Abstract
The basal ganglia are involved not only with motor processes such as posture, pre-movement planning and movement initiation, but also with the processing and modulation of nociceptive somatosensory information. In the current studies, unilateral, intrastriatal 6-hydroxydopamine (6-OHDA) was used to investigate how dopamine depletion alters nociceptive behavioral response to chemical, thermal and mechanical stimulation in rats. Compared to control rats injected with intrastriatal saline, rats depleted of dopamine displayed increased nociceptive responses to chemical stimulation of the face and hyperalgesic responses to thermal stimulation of the hind paw without alterations in rearing behavior or body weight gain. Minor changes were observed in the response to mechanical stimulation of the hind paws and face. These data provide further evidence that the dopaminergic nigrostriatal pathway plays a role in the modulation of nociceptive information.
Section: Sensory and Motor Systems
Keywords: dopamine, nociception, Parkinson’s disease, striatum, substantia nigra, Formalin
1. Introduction
Physiological, pharmacological, behavioral and clinical data provide evidence that the basal ganglia are involved not only with motor processes such as posture, pre-movement planning and movement initiation, but also with the processing of nociceptive and non-nociceptive somatosensory information (for review, see Chudler and Dong, 1995). For example, neurons in the caudate-putamen (CPu), globus pallidus (GP) and substantia nigra (SN) can respond to low-threshold, non-noxious somatosensory stimuli (Carelli and West, 1991; Chudler et al., 1995; Levine et al., 1987; Lidsky et al., 1979; Schneider and Lidsky, 1981; Schneider et al., 1982; Schneider et al., 1985) as well as to high-threshold, noxious, electrical, thermal and mechanical stimuli (Bernard et al., 1992; Chudler et al., 1993; Koyama et al., 2000; Lidsky et al., 1979; Lin et al., 1985; Richards and Taylor, 1982).
Recent evidence also suggests that dopaminergic systems within the basal ganglia play a role in pain modulation. For example, Parkinson's disease is characterized by severe degeneration of the zona compacta region of the substantia nigra and subsequent degeneration of the dopaminergic nigrostriatal pathway (for review, see Wichmann and DeLong, 1996). Although motor abnormalities are the most obvious symptoms of Parkinson’s disease, up to 63% of patients with Parkinson’s disease exhibit spontaneous tactile sensory sensations described as intermittent, poorly localized, cramp-like, aching and burning (Bulpitt et al., 1985; Goetz et al., 1986; Hillen and Sage, 1996; Koller, 1984; Koller, 1992; Olanow et al., 2001; Riley and Lang, 1993; Scherder et al., 2005; Schestatsky et al., 2007; Schott, 1985; Shulman et al., 2001; Snider et al., 1976; Stein and Read, 1997; Waseem and Gwinn-Hardy, 2001; Witjas et al., 2002). These painful sensory symptoms can often precede the diagnosis of Parkinson’s disease (Koller, 1984; Koller, 1992; Quinn et al., 1986; Schott, 1985; Snider et al., 1976; Waseem and Gwinn-Hardy, 2001) and may drive some patients with Parkinson’s disease to the emergency room (Factor and Molho, 2000). Patients with Parkinson’s disease (Djaldetti et al., 2004; Schestatsky et al., 2007) or lesions in the striatum (Peyron et al., 2004) may also have reduced pain thresholds or allodynia. Furthermore, the reduced pain thresholds exhibited by patients with Parkinson’s disease return to normal levels after the administration of levodopa (Gerdelat-Mas et al., 2007).
Electrical and chemical (i.e., dopamine agonists and antagonists, opioids) stimulation and electrolytic and chemical lesions of the CPu, GP and SN provide evidence that the basal ganglia can modify behavioral responses to noxious stimulation. For example, dopamine depletion by 6-hydroxydopamine injection into the medial forebrain bundle, CPu and SN results in hypersensitivity to mechanical (Saade et al., 1997; Takeda et al., 2005), electrical (Carey 1986) and thermal stimulation (Lin et al., 1985; Saade et al., 1997) and an earlier time of autotomy after a peripheral nerve injury (Saade et al., 1997). A recent study (Tassorelli et al., 2007) found hyperalgesic responses to painful chemical stimulation of the hind paw ipsilateral to intrastriatal 6-OHDA administration. These data are consistent with the observation that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice exhibit significant thermal hyperalgesia (Rosland et al., 1992). Magnusson and Fisher (Magnusson and Fisher, 2000) reported that microinjection of selective D2 receptor agonists into the dorsolateral striatum of rats can suppress pain behavior as measured with the Formalin test without disturbing motor behavior. Although D1 receptor agonists have no effect on pain behavior measured with the Formalin test (Magnusson and Fisher, 2000), these drugs can result in anti-hyperalgesic behavior in rats with paws injected with carrageenan (Gao et al., 2000). The involvement of striatal D2 receptors in pain is also suggested by the significant correlation between striatal D2 receptor binding and pain thresholds (Hagelberg et al., 2002; Hagelberg et al., 2004; Martikainen et al., 2005).
Few studies have examined how the basal ganglia to modulate nociceptive information originating from the trigeminal region. The majority of cutaneous nociceptive CPu and GP neurons have large trigeminal receptive fields, sometimes incorporating the ipsilateral side of the face or much of the entire body (Chudler et al., 1993; Chudler, 1998). Electrical stimulation of the CPu can inhibit nociceptive neurons in the spinal trigeminal nucleus (Belforte and Pazo, 2005) and reduce the jaw opening reflex in rats (Belforte et al., 2001). However, the effects of dopamine depletion on tonic persistent trigeminal pain have not been performed. The present study examines the effects of dopamine depletion by unilateral, striatal administration of 6-hydroxydopamine on the behavioral responses of rats to chemical and mechanical stimulation of face and hind paws and to thermal stimulation of the hind paws.
2. Results
All rats lost body weight during the first week after surgery. The maximum weight loss (mean ± se) was 24.8 g (± 3.7 g) and 21.9 g (± 3.1 g) for rats receiving saline injections and 6-OHDA injections, respectively. Saline- and 6-OHDA-injected rats gained body weight at similar rates and the total body weight gained by rats in these groups was not significantly different three weeks after surgery (67.6 g ± 4.2 g for saline-injected rats; 66.4 ± 8.5 g for 6-OHDA-injected rats; mean ± se; t-test, not significant). There were no gross abnormalities in locomotor ability or in the number of rears performed by the rats during the first five minutes of the Formalin test (saline-injected rats, 6.7 ± 2.2 rears; 6-OHDA-injected rats, 8.1 ± 1.0 rears; mean ± se; t-test, not significant). All rats groomed normally and maintained a healthy fur coat.
Injection of 6-OHDA into the CPu resulted in severe loss of TH reaction product in the ipsilateral substantia nigra (pars compacta) three weeks after injection. As shown in Fig. 1, TH reaction product was absent or reduced in the SNc ipsilateral (left side) to the injection and abundant in the SNc contralateral (right side) to the injection. No TH reaction product was observed in control sections processed without antibodies or with the primary antibody only.
Figure 1.
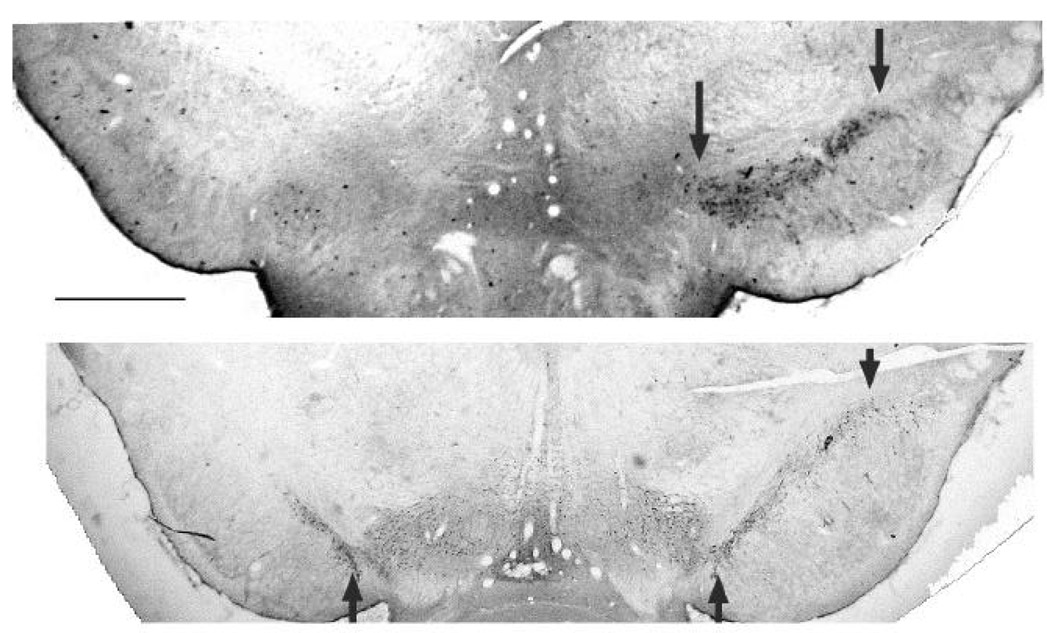
Loss of tyrosine hydroxylase (TH) immunostaining in the substantia nigra after unilateral, intrastriatal 6-OHDA administration. Top and bottom, respectively: Severe and partial loss of TH reaction product on the side ipsilateral (left side) to the 6-OHDA injection in two rats. Arrows indicate the substantia nigra, pars compacta. Scale bar = 1 mm.
Injection of 2.5% Formalin into the upper lip of rats treated with intrastriatal saline resulted in a biphasic increase in facial scratching (Fig. 2A). Immediately after Formalin injection, control rats displayed an increase in facial scratching that lasted approximately 9 min (Phase 1). This first phase was followed by a second increase starting 12 min after Formalin injection and lasting 24 min after injection (Phase 2). The peak increase in facial scratching in saline-injected rats occurred in the sixth time block (15–18 min after Formalin injection). Animals treated with intrastriatal 6-OHDA also showed two increases (Phase 1 and Phase 2) in facial scratching after Formalin injections. However, facial scratching remained elevated after 24 min. For data analysis purposes, this prolonged period of increased scratching between 27 and 36 min after Formalin injection was called Phase 3. In 6-OHDA-treated rats, the peak increase in facial scratching occurred in the fifth time block (12–15 min after Formalin injection). Little facial scratching was observed in any rats 45 to 60 min after Formalin injection. The times that 6-OHDA-injected and saline-injected rats spent scratching during Phase 1 were not significantly different from each other (Fig. 2B). However, 6-OHDA-injected rats spent significantly more time scratching during Phase 2 and 3 than did saline-injected rats (p < 0.05).
Figure 2.
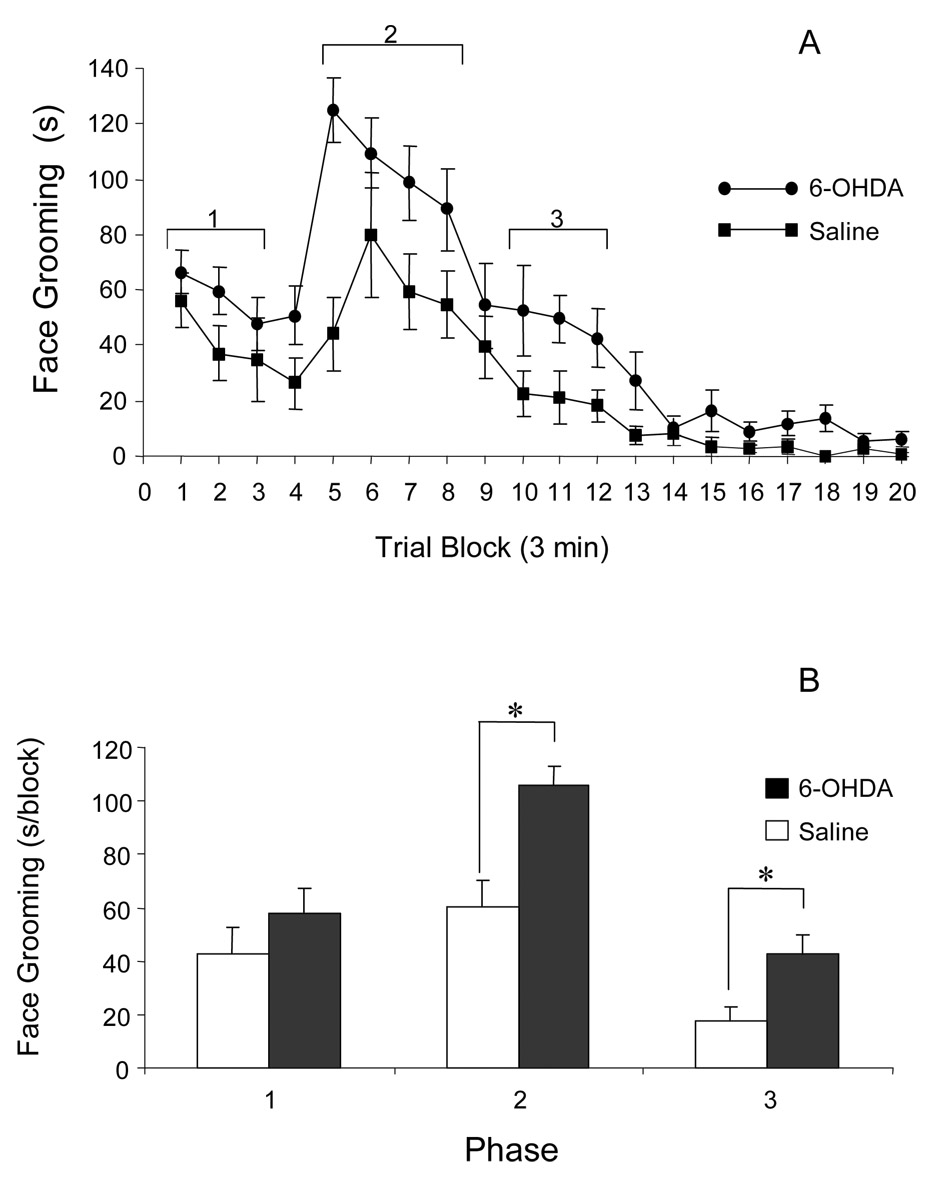
Effect of unilateral, intrastriatal 6-OHDA adminstration on Formalin-induced facial scratching. A: Time course of facial scratching and grooming represented in blocks of 3-minute intervals after the Formalin was injected into the upper lip. The duration of Phase 1, 2 and 3 are indicated brackets above different data points. B. Averaged facial scratching and grooming during Phase 1, 2 and 3 after Formalin was injected into the upper lip. Data are presented as the mean ± se; (* = p< 0.05, t-test.)
Thermal stimulation of the hind paw resulted in rapid (~11 s) limb withdrawal in all rats. There were no significant changes in hind paw withdrawal latency in rats one week after 6-OHDA or saline injection (Fig. 3). Two weeks after injections, 6-OHDA-injected and saline-injected rats demonstrated faster withdrawal times (less than 100% baseline latency), but these changes were not statistically different from values one week after injection and there were no differences in the values between the 6-OHDA-injected and saline-injected animals. Further reductions in withdrawal latencies were seen three weeks after injections and rats injected with 6-OHDA had significantly faster right hind paw (contralateral to injection) withdrawal latencies than rats injected with saline. Left hind paw (ipsilateral) withdrawal latencies were not significantly different between 6-OHDA-injected and saline-injected rats. Also, the withdrawal latency of the contralateral hind paw in 6-OHDA-injected rats at three weeks was significantly faster than the withdrawal latency of these same rats one week after surgery.
Figure 3.
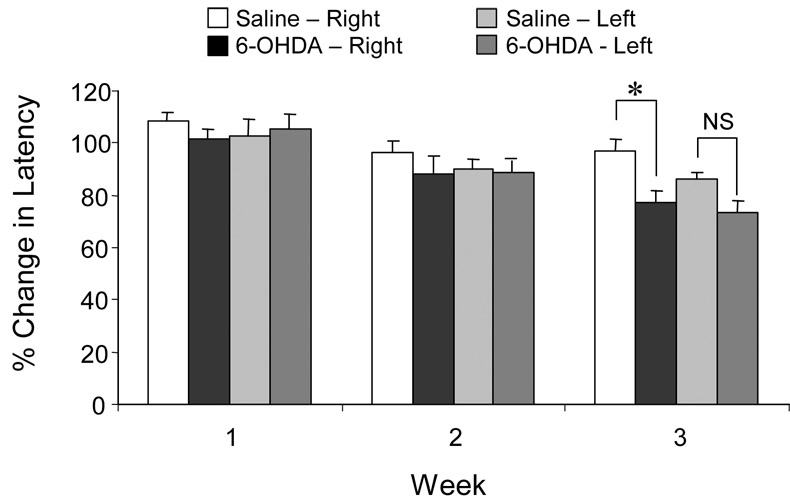
Effect of unilateral, intrastriatal 6-OHDA adminstration on hind paw withdrawal latency to thermal stimulation. Changes in thermal withdrawal latency of the right (contralateral to injection) and left (ipsilateral to injection) hind paws from baseline levels (100%) are presented as weekly means ± se for rats injected with 6-OHDA or saline. (* = p< 0.05, t-test.)
Although the response thresholds to mechanical stimulation of the face increased in 6-OHDA-injected rats, the change in threshold was not significantly different than that in saline-injected rats during the three-week observation period (Fig. 4A). Mechanical response thresholds to stimulation of the right and left hind paws were reduced in 6-OHDA-injected rats (Fig. 4B). The response thresholds for these rats were significantly reduced at three weeks compared to those at one and two weeks. However, there were no differences between the hind paw response thresholds of 6-OHDA-treated and saline-injected rats at any time.
Figure 4.
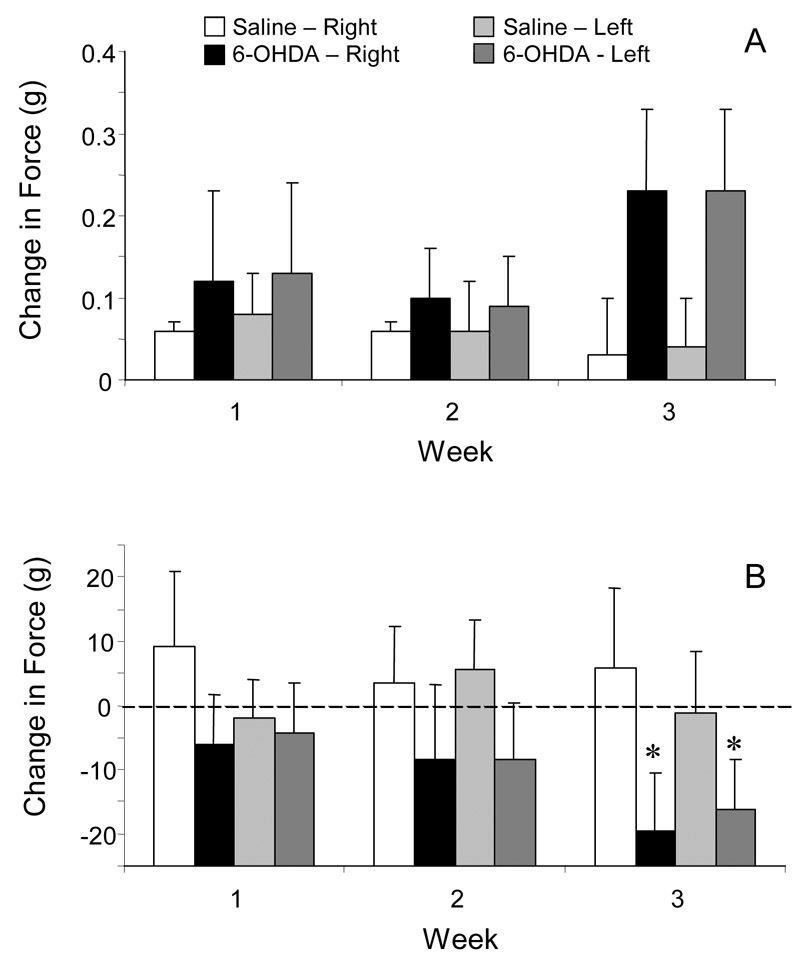
Effect of unilateral, intrastriatal 6-OHDA adminstration on the force necessary to evoke a withdrawal to mechanical stimulation of the face (A) and hind paw (B). Data are presented as weekly mean (± se) changes from baseline levels of gram force necessary to cause a withdrawal response. (* = p< 0.05, t-test, week three compared to week one.)
3. Discussion
In the current experiments, unilateral depletion of dopamine in the substantia nigra by intrastriatal administration of 6-OHDA altered the behavioral response to chemical, thermal and mechanical stimulation. It is unlikely that the changes observed after 6-OHDA injection were related to motor dysfunction because rats moved, explored and gained weight normally. Moreover, rats exhibited increased, not decreased, responsiveness to chemical stimulation of the face and faster responses to thermal stimulation of the hind paws. The ability of unilateral, intrastriatal 6-OHDA injections to alter movement and affect the nigrostriatal pathway is highly dependent on the dose and location of the neurotoxin injection. The magnitude of the movement deficit is most significant when 6-OHDA is injected into three or four locations (Kirik et al., 1998). However, single or two site injections of 6-OHDA have little and no effect on forelimb stepping and skilled paw use. As demonstrated in the current experiments, the behavioral responses to noxious sensory stimuli can be modified even with these smaller injections of neurotoxin.
Rats treated with 6-OHDA exhibited more nociceptive behavior (increased face scratching) when Formalin was injected in the face contralateral to the 6-OHDA injection when compared to those rats treated with saline. This difference was observed during the late phase, but not during the early phase, of the orofacial Formalin test. These results are in agreement with those of Magnusson and Fisher (Magnusson and Fisher, 2000) who showed that intrastriatal injection of eticlopride, a D2 antagonist, increased contralateral nociceptive responses and intrastriatal injection of quinpirole, a D2 agonist, reduced contralateral nociceptive responses in the late phase, but not early phase, of the hind paw Formalin test. In contrast to these results, Tassorelli et al. (Tassorelli et al., 2007) found that unilateral 6-OHDA administration caused a significant early phase and delayed late phase hyperalgesic response to Formalin injected into the ipsilateral hind paw. It is possible that the differences in these studies are related to differences in the site of chemical injections, methods used to measure nociceptive behavior, and in physiological and anatomical differences between the trigeminal and spinal sensory systems (for review, see Sessle 2005). The biphasic nature of the Formalin test is well documented (Cadet et al., 1998; Clavelou et al., 1989; Clavelou et al., 1995; Raboisson and Dallel, 2004) and the early response of the Formalin tests appears to be mediated by C-nerve fiber activation, while the late response is caused by local inflammatory and spinal cord changes (Tjolsen et al., 1992). Because 6-OHDA alters the late response but not the early response to chemical stimulation of the face, dopamine appears to play a role in modulating inflammatory pain, but not in C-nerve fiber mediated trigeminal pain.
The response to thermal stimulation was also modified by intrastriatal 6-OHDA administration. Paw withdrawal latencies to thermal stimulation were significantly faster in rats injected with 6-OHDA compared to paw withdrawal latencies of saline-injected rats on the side contralateral to dopamine depletion. The hyperalgesic response was not observed after thermal stimulation of the hind paw ipsilateral to dopamine administration. These data agree with those of Saade et al (Saade et al., 1997) who found that 6-OHDA or kainic acid administration into the striatum or substantia nigra reduces nociceptive response latencies of the contralateral hind paw on the hot plate and tail flick tests. Significant, inverse correlations between cold pain thresholds and D2 binding potential in the contralateral putamen (Hagelberg et al., 2002) and heat pain thresholds and D2/D3 binding potential in the ipsilateral putamen (Pertovaara et al., 2004) of humans have also been reported. However, patients with unilateral Parkinson’s disease do not display consistent lateralized pain symptoms. Of 21 patients with unilateral Parkinson’s disease, 7 had pain on the ipsilateral side, 2 had pain on the contralateral side and 12 had pain on both sides (Djaldetti et al., 2004).
Alterations in the behavioral response to mechanical stimuli were less evident. Although the forces necessary to evoke withdrawal thresholds of the face were elevated in rats injected with 6-OHDA, these were not significantly different from rats injected with saline. Withdrawal thresholds to the mechanical stimulation of the hind paw contralateral to the side of 6-OHDA injection were significantly faster three weeks after injection compared to one week after injection, but these withdrawal latencies of dopamine-depleted and saline-injected rats were not significantly different from one another. In contrast to these results, Takeda et al. (Takeda et al., 2005) found that unilateral 6-OHDA injection into the medial forebrain bundle that reduced TH immunoreactivity in the substantia nigra and ventral tegmental area resulted in faster hind paw withdrawal responses to mechanical stimuli on the ipsilateral side. Anatomical and physiological differences between trigeminal and spinal sensory systems may account for some of the behavioral differences observed in these studies.
Although our vehicle injection did not contain 0.2% ascorbic acid, it is unlikely that this additive resulted in the anatomical and behavioral changes observed after intrastriatal 6-OHDA administration. Alterations in intrastriatal ascorbic acid levels do not result in degeneration of the dopaminergic nigrostriatal pathway, but they can alter movement (Rebec and Wang, 2001). However, these behavioral changes occur within 20 min after changes in ascorbic acid levels. In the current experiments, 6-OHDA/0.2% ascorbic acid injections into the striatum did not affect any behavior measured for several weeks after it was administered.
Variation in the effectiveness of 6-OHDA to alter the behavioral responses to chemical, thermal and mechanical stimuli may be due to the divergent pathways that underlie each of these modalities. Alternatively, the relatively low forces used during mechanical testing may not activate basal ganglia somatosensory systems. This hypothesis is supported by the finding that patients with Parkinson’s disease have similar non-painful mechanical and warmth thresholds as control subjects, but lower heat pain thresholds (Djaldetti et al., 2004). Modality-specific differences in brain activation in patients with chronic neuropathic pain to cutaneous tactile, cold and heat pain stimuli have also been noted (Becerra et al., 2006). Also, the majority of neurons recorded in the CPu area where the 6-OHDA injections were made respond primarily to nociceptive stimulation (Chudler et al., 1993; Chudler, 1998). Although significant correlations between cutaneous heat pain thresholds and D2/D3 receptor binding potential in the putamen have been reported, similar correlations between non-noxious tactile stimuli and D2/D3 receptor binding have not been found (Martikainen et al., 2005).
The role of the basal ganglia in pain and nociception is strengthened by observations of patients with Parkinson’s disease. For example, studies to evaluate the quality of life in patients with Parkinson’s disease who have undergone pallidotomy procedures have revealed significant reductions in Parkinson’s disease-related pain (Favre et al., 2000; Honey et al., 1999; Martinez-Martin et al., 2000). Honey et al. (Honey et al., 1999) found significant pain reductions (as measured with a simple ordinal pain scale) at six weeks and one year after pallidotomy in the majority of their patients. However, two patients with "dysesthetic" type pain reported that their pain was the same or worse one-year postoperatively. The absence of pain relief in response to sympathetic nerve blockade in patients with (Sage et al., 1990) and the normal clinical and electrophysiological status of peripheral nerves and roots (Schestatsky et al., 2007) in patients with Parkinson’s disease argue against a muscular source of pain in these patients. It has been hypothesized that some of the motor phenomena observed in Parkinson’s disease patients are related to the inability to utilize somatosensory cues (Rothblat and Schneider, 1993; Tatton et al., 1984). For example, deficiencies in the ability to use environmental cues may result in delayed signals for the initiation or preparation of movement. Altered pain sensibility in patients with Parkinson’s disease may also contribute to these motor phenomena.
Numerous brain imaging studies (i.e., positron emission tomography and functional magnetic resonance imaging) have provided additional evidence that the basal ganglia play a role in nociception (for reviews, see (Kurata, 2002; Peyron et al., 2000). These studies demonstrate that blood flow within the caudate nucleus, globus pallidus and putamen can be altered by experimentally-induced pain (i.e., noxious chemical and thermal stimuli) and during clinical pain states such as headache, restless legs syndrome, burning mouth syndrome, and fibromyalgia (Bingel et al., 2002; Chudler and Bonica, 2001; Jaaskelainen et al., 2001; San Pedro et al., 1998). Moreover, in rats, glucose utilization within the CPu increases after chronic constriction of the sciatic nerve, a neuropathic pain model (Mao et al., 1993).
The pathways by which the basal ganglia exert their modulatory influence on nociceptive information are still unclear. Nociceptive information may reach the basal ganglia through multiple pathways (Chudler and Dong, 1995) and it may be that ascending sensory signals are modified relatively early in the information processing sequence. Alternatively, descending modulation of nociceptive neurons in the medullary dorsal horn by intrastriatal dopaminergic agonists has been demonstrated (Belforte and Pazo, 2005). These mechanisms for basal ganglia modulation of nociceptive information are not mutually exclusive and further electrophysiological and behavioral experiments should provide new information to resolve this issue.
4. Experimental Procedure
4.1 Experimental Animals and Surgery
Adult, male Sprague-Dawley rats (N=17; 287–405 g body weight) were used in these experiments. Rats were maintained on a 12 hr dark/12 hr light cycle and had ad libitum access to food and water. Body weight measurements were made prior to surgery and for three weeks after surgery. All experiments were approved by the University of Washington Institutional Animal Care and Use Committee and conform to the guidelines outlined by the International Association for the Study of Pain (Zimmermann, 1983).
After baseline thermal and mechanical behavioral testing, rats were pretreated with desipramine hydrochloride (25 mg/kg, i.p.) to protect noradrenergic pathways and atropine sulfate (0.4 mg/kg, i.m.) 30 min before being anesthetized with sodium pentobarbital (40 mg/kg, i.p.). A midline incision and craniectomy to expose the cerebral cortex overlying the left caudate-putamen (CPu) was then performed. A solution of 6-hydroxydopamine hydrobromide (6-OHDA) in 0.9% saline with 0.2% ascorbic acid (Sigma Co., #H116) or 0.9% saline was placed in a 5 µl or 10 µl Hamilton microsyringe. The microsyringe was then lowered into the CPu (AP:−0.8; LR: 4.0; H:5.5 (Paxinos and Watson, 1986).
A total of 5 µg (2 µl) of 6-OHDA was injected at this site into three rats and an additional 5 µg (2 µl) 6-OHDA was injected at a depth of 6.5 mm into eight rats at a rate of approximately 0.5 µl/min. The microsyringe was left in place for 4 min after each injection. Control injections of saline (0.5 µl/min; 2 µl; 1 rat; 4 µl; 5 rats) were made into the same area of the CPu. Rats were allowed to recover for two days before behavioral testing.
4.2 Behavioral Testing
For two days prior to surgery and every two days after surgery, rats were tested for their responses to mechanical stimulation of the face and hind paws and to thermal stimulation of the hind paws. For mechanical stimulation testing, rats were placed in a cage with a wire floor. Calibrated Semmes-Weinstein monofilaments (North Coast Medical, Inc.) were applied to the vibrissae region of the face and through the wire floor onto the plantar surface of the rats’ hind paws in an ascending series. The minimal force (g) required to produce head or hind paw withdrawal on three applications was defined as the threshold strength. The interstimulus interval for mechanical stimuli betweens paws was 3 min. For thermal stimulation testing, a Hargreaves Thermal Stimulator (IITC, Inc., Model 33; beam setting = 7) was used. Rats were placed in clear Plexiglass boxes on a glass floor. Each hind paw was tested three times (intertrial interval, 3 min) and the averaged latency to evoke a paw withdrawal reflex was determined. The orofacial Formalin test (Raboisson and Dallel 2004) was used to measure the response of each rat to chemical stimulation three weeks after 6-OHDA or saline injection. For this test, rats were restrained lightly and injected subcutaneously (50 µl) with Formalin (2.5% solution) into the right upper lip (contralateral to the side of intrastriatal 6-OHDA injection). After the injection, rats were placed in a clear Plexiglas box (width, 25 cm; height, 21 cm; length 47 cm) and their behavior was recorded using a digital video camera for one hour. The amount of time each rat spent rubbing or scratching its perinasal area using its fore paws and hind paws was determined for three-minute intervals during a one-hour observation period. The number of times each rat reared up on two hind paws to extend its snout over the top of the Plexiglass box was counted during the first five minutes of the Formalin test to provide a measure of motor activity.
4.3 Statistical Analysis
Mechanical response thresholds and thermal response latencies were compared to baseline values for each rat. Data were collated into weekly averages and analyzed using repeated measures ANOVA methods, followed by paired comparison with Student’s t-tests with a Bonferroni corrections. For all statistical tests, significance was set to p < 0.05.
4.4 Histological Analysis
Following the Formalin test (21 days post surgery), rats were overdosed with sodium pentobarbital and perfused through the heart with 4% paraformaldehyde in 0.1 M phosphate buffer. The brain of each rat was removed and postfixed in 4% paraformaldehyde solution and then placed in phosphate-buffered 30% sucrose. Dopaminergic degeneration was determined by examining tyrosine hydroxylase staining within the substantia nigra. Serial frozen, coronal sections (50 □m thickness) through tissue containing the microsyringe needle track (CPu) and the substantia nigra were made and processed for immunohistochemical staining of tyrosine hydroxylase (TH). Free-floating sections were incubated with a polyclonal TH antibody (Chemicon, #AB5986; 1:2500 dilution; overnight at room temperature). An avidin-biotin peroxidase method with a secondary biotinylated anti-rabbit IgG (1:500 dilution, 2 hours) and nickel enhanced 3,3’-diaminobenzidine with 0.0125% hydrogen peroxide was used to localize the TH antibody. Selected control sections were also processed in the absence of all antibodies and with the primary, but not secondary antibody. Stained sections were mounted on gelatin-coated slides, dehydrated and covered with cover glass.
Acknowledgments
We thank Drs. Margaret R. Byers and Gregory W. Terman for their careful reading of this manuscript and to Dr. Byers for assisting us with immunohistochemical procedures. This work was supported by National Institutes of Health-NIDCR grant DE017623-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Pazo JH. Striatal inhibition of nociceptive responses evoked in trigeminal sensory neurons by tooth pulp stimulation. J Neurophysiol. 2005;93:1730–1741. doi: 10.1152/jn.00496.2004. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Barcelo AC, Pazo JH. Striatal modulation of the jaw opening reflex. Brain Res. 2001;891:138–147. doi: 10.1016/s0006-8993(00)03184-x. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: Electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: Evidence from single-trial fMRI. Pain. 2002;99:313–321. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Bulpitt CJ, Shaw K, Clifton P, Stern G, Davies JB, Reid JL. The symptoms of patients treated for parkinson's disease. Clin Neuropharmacol. 1985;8:175–183. doi: 10.1097/00002826-198506000-00007. [DOI] [PubMed] [Google Scholar]
- Cadet R, Pajot J, Papon A, Woda A. Is there a correlation between scores of nociceptive behavioral responses to formalin injections given at different body sites in the rat? Neurosci Lett. 1998;242:123–126. doi: 10.1016/s0304-3940(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Carelli RM, West MO. Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J Comp Neurol. 1991;309:231–249. doi: 10.1002/cne.903090205. [DOI] [PubMed] [Google Scholar]
- Carey RJ. Acute ipsilateral hyperalgesia and chronic contralateral hypoalgesia after unilateral 6-hydroxydopamine lesions of the substantia nigra. Exp Neurol. 1986;91:277–284. doi: 10.1016/0014-4886(86)90068-3. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Bonica JJ. Supraspinal processing of nociceptive information. In: Loeser JD, editor. Bonica’s Management of Pain. 3rd edition. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 153–179. [Google Scholar]
- Chudler EH. Response properties of neurons in the caudate-putamen and globus pallidus to noxious and non-noxious thermal stimulation in anesthetized rats. Brain Res. 1998;812:283–288. doi: 10.1016/s0006-8993(98)00971-8. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60:3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Sugiyama K, Dong WK. Multisensory convergence and integration in the neostriatum and globus pallidus of the rat. Brain Res. 1995;674:33–45. doi: 10.1016/0006-8993(94)01427-j. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Sugiyama K, Dong WK. Nociceptive responses in the neostriatum and globus pallidus of the anesthetized rat. J Neurophysiol. 1993;69:1890–1903. doi: 10.1152/jn.1993.69.6.1890. [DOI] [PubMed] [Google Scholar]
- Clavelou P, Dallel R, Orliaguet T, Woda A, Raboisson P. The orofacial formalin test in rats: Effects of different formalin concentrations. Pain. 1995;62:295–301. doi: 10.1016/0304-3959(94)00273-H. [DOI] [PubMed] [Google Scholar]
- Clavelou P, Pajot J, Dallel R, Raboisson P. Application of the formalin test to the study of orofacial pain in the rat. Neurosci Lett. 1989;103:349–353. doi: 10.1016/0304-3940(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D. Quantitative measurement of pain sensation in patients with parkinson disease. Neurology. 2004;62:2171–2175. doi: 10.1212/01.wnl.0000130455.38550.9d. [DOI] [PubMed] [Google Scholar]
- Factor SA, Molho ES. Emergency department presentations of patients with parkinson's disease. Am J Emerg Med. 2000;18:209–215. doi: 10.1016/s0735-6757(00)90023-8. [DOI] [PubMed] [Google Scholar]
- Favre J, Burchiel KJ, Taha JM, Hammerstad J. Outcome of unilateral and bilateral pallidotomy for parkinson's disease: Patient assessment. Neurosurgery. 2000;46:344–353. doi: 10.1097/00006123-200002000-00017. discussion 353–5. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Wu G. Effects of dopaminergic agents on carrageenan hyperalgesia in rats. Eur J Pharmacol. 2000;406:53–58. doi: 10.1016/s0014-2999(00)00649-x. [DOI] [PubMed] [Google Scholar]
- Gerdelat-Mas A, Simonetta-Moreau M, Thalamas C, Ory-Magne F, Slaoui T, Rascol O, Brefel-Courbon C. Levodopa raises objective pain threshold in parkinson's disease: A RIII reflex study. J Neurol Neurosurg Psychiatry. 2007;78:1140–1142. doi: 10.1136/jnnp.2007.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tanner CM, Levy M, Wilson RS, Garron DC. Pain in parkinson's disease. Mov Disord. 1986;1:45–49. doi: 10.1002/mds.870010106. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Jaaskelainen SK, Martikainen IK, Mansikka H, Forssell H, Scheinin H, Hietala J, Pertovaara A. Striatal dopamine D2 receptors in modulation of pain in humans: A review. Eur J Pharmacol. 2004;500:187–192. doi: 10.1016/j.ejphar.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Martikainen IK, Mansikka H, Hinkka S, Nagren K, Hietala J, Scheinin H, Pertovaara A. Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain. 2002;99:273–279. doi: 10.1016/s0304-3959(02)00121-5. [DOI] [PubMed] [Google Scholar]
- Hillen ME, Sage JI. Nonmotor fluctuations in patients with parkinson's disease. Neurology. 1996;47:1180–1183. doi: 10.1212/wnl.47.5.1180. [DOI] [PubMed] [Google Scholar]
- Honey CR, Stoessl AJ, Tsui JK, Schulzer M, Calne DB. Unilateral pallidotomy for reduction of parkinsonian pain. J Neurosurg. 1999;91:198–201. doi: 10.3171/jns.1999.91.2.0198. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J. Role of the dopaminergic system in chronic pain -- a fluorodopa-PET study. Pain. 2001;90:257–260. doi: 10.1016/S0304-3959(00)00409-7. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjöklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Koller WC. When does parkinson's disease begin? Neurology. 1992;42:27–31. discussion 41–8. [PubMed] [Google Scholar]
- Koller WC. Sensory symptoms in parkinson's disease. Neurology. 1984;34:957–959. doi: 10.1212/wnl.34.7.957. [DOI] [PubMed] [Google Scholar]
- Koyama T, Kato K, Mikami A. During pain-avoidance neurons activated in the macaque anterior cingulate and caudate. Neurosci Lett. 2000;283:17–20. doi: 10.1016/s0304-3940(00)00894-6. [DOI] [PubMed] [Google Scholar]
- Kurata J. Functional magnetic resonance imaging explained for pain research and medicine. Reg Anesth Pain Med. 2002;27:68–71. doi: 10.1053/rapm.2002.29106. [DOI] [PubMed] [Google Scholar]
- Levine MS, Schneider JS, Lloyd RL, Hull CD, Buchwald NA. Aging reduces somatosensory responsiveness of caudate neurons in the awake cat. Brain Res. 1987;405:389–394. doi: 10.1016/0006-8993(87)90312-x. [DOI] [PubMed] [Google Scholar]
- Lidsky TI, Labuszewski T, Avitable MJ, Robinson JH. The effects of stimulation of trigeminal sensory afferents upon caudate units in cats. Brain Res Bull. 1979;4:9–14. doi: 10.1016/0361-9230(79)90051-0. [DOI] [PubMed] [Google Scholar]
- Lin MT, Uang WN, Chan HK. Both thermal and nociceptive afferents influence the unit activity of the neurons in the corpus striatum. Experientia. 1985;41:120–122. doi: 10.1007/BF02005908. [DOI] [PubMed] [Google Scholar]
- Magnusson JE, Fisher K. The involvement of dopamine in nociception: The role of D(1) and D(2) receptors in the dorsolateral striatum. Brain Res. 2000;855:260–266. doi: 10.1016/s0006-8993(99)02396-3. [DOI] [PubMed] [Google Scholar]
- Mao J, Mayer DJ, Price DD. Patterns of increased brain activity indicative of pain in a rat model of peripheral mononeuropathy. J Neurosci. 1993;13:2689–2702. doi: 10.1523/JNEUROSCI.13-06-02689.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen IK, Hagelberg N, Mansikka H, Hietala J, Nagren K, Scheinin H, Pertovaara A. Association of striatal dopamine D2/D3 receptor binding potential with pain but not tactile sensitivity or placebo analgesia. Neurosci Lett. 2005;376:149–153. doi: 10.1016/j.neulet.2004.11.045. [DOI] [PubMed] [Google Scholar]
- Martinez-Martin P, Valldeoriola F, Molinuevo JL, Nobbe FA, Rumia J, Tolosa E. Pallidotomy and quality of life in patients with parkinson's disease: An early study. Mov Disord. 2000;15:65–70. doi: 10.1002/1531-8257(200001)15:1<65::aid-mds1011>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of parkinson's disease (2001): Treatment guidelines. Neurology. 2001;56:S1–S88. doi: 10.1212/wnl.56.suppl_5.s1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Pertovaara A, Martikainen IK, Hagelberg N, Mansikka H, Nagren K, Hietala J, Scheinin H. Striatal dopamine D2/D3 receptor availability correlates with individual response characteristics to pain. Eur J Neurosci. 2004;20:1587–1592. doi: 10.1111/j.1460-9568.2004.03622.x. [DOI] [PubMed] [Google Scholar]
- Peyron R, Schneider F, Faillenot I, Convers P, Barral FG, Garcia-Larrea L, Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Quinn NP, Koller WC, Lang AE, Marsden CD. Painful parkinson's disease. Lancet. 1986;1:1366–1369. doi: 10.1016/s0140-6736(86)91674-0. [DOI] [PubMed] [Google Scholar]
- Raboisson P, Dallel R. The orofacial formalin test. Neurosci Biobehav Rev. 2004;28:219–226. doi: 10.1016/j.neubiorev.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Wang Z. Behavioral activation in rats requires endogenous ascorbate release in striatum. J. Neurosci. 2001;21:668–675. doi: 10.1523/JNEUROSCI.21-02-00668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CD, Taylor DC. Electrophysiological evidence for a somatotopic sensory projection to the striatum of the rat. Neurosci Lett. 1982;30:235–240. doi: 10.1016/0304-3940(82)90405-0. [DOI] [PubMed] [Google Scholar]
- Riley DE, Lang AE. The spectrum of levodopa-related fluctuations in parkinson's disease. Neurology. 1993;43:1459–1464. doi: 10.1212/wnl.43.8.1459. [DOI] [PubMed] [Google Scholar]
- Rosland JH, Hunskaar S, Broch OJ, Hole K. Acute and long term effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in tests of nociception in mice. Pharmacol Toxicol. 1992;70:31–37. doi: 10.1111/j.1600-0773.1992.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Rothblat DS, Schneider JS. Response of caudate neurons to stimulation of intrinsic and peripheral afferents in normal, symptomatic, and recovered MPTP-treated cats. J Neurosci. 1993;13:4372–4378. doi: 10.1523/JNEUROSCI.13-10-04372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saade NE, Atweh SF, Bahuth NB, Jabbur SJ. Augmentation of nociceptive reflexes and chronic deafferentation pain by chemical lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain Res. 1997;751:1–12. doi: 10.1016/s0006-8993(96)01164-x. [DOI] [PubMed] [Google Scholar]
- Sage JI, Kortis HI, Sommer W. Evidence for the role of spinal cord systems in parkinson's disease-associated pain. Clin Neuropharmacol. 1990;13:171–174. doi: 10.1097/00002826-199004000-00006. [DOI] [PubMed] [Google Scholar]
- San Pedro EC, Mountz JM, Mountz JD, Liu HG, Katholi CR, Deutsch G. Familial painful restless legs syndrome correlates with pain dependent variation of blood flow to the caudate, thalamus, and anterior cingulate gyrus. J Rheumatol. 1998;25:2270–2275. [PubMed] [Google Scholar]
- Scherder E, Wolters E, Polman C, Sergeant J, Swaab D. Pain in parkinson's disease and multiple sclerosis: Its relation to the medial and lateral pain systems. Neurosci Biobehav Rev. 2005;29:1047–1056. doi: 10.1016/j.neubiorev.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Schestatsky P, Kumru H, Valls-Sole J, Valldeoriola F, Marti MJ, Tolosa E, Chaves ML. Neurophysiologic study of central pain in patients with parkinson disease. Neurology. 2007;69:2162–2169. doi: 10.1212/01.wnl.0000295669.12443.d3. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Levine MS, Hull CD, Buchwald NA. Development of somatosensory responsiveness in the basal ganglia in awake cats. J Neurophysiol. 1985;54:143–154. doi: 10.1152/jn.1985.54.1.143. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Morse JR, Lidsky TI. Somatosensory properties of globus pallidus neurons in awake cats. Exp Brain Res. 1982;46:311–314. doi: 10.1007/BF00237190. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Lidsky TI. Processing of somatosensory information in striatum of behaving cats. J Neurophysiol. 1981;45:841–851. doi: 10.1152/jn.1981.45.5.841. [DOI] [PubMed] [Google Scholar]
- Schott GD. Pain in parkinson's disease. Pain. 1985;22:407–411. doi: 10.1016/0304-3959(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. Orofacial pain. In: Merskey H, Loeser JD, Dubner R, editors. The Paths of Pain, 1975–2005. Seattle: IASP Press; 2005. pp. 131–150. [Google Scholar]
- Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of parkinson's disease. Mov Disord. 2001;16:507–510. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- Snider SR, Fahn S, Isgreen WP, Cote LJ. Primary sensory symptoms in parkinsonism. Neurology. 1976;26:423–429. doi: 10.1212/wnl.26.5.423. [DOI] [PubMed] [Google Scholar]
- Stein WM, Read S. Chronic pain in the setting of parkinson's disease and depression. J Pain Symptom Manage. 1997;14:255–258. doi: 10.1016/s0885-3924(97)00176-0. [DOI] [PubMed] [Google Scholar]
- Takeda R, Ikeda T, Tsuda F, Abe H, Hashiguchi H, Ishida Y, Nishimori T. Unilateral lesions of mesostriatal dopaminergic pathway alters the withdrawal response of the rat hindpaw to mechanical stimulation. Neurosci Res. 2005;52:31–36. doi: 10.1016/j.neures.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Armentero MT, Greco R, Fancellu R, Sandrini G, Nappi G, Blandini F. Behavioral responses and fos activation following painful stimuli in a rodent model of parkinson's disease. Brain Res. 2007;1176:53–61. doi: 10.1016/j.brainres.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Eastman MJ, Bedingham W, Verrier MC, Bruce IC. Defective utilization of sensory input as the basis for bradykinesia, rigidity and decreased movement repertoire in parkinson's disease: A hypothesis. Can J Neurol Sci. 1984;11:136–143. doi: 10.1017/s0317167100046291. [DOI] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: An evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Waseem S, Gwinn-Hardy K. Pain in parkinson's disease. common yet seldom recognized symptom is treatable. Postgrad Med. 2001;110:33–34. 39–40, 46. doi: 10.3810/pgm.2001.12.1063. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Cherif AA. Nonmotor fluctuations in parkinson's disease: Frequent and disabling. Neurology. 2002;59:408–413. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


