdom.
Can we select different chemotherapy regimens for different cancers?
Adjuvant chemotherapy trials have examined all breast cancer subtypes together (fig 1), although the optimal chemotherapy may vary between the different subtypes. In the not too distant future, molecular predictive tests may be used to individualise the chemotherapy regimen—for example, anthracylines may be particularly effective in the HER2 positive subtype of breast cancer.27 Anthracylines have rare but potentially serious long term side effects, including cardiac damage and secondary leukaemia, and several alternative, highly effective chemotherapy regimens are now available.
Adjuvant trastuzumab (Herceptin)
About a quarter of breast cancers have amplification of the HER2 gene (HER2 positive), and over-expression of HER2 has a dominant effect on the biology of these cancers. The antibody trastuzumab, which targets HER2 and is generally given for 12 months, reduces the risk of relapse by a further 35-52%5 28 compared with that achieved with chemotherapy and hormonal therapy (fig 2). This translates to an additional absolute survival benefit of 2.7% after two years,5 but follow-up long term will determine whether this level of benefit is maintained. A recognised side effect of trastuzumab is cardiac dysfunction, which is usually reversible when the drug is stopped.29
Neoadjuvant therapy
Neoadjuvant therapy—presurgical treatment with chemotherapy or hormones—can be used to “downstage” large tumours before operation. Trials in the adjuvant setting, after surgery, often require many thousands of patients, are costly, and require many years of follow-up. Increasingly the neoadjuvant setting is seen as a research opportunity to assess new treatments quickly and with fewer patients. Neoadjuvant studies rely on surrogate end points, although pathological complete response (the complete disappearance of a tumour)at surgery predicts accurately for long term survival.
Treatment of advanced and metastatic disease
Survival of patients with metastatic disease has been improving over the decades as treatments have improved. For many women, treatment now resembles that for a chronic disease, with sequential use of different chemotherapy and hormonal therapies to control disease and maintain quality of life. With a wide range of newer cytotoxic drugs available, clinical decisions depend on prior adjuvant therapy as well as on patient factors.
The greatest interest is in targeted therapies directed at the underlying biology of the cancer. For HER2 positive cancers, trastuzumab combined with chemotherapy remains the standard of care, or benchmark, although there are many new drugs that target HER2. One of these new drugs, lapatanib, an oral inhibitor of the HER2 protein, when given in combination with the chemotherapy capecitabine, improves progression-free survival by four months in women progressing after trastuzumab.30 Epidermal growth factor receptor (EGFR, HER1) may be a target in basal-like breast cancer (fig 1), although early clinical trials are not encouraging.
Evidence is growing that anti-angiogenic approaches targeting blood vessel formation are effective in breast cancer. Bevacizumab, an antibody to vascular endothelial growth factor, when combined with the chemotherapy drug paclitaxel, prolongs time to progression by six months31 in first line metastatic breast cancer. Other anti-angiogenic drugs are being tested in clinical trials.
Many other new drugs are in an early stage of clinical development, and access to these drugs has improved greatly in the UK through the development of experimental cancer centres. With targeted therapies, important questions remain over optimal duration and timing of treatment, with evidence of greatest benefit in the early stages of the disease.
Conclusion
Further advances in treatment of breast cancer will come from a greater understanding of the key molecular changes that drive tumour growth and from the development of treatment and preventive strategies that target these changes. However, an important barrier to the development of targeted therapy is the high cost of treatment, so parallel improvements in molecular pathology will be needed to better identify the population that will benefit from treatment.
Additional educational resources
Resources for healthcare professionals
See Journal of Oncology 2008;26:5 (http://jco.ascopubs.org/content/vol26/issue5/). A review issue devoted to in-depth discussions on the current issues of breast cancer
Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717
Resources for patients
Breakthrough (www.breakthrough.org.uk)—Charity tackling breast cancer through research, campaigning, and education
CancerHelp UK (www.cancerhelp.org.uk/)—The patient information website of Cancer Research UK. It provides a free information service about cancer and cancer care for people with cancer and their families
US National Cancer Institute (www.cancer.gov/)—Provides comprehensive information about cancer for patients
Ongoing research
Optimal sequence of hormonal therapies in postmenopausal women
Use of gonadotrophin releasing hormone agonists in premenopausal women, and whether these drugs allow the use of aromotase inhibitors in premenopausal women
Optimal chemotherapy regimen and whether this differs according to cancer subtype
Validation of molecular tests to enable selection of patients for chemotherapy
Multiple new approaches to tumour targeted therapy
Contributors: Both authors planned and wrote this review and act as guarantors.
Competing interests: ALJ has received consultancy and lecturing fees from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Roche, and Sanofi-Aventis.
Provenance and peer review: Commissioned; externally peer reviewed
Cite this as: BMJ 2008;337:a540
References
- 1.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 2003;100:8418-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Winer EP. Advances in adjuvant endocrine therapy for postmenopausal women. J Clin Oncol 2008;26:798-805. [DOI] [PubMed] [Google Scholar]
- 4.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 2008;9:45-53. [DOI] [PubMed] [Google Scholar]
- 5.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 2007;369:29-36. [DOI] [PubMed] [Google Scholar]
- 6.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365:60-2. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the intergroup exemestane study (IES): a randomised controlled study. Lancet Oncol 2007;8:119-27. [DOI] [PubMed] [Google Scholar]
- 8.Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 2008;26:1051-7. [DOI] [PubMed] [Google Scholar]
- 9.Bundred NJ, Campbell ID, Davidson N, Deboer RH, Eidtmann H, Monnier A, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST study results. Cancer 2008;112:1001-10. [DOI] [PubMed] [Google Scholar]
- 10.Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, et al. Relationship Between Quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, tamoxifen, alone or in combination trial. J Clin Oncol 2008;26(7):1059-65. [DOI] [PubMed] [Google Scholar]
- 11.Smith IE, Dowsett M, Yap YS, Walsh G, Lonning PE, Santen RJ, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol 2006;24:2444-7. [DOI] [PubMed] [Google Scholar]
- 12.Lim HS, Ju Lee H, Seok Lee K, Sook Lee E, Jang IJ, Ro J. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol 2007;25:3837-45. [DOI] [PubMed] [Google Scholar]
- 13.Schroth W, Antoniadou L, Fritz P, Schwab M, Muerdter T, Zanger UM, et al. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol 2007;25:5187-93. [DOI] [PubMed] [Google Scholar]
- 14.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003;95:1758-64. [DOI] [PubMed] [Google Scholar]
- 15.Cuzick J. Hot flushes and the risk of recurrence—retrospective, exploratory results from the ATAC trial. Breast Cancer Research and Treatment 2007;106(suppl 1):abstract 2069. [Google Scholar]
- 16.Adjuvant Breast Cancer Trials Collaborative Group. Ovarian ablation or suppression in premenopausal early breast cancer: results from the international adjuvant breast cancer ovarian ablation or suppression randomized trial. J Natl Cancer Inst 2007;99:516-25. [DOI] [PubMed] [Google Scholar]
- 17.Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2000;355:1757-70. [PubMed] [Google Scholar]
- 18.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262-71. [DOI] [PubMed] [Google Scholar]
- 19.Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group trial 6a. J Natl Cancer Inst 2007;99:1845-53. [DOI] [PubMed] [Google Scholar]
- 20.Peto R, Davies C, on Behalf of the ATLAS Collaboration. ATLAS (adjuvant tamoxifen, longer against shorter): international randomized trial of 10 versus 5 years of adjuvant tamoxifen among 11,500 women—preliminary results. Breast Cancer Res Treat 2007;106(suppl 1):abstract 48. [Google Scholar]
- 21.De Laurentiis M, Cancello G, D’Agostino D, Giuliano M, Giordano A, Montagna E, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 2008;26:44-53. [DOI] [PubMed] [Google Scholar]
- 22.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726-34. [DOI] [PubMed] [Google Scholar]
- 23.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817-26. [DOI] [PubMed] [Google Scholar]
- 24.Van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999-2009. [DOI] [PubMed] [Google Scholar]
- 25.Ma XJ, Wang Z, Ryan PD, Isakoff SJ, Barmettler A, Fuller A, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 2004;5:607-16. [DOI] [PubMed] [Google Scholar]
- 26.Fan C, Oh DS, Wessels L, Weigelt B, Nuyten DS, Nobel AB, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med 2006;355:560-9. [DOI] [PubMed] [Google Scholar]
- 27.Pritchard KI, Messersmith H, Elavathil L, Trudeau M, O’Malley F, Dhesy-Thind B. HER-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol 2008;26:736-44. [DOI] [PubMed] [Google Scholar]
- 28.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr., Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. [DOI] [PubMed] [Google Scholar]
- 29.McArthur HL, Chia S. Cardiotoxicity of trastuzumab in clinical practice. N Engl J Med 2007;357:94-5. [DOI] [PubMed] [Google Scholar]
- 30.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43. [DOI] [PubMed] [Google Scholar]
- 31.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76. [DOI] [PubMed] [Google Scholar]