Abstract
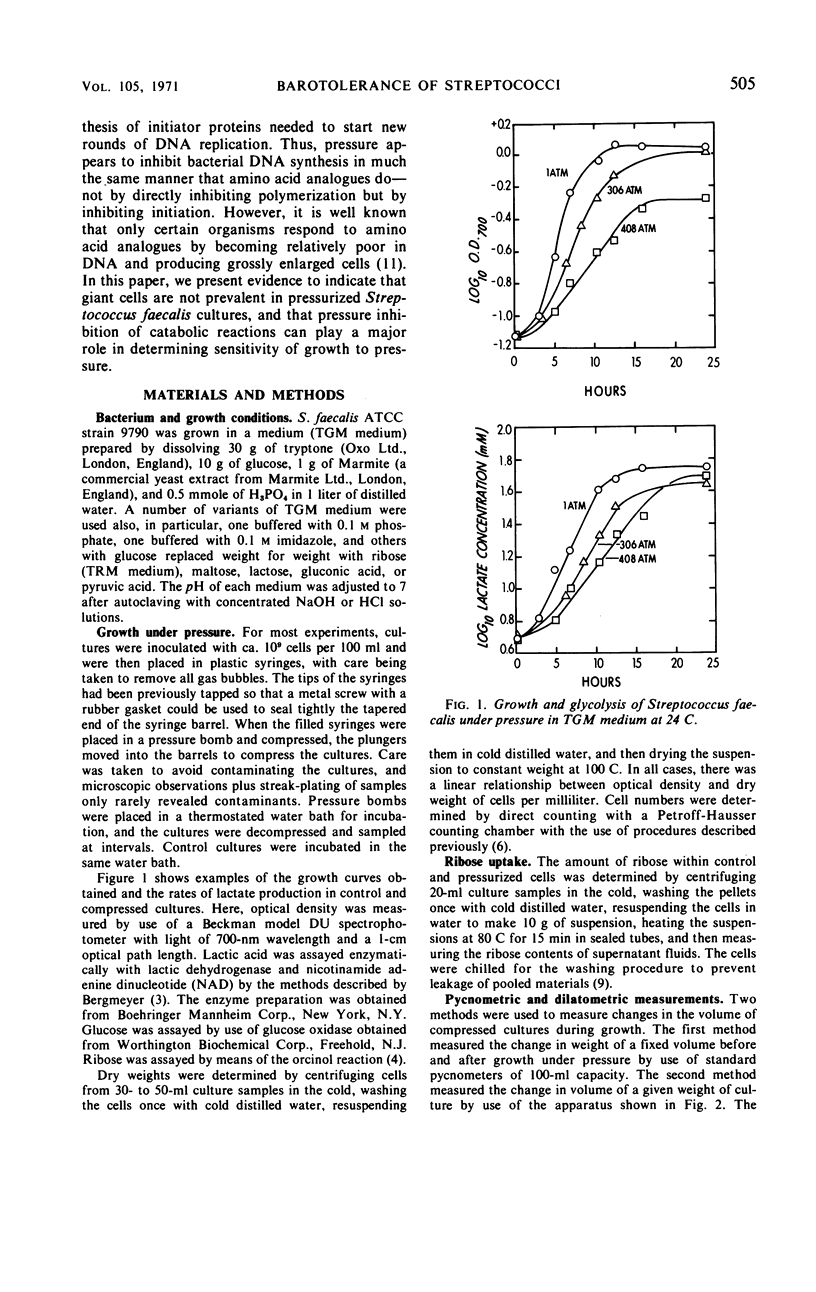
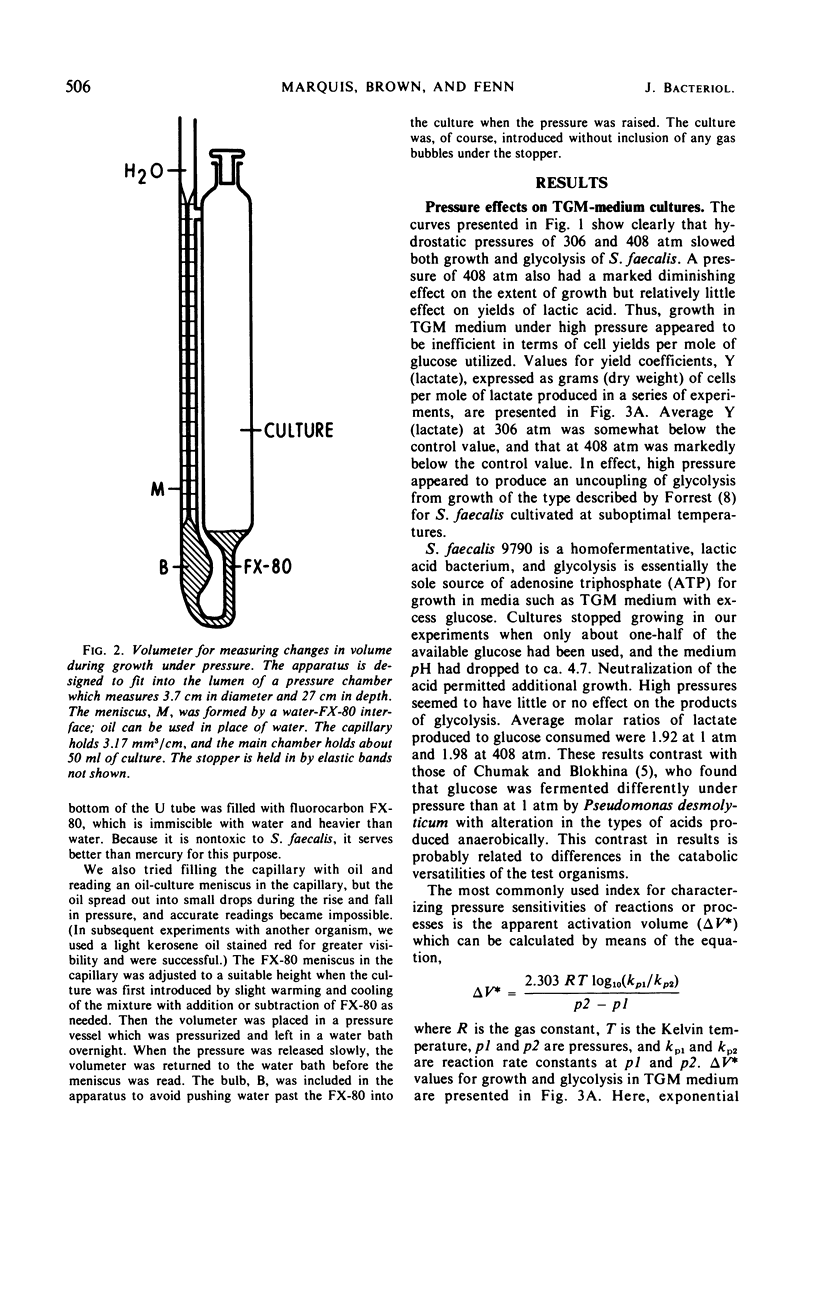
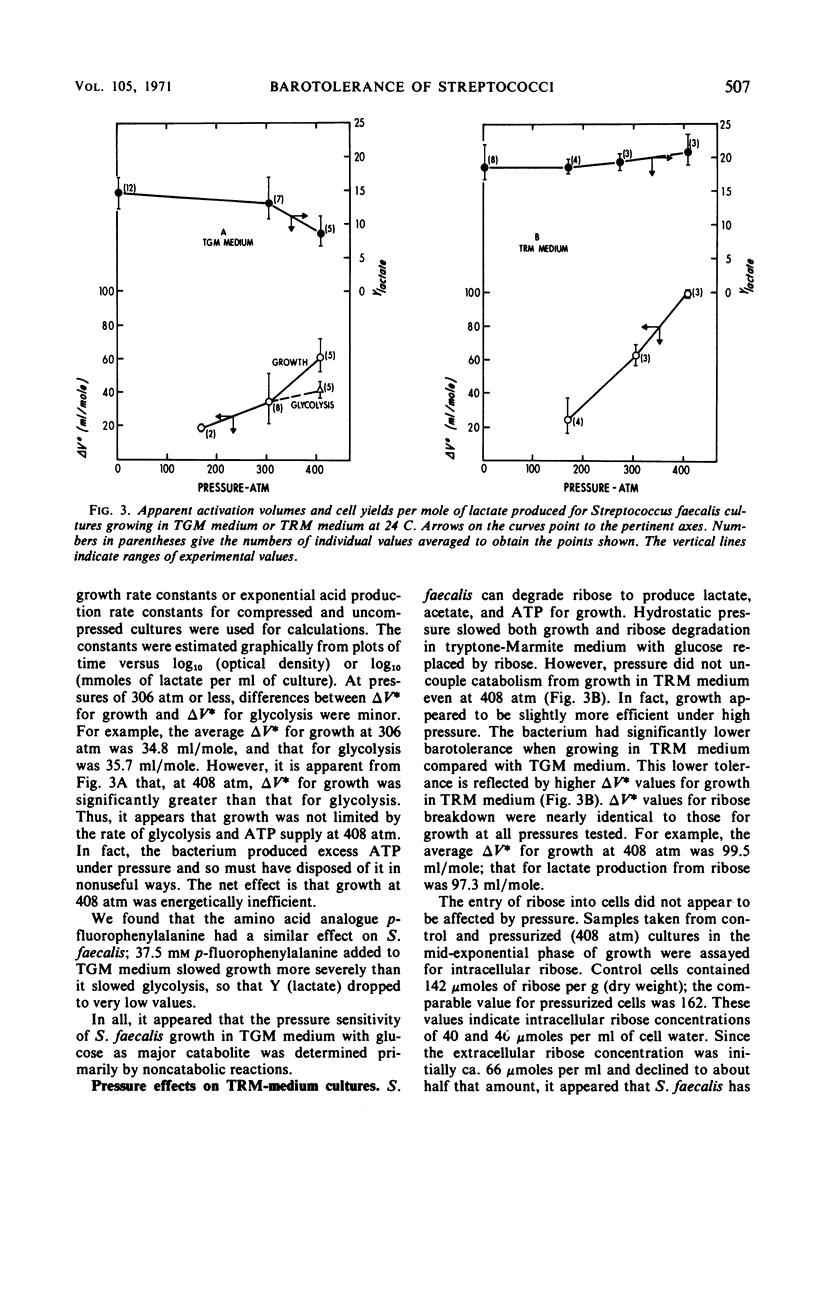
The sensitivity of Streptococcus faecalis growth to hydrostatic pressures ranging up to 550 atm was found to depend on the source of adenosine triphosphate for growth. Barotolerance of cultures growing in a complex medium with ribose as major catabolite appeared to be determined primarily by the pressure sensitivity of ribose-degrading enzymes. Apparent activation volumes for growth were nearly identical to those for lactate production from ribose, and yield coefficients per mole of ribose degraded were relatively independent of pressure. In contrast, cultures with glucose as main catabolite were less sensitive to pressure; glycolysis was less severely restricted under high pressure than was growth, and yield coefficients declined with pressure, especially above 400 atm. Thus, two distinct types of barotolerance could be defined—one dominated by catabolic reactions and one dominated by noncatabolic reactions. The results of experiments with a series of other catabolites further supported the view that catabolic reactions can determine streptococcal barotolerance. We also found that growing, glucose-degrading cultures increased in volume under pressure in the same manner that they do at 1 atm. Thus, it appeared that the bacterium has no alternative means of carrying out glycolysis under pressure without dilatation. Also, the observation that cultures grown under pressure did not contain abnormally large or morphologically deformed cells suggested that pressure did not inhibit cell division more than cell growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright L. J. Alternate pressurization-depressurization effects on growth and net protein, RNA and DNA synthesis by Escherichia coli and Vibrio marinus. Can J Microbiol. 1969 Oct;15(10):1237–1240. doi: 10.1139/m69-223. [DOI] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- Corner T. R., Marquis R. E. Why do bacterial protoplasts burst in hypotonic solutions? Biochim Biophys Acta. 1969;183(3):544–558. doi: 10.1016/0005-2736(69)90168-0. [DOI] [PubMed] [Google Scholar]
- Fenn W. O., Marquis R. E. Growth of Streptococcus faecalis under high hydrostatic pressure and high partial pressures of inert gases. J Gen Physiol. 1968 Nov;52(5):810–824. doi: 10.1085/jgp.52.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest W. W. Energies of activation and uncoupled growth in Streptococcus faecalis and Zymomonas mobilis. J Bacteriol. 1967 Nov;94(5):1459–1463. doi: 10.1128/jb.94.5.1459-1463.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Fenn W. O. Dilatometric study of streptococcal growth and metabolism. Can J Microbiol. 1969 Aug;15(8):933–940. doi: 10.1139/m69-164. [DOI] [PubMed] [Google Scholar]
- Pollard E. C., Weller P. K. The effect of hydrostatic pressure on the synthetic processes in bacteria. Bibl Laeger. 1966 Mar 14;112(3):573–580. doi: 10.1016/0926-6585(66)90261-5. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Hiramoto Y., Marsland D. Studies on the microtubules in heliozoa. 3. A pressure analysis of the role of these structures in the formation and maintenance of the axopodia of Actinosphaerium nucleofilum (Barrett). J Cell Biol. 1966 Apr;29(1):77–95. doi: 10.1083/jcb.29.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A., Pollard E. C. A study of the effects of hydrostatic pressure on macromolecular synthesis in Escherichia coli. Biophys J. 1969 Dec;9(12):1464–1482. doi: 10.1016/S0006-3495(69)86466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN A. M., MARSLAND D. CELL DIVISION: EFFECTS OF PRESSURE ON THE MITOTIC MECHANISMS OF MARINE EGGS (ARBACIA PUNCTULATA). Exp Cell Res. 1964 Jul;35:293–302. doi: 10.1016/0014-4827(64)90096-5. [DOI] [PubMed] [Google Scholar]
- ZOBELL C. E., COBET A. B. FILAMENT FORMATION BY ESCHERICHIA COLI AT INCREASED HYDROSTATIC PRESSURES. J Bacteriol. 1964 Mar;87:710–719. doi: 10.1128/jb.87.3.710-719.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOBELL C. E., OPPENHEIMER C. H. Some effects of hydrostatic pressure on the multiplication and morphology of marine bacteria. J Bacteriol. 1950 Dec;60(6):771–781. doi: 10.1128/jb.60.6.771-781.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]



