Abstract
Numerous components and pathways are involved in the complex interplay between cancer cells and their environment. The family of glycophosphoproteins comprising osteopontin, bone sialoprotein, dentin matrix protein 1, dentin sialophosphoprotein and matrix extracellular phosphoglycoprotein — small integrin-binding ligand N-linked glycoproteins (SIBLINGs) — are emerging as important players in many stages of cancer progression. From their detection in various human cancers to the demonstration of their key functional roles during malignant transformation, invasion and metastasis, the SIBLINGs are proteins with potential as diagnostic and prognostic tools, as well as new therapeutic targets.
Introduction
The progression of malignant cells requires complex interactions with the host tissues. Tumour survival and metastasis necessitate overcoming immune surveillance, extracellular matrix barriers and limiting nutrients. Effectively stopping cancer progression appears to require the use of a panel of therapeutic modalities able to interfere with the multiple stages of cancer cell invasion and dissemination. An alternative to targeting specific stages in progression (for example, angiogenesis and metastasis) would be to target select molecules that have key roles in multiple stages of cancer development.
Small integrin-binding ligand N-linked glycoproteins (SIBLINGs1), a family of five integrin-binding glycophosphoproteins comprising osteopontin (OPN), bone sialoprotein (BSP), dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP) and matrix extracellular phosphoglycoprotein (MEPE), are an emerging group of molecular tools that cancer cells use to facilitate their expansion. SIBLINGs are soluble, secreted proteins that can act as modulators of cell adhesion as well as autocrine and paracrine factors by their interaction with cell surface receptors such as integrins. OPN is the only SIBLING for which there is unequivocal evidence of its role in many steps of cancer development and progression, but accumulating data also implicate other family members, notably BSP and DSPP2–6. The involvement of SIBLINGs in many of the crucial steps for malignant progression makes them potentially valuable candidates for effective anticancer therapies. In this Review, we describe the major characteristics of SIBLINGs, including their proposed roles in normal tissue and the major activities they display during malignant progression. Finally, we discuss their potential as therapeutic targets and prognostic markers.
Discovery and characteristics of SIBLINGs
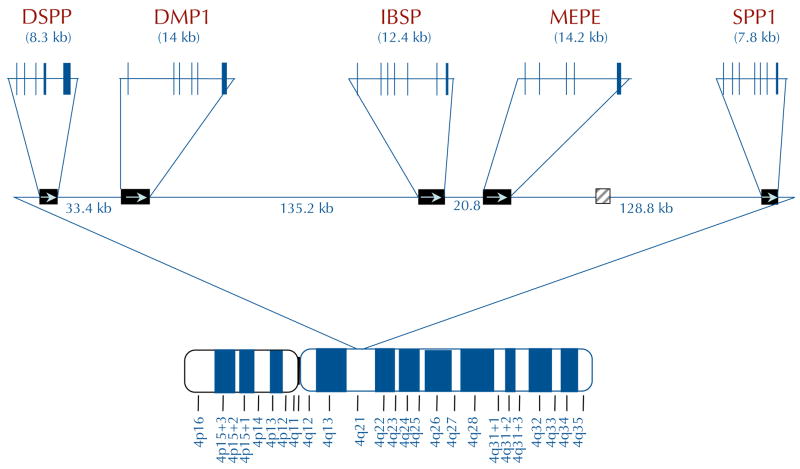
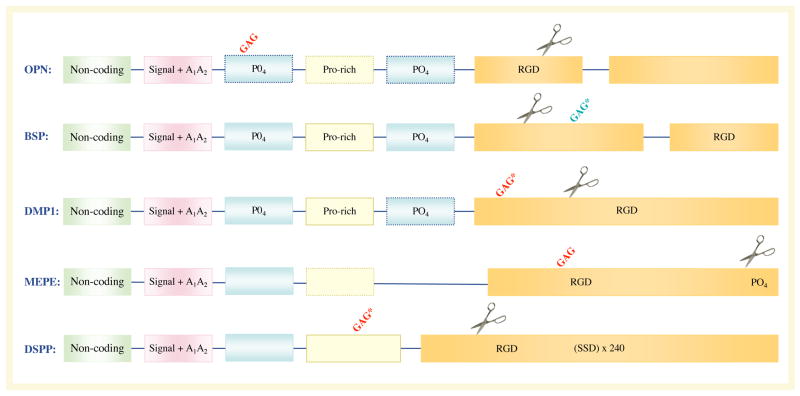
SIBLINGs are currently a family of five identically orientated tandem genes within a 375,000 bp region on chromosome 4 (FIG. 1a). The genes (DMP1, DSPP, integrin-binding sialoprotein (IBSP, which encodes BSP), MEPE and secreted phosphoprotein 1 (SPP1, which encodes OPN)) are probably a result of an early gene duplication and divergence1. The term SIBLING refers to the gene family’s unifying genetic and biochemical characteristics in general, not to functional activity. SIBLINGs are defined as small, soluble RGD motif containing, integrin-binding ligands to distinguish them from large extracellular matrix proteins such as fibronectin (FN1), collagen and thrombospondin (THBS1). Comparison of any one SIBLING to itself at the amino acid sequence level throughout evolution (predominantly in birds and mammals) shows that they are poorly conserved. Each SIBLING member appears to be able to drift substantially as long as it remains hydrophilic and flexible, and retains a number of motifs and member-related short amino acid sequences. For example, using standard sequence comparison basic local alignment search tool (BLAST) programs7, human and mouse OPN are identical at only 63% of the amino acid positions and the comparison with chicken drops to 30% identity, although all retain, for example, at least one RGD and N-linked oligosaccharide motif each. Therefore, the SIBLING family was defined structurally by the conserved motifs within their exons, including an abundance of acidic amino acids, the RGD motif, similar post-translational modification motifs (for example, casein kinase phosphorylation and various glycosylation events) and more recently the recognition that at least one site of controlled proteolysis appears to be important in all members (FIG. 1b). The SIBLINGs that have had their three-dimensional structure solved by NMR analysis (BSP and OPN) are extended and flexible in solution, a property shared by a number of proteins that have multiple binding partners and that are involved in bridging macromolecular components (for example, certain ribosomal proteins). Consistent with that observation, SIBLINGs can bind to a number of different protein families, including integrins (through both RGD and non-RGD motifs) and other cell-surface proteins, members of the matrix metalloproteinase (MMP) family and complement factor H (CFH). These interactions enable cell surface localization and sequestration of MMPs and CFH by at least OPN, BSP and DMP1, which in turn enables their biological activities (extracellular matrix degradation and evasion of complement-mediated lysis for example).
Figure 1. Chromosomal localization and exon–intron similarities of human SIBLInG genes.
a| The genes are clustered within a 375 kb region of chromosome 4 and are similarly arranged in all completed mammalian genomes to date. Except for an apparent pseudogene (HSP90AB177) between matrix extracellular phosphoglycoprotein (MEPE) and secreted phosphoprotein 1 (SPP1) in humans and chimps only (light grey box), there are no other significant open-reading frames within this region. Integrin-binding sialoprotein (IBSP) encodes bone sialoprotein (BSP) and SPP1 encodes osteopontin (OPN). Vertical lines represent exons. b | The transcripts of small integrin-binding ligand N-linked glycoprotein (SIBLING) genes. The SIBLINGs, which are composed almost exclusively of hydrophilic amino acids, are likely to be flexible, extended structures in solution. The exons (boxes; not drawn to scale) often have similar motifs and properties and are separated by type 0 introns. The first exon is non-coding. The second exon contains the start codon, the hydrophobic signal peptide and the first two amino acids of the mature protein (A1A2). Exons 3 and 5 frequently contain consensus sequences for serine phosphorylation (PO4). Exon 4 can be relatively proline-rich and, like the other small exons (3 and 5), has been shown in some cases to be spliced out of a subset of mRNA (exons with dashed borders). The integrinbinding tripeptide, Arg–Gly–Asp (RGD), is found within the last one or two large exons (which typically encode >80% of the protein). All SIBLINGs contain variously located N- and/or O-linked oligosaccharides, but only the observed (GAG*) and proposed (GAG) consensus attachment sites of the relatively long chain glycosaminoglycans are shown. (Orange GAG indicates chondroitin or dermatan sulphate chains and green GAG indicates keratan sulphate chains.) Cleavage of SIBLINGs (scissors) by specific proteases (bone morphogenetic protein 1 (BMP1), thrombin, matrix metallopeptidases and so on) is thought to be important, although whether this activates and/or inactivates specific SIBLING functions is currently under investigation. Human DSPP also contains ~240 tandem repeats of the phosphorylated nominal Ser–Ser–Asp (SSD) tripeptide. (For summary of some of the post-translational modifications and protease cleavage sites, see REF. 159.) DMP1, dentin matrix protein 1; DSPP, dentin sialophosphoprotein.
Four acidic members (BSP8, DMP1 (REF. 9), DSPP10 and OPN11) were discovered as abundant proteins trapped within the mineralized matrices of bone and dentin. In the early years of study, each of these proteins was thought to be both skeletal tissue-specific and to have a role in directly nucleating hydroxyapatite crystals within mesenchymal tissues through their phosphate groups and/or polyacidic amino acid domains9,12. From the 1990s, various combinations of SIBLING proteins were discovered to be significantly upregulated in a number of epithelial tumours that are known to frequently exhibit pathological microcalcifications and to have strong propensities to metastasize to bone13. More recently it has been shown that all five of the SIBLINGs are also expressed in the epithelial cells of metabolically active normal ducts of the salivary gland and kidney14,15, but not in metabolically passive normal ducts16. SIBLINGs are secreted proteins that can be localized through interactions with receptors either on the cell’s own surface, enabling autocrine activities, or by diffusing short distances to nearby cells where they may function in paracrine signalling. SIBLINGs propagate biological signals by initiating integrin signalling and by binding and sequestering other proteins to the cell surface.
Roles of SIBLINGs in normal tissues
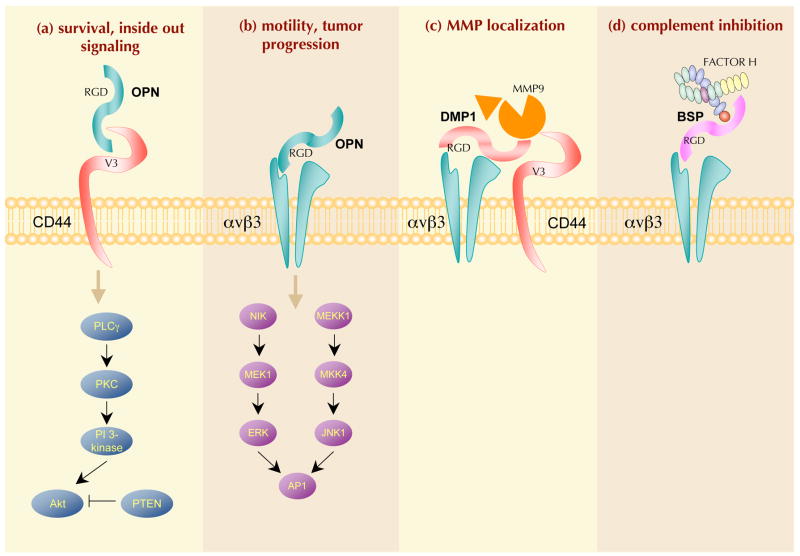
SIBLINGs bind to cell surface integrins and sometimes CD44 in normal tissues and function as signal transducers to promote cell adhesion, motility and survival (FIG. 2) through activating kinase cascades and transcription factors. The biological activities of SIBLINGs are also modulated by proteolytic processing, which can reveal cryptic binding sites and can remove or separate functional domains, thereby modulating cell adhesion and migration (FIG. 1b).
Figure 2. SIBLINGs mediate cell–matrix interactions and cellular signalling.
Small integrin-binding ligand N-linked glycoproteins (SIBLINGs; bone sialoprotein (BSP), dentin matrix protein 1 (DMP1) and osteopontin (OPN) are shown) can initiate Arg–Gly–Asp (RGD)-dependent and RGD-independent interactions with several integrins (such as αvβ3 and a9β1, respectively). OPN (and perhaps DMP1) can also interact with the CD44 family of receptors. Some of these complexes are able to mediate the following functions: (a) cell survival through phospholipase C-γ (PLCγ)–protein kinase C (PKC)–phosphatidylinositol 3-kinase (PI3K)–Akt pathway activation that leads to anti-apoptotic signals in tumour cells. OPN-induced Akt phosphorylation can be blocked by the tumour suppressor PTEN (phosphatase and tensin homologue). However, PTEN is frequently mutated and thus rendered inactive in cancer cells such as melanoma and glioma; (b) motility through the activation of the canonical αvβ3 integrin pathway where both nuclear factorinducing kinase (NIK)–ERK (extracellular signal-related kinase) and MEKK1 (also known as mitogen-activated protein kinase kinase kinase 1 (MAP3K1)–JNK1 (also known as MAPK8) signalling promote cell migration by activating AP1- dependent gene expression (for a review see REF. 178). Upon binding to αvβ3, OPN also stimulates epidermal growth factor receptor (EGFR) transactivation, ERK phosphorylation and AP1 activation; (c) bridging of otherwise soluble matrix metalloproteinases (MMPs) to cell membranes and their activation, enabling digestion of local extracellular matrix and thereby aiding tissue remodelling and cell migration through the extracellular matrix, a key step for cancer cell invasion; and (d) bridging and activation of complement factor H (CFH) to receptors including αvβ3 integrin. By promoting the degradation of the C3 convertase complex C3bBb, SIBLING-activated CFH disables the formation of the membrane attack complex (MAC) and the subsequent lysis of cancer cells, thus favouring their escape from host immune defence. The ? illustrates that it is not known if all binding of SIBLINGs (with or without ligands) necessarily results in signal transduction. MKK4, MAP kinase kinase 4.
For example, OPN interacts with a variety of integrins, including αvβ3, αvβ5, αvβ1, α4β1, α8β1 and α9β1, as well as CD44 splice variants. Integrin-mediated cell adhesion and migration are stimulated in assays in which full-length OPN is immobilized on tissue culture dishes. Thrombin cleavage of OPN separates the integrin- and CD44-binding domains, which in some cases promotes adhesion over migration. For example, the thrombin-generated amino-terminal OPN fragment binds to αvβ3 and αvβ5 integrins (through RGD17) or to α9β1 and α4β1 integrins (through the cryptic SVVYGLR sequence18) and promotes cell adhesion. The carboxy-terminal fragment binds to CD44 variant 6 (CD44v6) — and sometimes to CD44v3 by a heparin bridge — and promotes the formation of foci, invasion and tumorigenesis19. under specific conditions, OPN is also a substrate for MMP3 and MMP7, and the resulting OPN fragments facilitate adhesion and migration in vitro through activation of β1-containing integrins20. OPN has also been shown to be a substrate for liver transglutaminase and plasma transglutaminase factor IIIa, resulting in protein crosslinking21 and enhanced cell adhesion, spreading, focal contact formation and migration22. Through interactions with cell-surface receptors, OPN and its proteolytic fragments modulate cell adhesion and migration.
Through their action on the transcription factor nuclear factor κB (NFκB), SIBLINGs can also affect cellular proliferation, differentiation and apoptosis in normal tissues. BSP increases survival and decreases apoptosis of bone marrow-derived monocytes and macrophages through enhanced NFκB signalling23. BSP can also induce NFκB -dependent bone resorption by inducing osteoclastogenesis and osteoclast survival23. OPN activation of NFκB promotes survival of activated T cells through phosphorylation of the kinase IKKβ (also known as inhibitor of NFκB kinase β (IKBKB)) and inhibition of the transcription factor FOXO3 (REF. 24). A role for OPN-induced activation of NFκB in the survival of dopaminergic neurons25, endothelial cells26 and dendritic cells27 has also been reported.
The above-mentioned pathways (migration, adhesion and apoptosis evasion) are crucial in the development and progression of cancer as nascent neoplasms must successfully navigate these pathways to survive. Therefore it seems possible that the effects of SIBLINGs in cancer biology are due in part to modulation of these pathways.
SIBLINGs and tumour progression
Tumour progression involves a sequential series of events that confer a survival advantage to transformed cells. These events begin with neoplastic transformation and continue through the subversion of proliferation blockades, growth restriction, physical barriers and host defense systems. Cancer cell survival requires proliferation, interaction with the extracellular matrix to create space to grow, pathways for nutrient access and escape of the cells to a new environment. Successful progression also involves cellular responses and evasion of immune surveillance.
Cancer cell adhesion and proliferation
Cancer cells bind to SIBLINGs and their various proteolytic fragments through a variety of integrin receptors by both RGD-dependent and RGD-independent interactions. OPN, and perhaps DMP1, can also interact with specific splice variants of CD44 that are expressed by cancer cells. Interestingly, OPN binding to CD44v6 results in the propagation of cytosolic signals that enhance integrin activation and thus migration (an example of inside-out signaling) in colon HT29 cells28. Tumour cells were stimulated to spread following the interaction of CD44 and OPN apparently through β1 integrins, which have well-characterized roles in enabling cell adhesion29,30. OPN exhibiting reduced serine/threonine phosphorylation by casein kinase induces the adhesion of human breast cancer cells almost sixfold more than hyperphosphorylated OPN31, highlighting the possible modifying roles of the many post-translational events on SIBLING functions.
The adhesive properties of the SIBLINGs have also been investigated in the context of bone targeting and recognition by metastasizing cancer cells, and these molecules have been implicated in enhancing the affinity of metastatic cancer cells for bone (discussed in more detail below). Breast cancer cells expressing active αvβ3 integrin adhere to BSP-enriched mineralized bone as well as to recombinant BSP during in vitro adhesion and invasion assays32. Thus, exogenously added BSP peptides strongly inhibited breast cancer cell adhesion to extracellular bone matrix at micromolar concentrations33. Furthermore, multiple myeloma cells adhere to OPN, indicating that the increased stromal expression of OPN that is associated with this disease might be one of the factors enhancing the retention of these cells in the bone marrow34. Although the RGD domain of DMP1 has been shown to mediate the adhesion and spreading of dental pulp cells in vitro35, no data about DMP1-mediated cancer cell adhesion are available.
SIBLINGs may also affect cancer cell proliferation. BSP accelerates the proliferation of breast cancer cells in vitro6. Furthermore, IBSP-transfected breast cancer cells show increased primary tumour growth following injection into the mammary fat pad of nude mice36. OPN stimulates human prostate cancer cell line proliferation when transferred to a mouse xenograft model system37. OPN-induced proliferative responses mainly occur through activation of the epidermal growth factor receptor (EGFR)38 and integrin-mediated intracellular Ca2+ signalling39. The intracellular signalling pathways operant in OPN modulation of cell proliferation and migration have been well characterized (for a review see REF. 18). The binding of OPN to CD44 promotes cell migration through kinase cascades involving phospholipase Cγ, protein kinase C, phosphatidylinositiol 3-kinase (PI3K) and Akt, a serine/threonine kinase that regulates cell cycle progression, growth factor-mediated survival and cell migration (FIG. 2a). The binding of αvβ3 by OPN is associated with SRC kinase-mediated complex formation between αvβ3 and EGFR, which activates the mitogen-activated protein kinase (MAPK) pathway and results in the promotion of tumour growth. The potential effects of SIBLINGs other than BSP and OPN on cell proliferation have not been investigated.
Invasion and extracellular matrix degradation
High cancer cell motility combined with increased expression of proteases that degrade the extracellular matrix is generally predictive of invasive capability40,41. OPN and BSP are expressed at high levels by numerous cancers and might contribute to their invasive potential. Functional studies using over-expression of OPN in two prostate cancer cell lines reveal that OPN increases invasion and enhances the ability of cancer cells to intravasate into blood vessels in a mouse neoplastic model37. OPN also enhances in vitro migration of various types of cancer cells including melanoma42, breast43, and multiple myeloma44. Similarly, transfection of a breast cancer cell line with IBSP cDNA stimulated migration and invasion in vitro36.
Invasive cells have the capacity to degrade the extracellular matrix through at least two pathways of controlled proteolysis: the urokinase-type plasminogen activator (uPA, also known as PLAU) pathway and the MMP pathway. Several studies using recombinant OPN show that this SIBLING significantly increases in vitro invasiveness. For example, OPN increases the invasiveness of pancreatic cancer cells45 and non-small cell lung carcinoma cells46. Treatment of breast cancer cells with OPN results in higher invasiveness through the basement membrane analogue, Matrigel, and increases both PLAU mRNA expression and urokinase activity47. In a metastatic murine mammary cancer cell lines model, the binding of OPN to integrin receptors induces MMP2 and uPA expression through integrin-linked kinase (ILK)-dependent AP1 activity48. It has recently been shown that OPN induces αvβ3 integrin-mediated AP1 activity and uPA secretion by activating SRC–EGFR–ERK (extracellular signal-regulated kinase) signalling pathways and further demonstrates a functional molecular link between OPN-induced integrin- and SRC-dependent EGFR phosphorylation and ERK- and AP1-mediated uPA secretion, and all of these ultimately control the motility of breast cancer cells49. Thus, both increased cell motility and induction of uPA expression are possible mechanisms of increased invasiveness of breast epithelial cells in response to OPN.
Potential mechanisms for SIBLING-enhanced matrix degradation have been described. BSP and OPN induce the activation of MMP2 in GCT23 giant cell tumour cells50. OPN binding to αvβ3 is associated with PI3Kmediated NFκB activation and nuclear factor-inducing kinase (NIK, also known as mitogen-activated protein kinase kinase kinase 14 (MAP3K14)) activation of AP1 and NFκB, which stimulate uPA-dependent MMP9 activation51. Thus, at least two SIBLINGs can induce MMP expression. Interestingly, NIK-dependent MMP9 activation has been recently implicated in melanoma growth and metastasis to lung52.
BSP, DMP1 and OPN bind to and modulate the activity of MMP2, MMP9 and MMP3, respectively53. SIBLING-mediated MMP activation includes both making the proMMPs enzymatically active to some degree and reactivating MMPs that are inhibited by tissue inhibitors of MMP (TIMPs)53. The expression of these three SIBLINGs and their cognate MMPs was correlated in a number of different cancer types54. BSP promoted invasion of several osteotropic cancer cell lines in vitro by apparently localizing MMP2 to the cell surface through αvβ3 (REF. 55). DMP1 enhanced the invasion potential of a colon cancer cell line by bridging MMP9 to integrins and, perhaps, CD44 (REF. 56). Another mechanism might involve enzymatic processing of SIBLINGs that alters invasion and migration properties. For example, increased hepatocellular carcinoma cell invasion was attributed to OPN peptides cleaved by MMP9 and thrombin57. Interestingly, transglutaminase crosslinking of OPN forms proteolysis-resistant inactive OPN polymers that reduced breast cancer cell invasion and migration in vitro58.
Metastasis
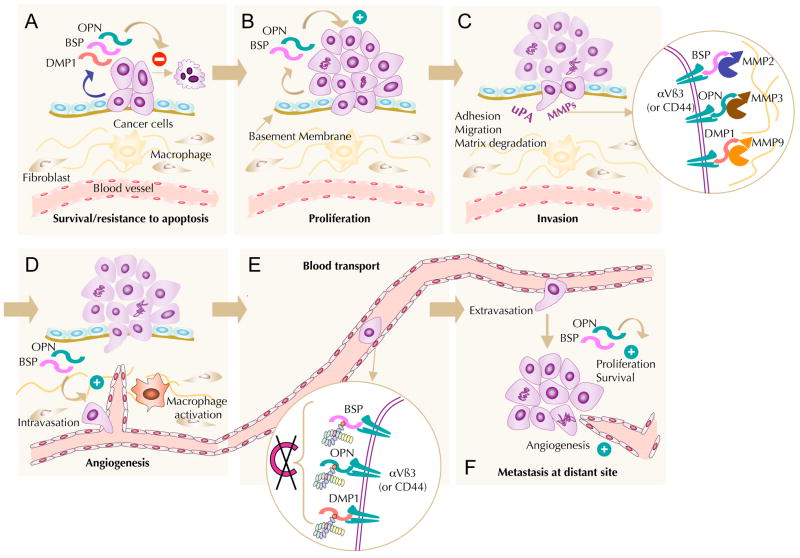
Metastasis is a complex process characterized by multiple stages: malignant cells proliferate and spread from the primary tumour mass, invade adjacent capillary or lymphatic vessels, resist immunological attacks and eventually gain access to secondary sites where they proliferate to form a new tumour (for a review see REF. 59). Throughout this multi-step progression, cancer cells interact with extracellular matrix proteins, endothelial cells, platelets, stromal cells and other organ-specific structures. Multifunctional extracellular matrix proteins such as the SIBLINGs are expected to have key roles in metastasis as they affect adhesion, migration and matrix degradation (FIG. 3).
Figure 3. The role of SIBLING proteins at different steps of the metastatic cascade.
a, b | At the primary site, cancer cells secrete high levels of small integrin-binding ligand, N-linked glycoproteins (SIBLINGs), which favour their proliferation (osteopontin (OPN) and bone sialoprotein (BSP)) and survival (OPN, BSP and dentin matrix protein 1 (DMP1)). c | Cancer cells with enhanced adhesive and migratory capabilities can detach from the primary tumour mass and degrade the basement membrane to invade the stroma. The associated proteolysis of the extracellular matrix (ECM) is mediated through matrix metalloproteinases (MMPs) and urokinase plasminogen activator (uPA). OPN enhances uPA activation, cell motility and invasion into the surrounding tissue. The insert shows BSP, DMP1 and OPN bound to their respective receptors (αvβ3 integrins and/or CD44), which may actively promote local proteolysis through binding specific MMPs (MMP2, MMP9 and MMP3, respectively). d | As ligands for αvβ3 integrin, OPN and BSP have roles in angiogenesis. The expression of these SIBLINGs by tumour cells promotes the migration and adhesion of activated endothelial cells, which are crucial during angiogenesis. OPN acts as a chemotactic and adhesion molecule for macrophages and promotes their infiltration of the tumour. e | The transport of cancer cells in the circulation is one of the limiting steps for metastasis to distant organs because they are confronted by the host immune system. The insert shows that, in this context, the expression and the presentation of BSP, DMP1 and OPN on the cancer cell surface enables them to sequester and activate complement factor H (CFH) and protect themselves from complement-mediated lysis. f | At distant site(s), cancer cell extravasation is followed by the formation of a secondary colony. Proliferative, survival and angiogenenic signals by newly formed metastatic colonies occur mainly through mechanisms similar to those that are used during the early steps of tumour progression with tumour-secreted SIBLINGs continuing to act as enhancing factors.
Compelling evidence from cancer cell transfection experiments and mouse xenograft models demonstrate that high-level OPN expression can confer a metastatic phenotype on cells that originally formed benign tumours60. Since this initial observation, numerous studies conducted with human biological tissue from various cancer types consistently report that tumours that are likely to progress to more advanced stages present with de novo or increased expression of SIBLINGs (TABLE 1). In particular, the correlations observed between high levels of OPN expression in tumour cells and their subsequent metastatic dissemination were supported by gain- and loss-of-function studies demonstrating the pro-metastatic role of OPN (for a review see REF. 61). Thus, the introduction of an OPN expression vector into non-metastatic rat mammary epithelial cells resulted in lung metastasis development in 55% of the inoculated animals that produced primary tumours60 and the antisense inhibition of OPN inhibited osteolytic metastases of human breast cancer cells62. Hostproduced OPN also appeared to be of importance for metastasis development, as melanoma cells that do not express OPN showed reduced lung and bone metastases when injected into OPN-deficient mice compared with wild-type mice63.
Table 1.
SIBLING expression in normal and malignant human tissues as well as correlation with disease progression
| Organ/tissue | OPN | BSP | DMP1 | DSPP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| normal* | Tumour‡ | Prog’n§ | normal | Tumour | Prog’n | normal | Tumour | Prog’n | normal | Tumour | Prog’n | |
| Bladder | Low164 | High179 | Yes180 | ND | High158 | ND | ND | ND | ND | ND | ND | ND |
| Bone | High164 | High181 | No181 | High182 | High183 | Yes183 | High184 | ND | ND | Low185 | ND | ND |
| Brain | Low179 | High186 | Yes186 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Breast | Low164 | High187 | Yes188 | Low64 | High64 | Yes135 | Low121 | High121 | Yes121 | ND | ND | ND |
| Cervix | Low164 | High189 | Yes127 | Low116 | High116 | Yes116 | ND | ND | ND | ND | ND | ND |
| Colon | Low164 | High107 | Yes190 | Low54 | High54 | ND | Low54 | High54 | ND | Low54 | Low54 | ND |
| Connective tissue | No164 | High191 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Gastric | No164 | High192 | Yes143 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Oral mucosa | No119 | High119 | Yes119 | No119 | High119 | No119 | No119 | No119 | No119 | No119 | High119 | Yes119 |
| Kidney | High15,164 | High193 | Yes126 | High15 | ND | ND | High15 | ND | ND | High15 | ND | ND |
| Bone marrow | No194 | High195 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Lung | No164 | High196 | Yes133 | Low54 | High66 | Yes136 | Low54,120 | High54,120 | ND | Low54 | High54 | ND |
| Lymphocytes | Low197 | High106 | Yes198 | ND | High67 | ND | ND | ND | ND | ND | ND | ND |
| Melanocytes | No199 | High200 | Yes200 | ND | High118 | ND | ND | ND | ND | ND | ND | ND |
| Oesophagus | ND | High179 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ovary | No164 | High201 | Yes125 | Low54 | Low66 | ND | ND | ND | ND | ND | ND | ND |
| Pancreas | No164 | High146 | Yes45 | Low117 | High117 | ND | ND | ND | ND | ND | ND | ND |
| Prostate | No164 | High202 | Yes203 | No54 | High65 | Yes65 | No54 | ND | ND | Low2 | High2 | Yes2 |
| Rectum | ND | High204 | Yes204 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Thyroid | No179 | High132 | ND | No115 | High115 | ND | Low54 | High54 | ND | ND | ND | ND |
Defines expression of the designated small integrin-binding ligand N-linked glycoprotein (SIBLING) at the protein or mRNA level in normal tissue.
Defines expression of the designated SIBLING at the protein or mRNA level in primary malignant lesions.
Defines a positive and significant association between the level of expression of the designated SIBLING in the primary malignant lesions and the subsequent development of distant metastases and/or poor disease survival.
Note that the association was negative for dentin matrix acidic phosphoprotein 1 (DMP1) expression in breast cancer and disease progression.
BSP, bone sialoprotein; DSPP, dentin sialophosphoprotein; ND, not determined; OPN, osteopontin; Prog’n, progression.
High expression of SIBLINGs is associated with osteotropic cancers including breast64, prostate65 and lung66 as well as multiple myeloma67. A recent microarray and functional genomic study in an experimental mouse model demonstrated a functional association between OPN, interleukin 11 and either chemokine receptor 4 (CXCR4) or connective tissue growth factor (CTGF) in breast cancer cells that favours bone metastasis68. Overexpression of BSP also enhanced experimental bone metastasis69. The affinity of BSP-expressing cancer cells for bone is emphasized in a study where the transfection of IBSP cDNA into a brain-metastasizing breast cancer cell line subclone was sufficient to induce bone metastases, although no bone lesions were observed with the control line70.
The expression of SIBLINGs by breast and prostate carcinoma prompted the hypothesis that osteotropic cancer cells can become ‘bone-like’ or express osteomimetic properties that favour ‘seeding’ in the skeleton by improving their adhesion, proliferation and/or survival in bone. It was shown that during the malignant transformation of prostate epithelium, a switch of gene expression that confers an osteoblastic phenotype (including expression of SIBLINGs) may occur71. Indeed, the expression of certain crucial transcription factors that are known to regulate the expression of bone-related genes, such as RUNX2 and MSX, is altered in prostate cancer cells in a way that favours the acquisition of an osteoblast-like profile by these cells (see below for details). The expression of ‘bone’ proteins by cancer cells does not necessarily target cell metastasis to bone, rather it is more likely that the expression of transcription factors regulating SIBLING genes such as RUNX2 produces a mesenchymal phenotype that finds in bone a fertile soil for survival. More recently, Notch signalling and ERK activation have been shown to be important for the osteomimetic properties of prostate cancer bone metastatic cell lines72. In good agreement with the osteomimicry theory, a parallel between the gene expression profiles of human breast cancer cells with a high propensity to metastasize to bone and differentiating osteoblast cells was revealed73. Interestingly, the osteomimicry gene expression profile observed in osteotropic breast cancer cells is comprised not only of SIBLINGs but also of other proteins that are associated with the acquisition of an osteoblastic phenotype, including core-binding factor β (CBFβ, a RUNX co-transcription factor) and the osteoblastic cell–cell adhesion protein cadherin 11 (CDH11)73.
The known biological activities of OPN and BSP support their role in promoting metastases to the bone, and to other organs. It is likely that future explorations will identify SIBLINGs as essential regulators of the metastatic phenotype. This phenotype is presumably influenced by stromal and inflammatory cells that are closely associated with primary and metastatic tumours. Identifying the specific roles of SIBLINGs in cancer–stroma interactions and signalling cascades involving growth factor–growth factor receptor and cell–matrix interactions could result in the development of additional novel and refined strategies for the prevention and treatment of metastases. Tumour cell survival (at both primary and distant sites) requires successful counter-responses to immune surveillance.
Inflammation and complement evasion
The interplay between inflammation and cancer is currently an area of intense research74. Inflammation is part of the innate immune system and can provide an immediate, although non-specific, response. SIBLINGs can have roles in immune cell migration into sites of matrix turnover and degradation as well as in infection and inflammation. One paradigm for SIBLING function and metabolism within the immune system is that OPN is secreted by activated macrophages, leukocytes and activated T lymphocytes75–77 and is also a chemotactic cytokine for macrophages78, dendritic cells27 and neutrophils79. It is possible that OPN expression by tumours actually promotes inflammation-induced cancer growth and progression through, for example, the promotion of macrophage and neutrophil infiltration. Tumour cells that secrete OPN might be propagating chronic inflammation, which can accelerate transformation and tumour progression. OPN might also enhance tumour survival by downregulating macrophage nitric oxide synthase expression and downstream production of nitric oxide80. Cytokines can also alter SIBLING levels. Studies have reported that both OPN and DSPP are upregulated by the macrophage inflammatory protein MIP3α (also known as CCL20), a chemokine involved in modulating cell-mediated immunity81, which is known to promote pancreatic cancer cell migration82. The overall action of OPN on the immune system is to regulate the function of macrophage and macrophage-derived cells (that is, osteoclasts). The expression and potential biological activities of the other SIBLINGs in immune cells have not been studied; however, given their adhesive properties and other shared biochemical properties with OPN, it is possible that they have similar as yet undiscovered modulatory roles in inflammation.
Another component of the innate immune response is the complement system, which is composed of about 26 proteins that combine with antibodies and/or cell surface molecules as part of the humoral response. The complement cascade has a role in inflammation, immune adherence, opsonization, viral neutralization, cell lysis and localization of antigen83. Nearly all cells are subject to constant low levels of complement attack, but only cells that do not express the correct cell surface proteins, thereby inactivating the early steps of the cascade, are killed. CFH is a major negative regulatory factor that quenches complement- mediated lysis. Cells that become transformed may escape the complement system during their transit through the patient’s circulation by upregulating genes that help to control this aspect of immune surveillance. As such, the expression of SIBLINGs (specifically OPN, DMP1 and BSP) by tumour cells might provide such a selective advantage for survival by mediating the binding of CFH to the cell surface through integrins and/or CD44. The activated CFH then inhibits the formation of the membrane attack complex and subsequent cell lysis (FIG. 2d). In vitro experiments have demonstrated that these three SIBLINGs can protect murine and human cancer cell lines from attack by complement84–86.
Angiogenesis
Angiogenesis promotes tumour growth as well as metastatic spread through a complex interplay of positive and negative mediators of extracellular matrix degradation and endothelial cell and vasculature recruitment. There is evidence that αvβ3 is a key angiogenesisassociated receptor that is significantly upregulated on the surface of activated endothelial cells87. OPN and BSP have been shown to act as pro-angiogenic factors and, based on their RGD motifs, it is likely that the other SIBLINGs may also interact with αvβ3 integrin and influence the behaviour of endothelial cells. OPN contributes to the genesis of new capillaries infiltrating the cancer lesion in several in vivo models88,89. BSP also promotes angiogenesis in the chicken chorioallantoic membrane assay through binding αvβ3 (REF. 90).
The integrin αvβ3 mediates the migration of activated endothelial cells during vessel formation. SIBLINGs, as ligands for αvβ3 through the RGD sequence, may stimulate endothelial cell migration. It is also possible that SIBLING modulation of protease activity (uPA or MMP) generates bioactive fragments of extracellular matrix components responsible for angiogenesis. Experimental evidence suggests that antagonizing the ligation of SIBLINGs to integrins is a promising approach for the inhibition of angiogenesis and associated tumour growth. For example, blocking the interaction between OPN and αvβ3 inhibits angiogenesis and stops lung cancer growth in mice88. The αvβ3 integrin was shown to be important for OPN-mediated NFκB induction and survival, as adding a neutralizing anti-β3 integrin antibody blocked NFκB activity and induced endothelial cell death when cells were plated on OPN26. A recent study demonstrates that OPN triggers vascular endothelial growth factor-dependent breast tumour growth and angiogenesis by autocrine and paracrine mechanisms91. Thus, it is possible that, through their interaction with αvβ3, the SIBLINGs may also cooperate with molecules that have important biological functions during angiogenesis and tumoural growth processes, including MMPs, growth factors and their receptors.
Microcalcification
All SIBLINGs are expressed by bones and teeth, and it was originally thought that they acted to directly regulate hydroxyapatite crystal formation92. Outside of the skeletal system, pathological dystrophic calcification associated with the upregulation of OPN and/or BSP has been observed. Notable among these are atherosclerotic vascular plaques, renal osteodystrophy and kidney stones93. Because of their earlier association with mineralization in bone, the expression of BSP and OPN has been studied in cancers such as breast and thyroid carcinomas, in which ectopic calcification occurs64,94,95. Although calcifications are usually associated with benign lesions, certain patterns of calcification — such as tight clusters with irregular shapes — may indicate the presence of a premalignant tumour. Tumours from the primary sites of bone-seeking cancers frequently contain foci of dystrophic calcifications in the form of hydroxyapatite microcalcifications. The cause(s) of these ectopic calcifications are ill-defined and the exact role of the SIBLINGs in the formation of such calcifications is not known. Although it was initially thought that mineral deposition might occur because of the increased local concentration of SIBLINGs (which have well-described characteristics of nucleators of mineralization) it has also been reported that OPN actually blocks ectopic calcification96. It is also possible that SIBLING association with ectopic calcification primarily controls and diminishes the immune and inflammatory response that is provoked by inappropriate crystal deposition.
Nevertheless, SIBLING-expressing tumours are more readily detectable clinically at early stages, on the basis of associated abnormal mammographic calcifications. Most of the breast calcifications detected at mammography are benign. Radiologists must be able to identify typically benign breast calcifications that do not require biopsy to prevent unnecessary procedures and to reduce patient anxiety. It will be interesting to determine whether the high expression of SIBLINGs that is associated with the detection of suspicious microcalcifications will help to identify lesions that are likely to evolve towards malignancy. The expression of SIBLINGs in premalignant lesions has not yet been investigated and is an interesting field of investigation to fully understand the role of these proteins during cancer progression.
Regulation of SIBLING genes in cancer cells The promoter regions of SPP1, IBSP, DMP1 and DSPP have been cloned in different species and they exhibit a number of consensus regulatory sequences, such as potential binding sites for AP1 and NFκB transcription factors. Regulation of SIBLING genes has been best studied in the context of osteoblastic and odontoblastic cell differentiation (BOX 1). RUNX2, a member of the RUNX transcription factor family, is a transcription factor that is crucial for the regulation of genes that support bone formation97 and as such it is involved in the control of OPN98, BSP99, DMP1 (REF. 100) and DSPP101 expression. All the RUNX proteins are intimately associated with tumour progression, invasion and metastasis102. Notably, RUNX2 is aberrantly expressed at high levels in breast and prostate tumours and cells that metastasize to the bone103. Interestingly, RUNX2 also transactivates SPP1 (REF. 104) and IBSP105 in breast cancer cells, suggesting that the expression of SIBLINGs might be subject to the same regulation both in normal osteoblasts and in cancer cells. Human myeloma cells with active RUNX2 protein produce OPN that is involved in the pathophysiology of multiple myeloma-induced angiogenesis106. In colorectal cancer, gene-profiling studies identified a positive correlation between metastatic colon tumours and increased OPN expression107. More recently, RUNX2 and ETS1 were identified as crucial transcriptional regulators of OPN expression in a murine colorectal cancer cell line and their suppression using antisense oligonucleotides resulted in significant downregulation of OPN108. Several additional signalling pathways and transcription factors that are associated with cancer progression regulate OPN expression in models of breast cancer, melanoma and leukaemia (for reviews see REFs 4,61). These include AP1, MYC, OCT1 (also known as POU2F1), upstream stimulating factor (USF), v-Src, transforming growth factor β (TGFβ)–BMP–SMAD–HOX (homeobox), WNT–β catenin–adenomatous polyposis coli (APC)– glycogen synthase kinase 3 (GSK3)–transcription factor 4 (TCF4), Ras–Ras response factor (RRF) and p53. The global picture of OPN gene transcriptional regulation in cancer cells is that of an intricate regulatory network. Studies of the proximal promoter regions of other SIBLING genes are needed to identify regulatory elements that could be responsible for their overexpression in cancer.
Box 1 Expression and distribution of SIBLINGs in normal tissues
With the exception of osteopontin (OPN), which was independently discovered in several tissues, the distribution of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family in normal tissues was originally believed to be limited to bones and teeth92. In these calcified tissues, the presumed function of the family was a role in the biomineralization of matrix and a number of published in vitro studies do support such a role (for a review see REF. 159). With one exception, the dentin of dentin sialophosphoprotein (Dspp)-null mice160, all of the SIBLING gene knockout mouse models have little if any significant change in basic matrix mineralization. Early reports indicated that OPN is also a component of human breast milk161, and is expressed in chronic inflammatory cells162 and kidney163, as well as some other epithelia164. Terasawa et al.165 reported the expression of dentin matrix protein 1 (DMP1) in several mouse soft tissues including liver, muscle, brain, pancreas and kidney. Matrix extracellular phosphoglycoprotein (MEPE) was originally discovered in tumours causing osteomalacia and was shown at that time to be expressed (mRNA only) predominantly in bone and brain with “very low levels of expression” in lung, kidney and placenta122. Recent studies have demonstrated that all five members of the SIBLING family are expressed in metabolically active ductal epithelial cells of the salivary gland and kidney14,15 and all but MEPE were expressed in eccrine sweat ducts16. MEPE expression appears to be limited to ductal cells that actively transport phosphate16. The role of the SIBLINGs in normal soft tissues is now a subject of intense investigation. SIBLINGs expression can also be transcriptionally regulated (see table). BSP, bone sialoprotein; DLX5, distalless homeobox 5; FRE, fibroblast growth factor response element; HDAC3, histone deacetylase 3; HOX, homeobox; PPARγ, peroxisome proliferator-activated receptor γ.
Consistent with its role in tumour initiation and progression, SPP1 expression is also repressed by tumour suppressors such as BRCA1 and phosphatase and tensin homologue (PTEN), and metastasis suppressors such as breast cancer metastasis suppressor 1 (BRMS1). BRCA1 expression inhibits SPP1 promoter transactivation and hence suppresses OPN expression109. A BRCA1 mutation in human primary breast cancer lesions is associated with OPN overexpression, suggesting that it may confer increased tissue-specific cancer risk, in part, by disruption of the suppression of OPN transcription by BRCA1 (REF. 109). The tumour suppressor PTEN antagonizes PI3K, which is responsible for promoting cell growth, survival and tumorigenesis, when over-stimulated in cancer cells. OPN was shown to act downstream of the PI3K pathway in melanoma and glioma cancer cells. A link has been found between OPN expression at both the mRNA and protein level that involves PI3K activation of OPN and may help explain how PTEN loss contributes to the development of these malignancies110,111. By contrast, BRMS1 inhibits OPN expression through the inactivation of NFκB and subsequent binding to the SPP1 promoter. Thus, downregulation of OPN might be one of the mechanisms of metastasis suppression by BRMS1 (REF. 112).
SIBLINGs as prognostic indicators in cancer
The first SIBLING found to be overexpressed in cancer was OPN113,114. Since then, large numbers of studies have established that increased expression of OPN is a consistent feature for most known human malignancies (TABLE 1). Increased expression of BSP was originally observed in human breast cancer64 and its putative role in the acquisition of an osteotropic phenotype by metastatic cancer cells soon led to the extension of this observation to other bone-seeking cancers such as prostate65, lung66, thyroid115 and cervical carcinoma116, as well as multiple myeloma67 (TABLE 1). Recently, BSP was detected in pancreas117, skin118 and oral119 carcinomas, a group of neoplastic lesions that are not particularly osteotropic when they metastasize. These observations suggest that, as is the case for OPN, BSP expression in cancer cells is not restricted to cancer cells metastasizing to bone but is a common feature of the malignant phenotype. The expression profile in cancer of the three other SIBLINGs — DMP1, DSPP and MEPE — has not been evaluated in detail to date, but data indicate that DMP1 and DSPP are upregulated in several human malignancies54. Immunohistochemical studies demonstrated increased expression of DSPP in human prostate2 and oral119 cancers, and high levels of DMP1 were observed in human lung120 and breast121 cancers. It is expected — based on the detection of DMP1 and DSPP in a variety of human malignancies compared with their normal corresponding tissues on a mRNA–cDNA array54 — that future studies will verify high expression of these proteins as a consistent feature of most human cancers (TABLE 1). By contrast, MEPE expression seems to be much more restricted than that of the other SIBLINGs, so far being found to be expressed in normal cells associated with phosphate transport16. Interestingly, only tumours that result in oncogenic hypophosphataemic osteomalacia appear to express MEPE122. Screening of MEPE expression at the mRNA level in a collection of human normal and cancer tissues revealed minimal expression in all tissues analysed54. This observation suggests that MEPE, unlike other SIBLINGs, does not intervene during cancer progression. The reasons for this are still unclear. Enhanced expression of SIBLINGs is not only associated with several tumour types, but their levels of expression are also often directly correlated to specific stages of clinical progression. Gene expression analyses have identified SPP1 to be among the most strongly upregulated genes in human colon cancer123. In this study, OPN expression was shown to be an independent prognostic marker for poor overall survival. These observations were supported by a recent study that found that colon cancer patients with tumours expressing high levels of OPN have significantly reduced survival124. In another study, OPN was shown to be a predictor of outcome that was independent of clinical characteristics (such as age, lesion size and histological type) in ovarian carcinoma. The prognosis for survival within 36 months was <5% for patients with increased OPN and 75% for those with no OPN increase125. Clear cell renal carcinoma patients with OPNpositive tumours also exhibited worse prognosis than patients who had tumours lacking OPN126. The prognosis for patients with increased OPN in cervical cancers is also extremely poor, as none survived within 24 months, compared with the 67% survival rate observed within the same time period for patients with no OPN increase127. Other cancers with a positive correlation between OPN expression and poor prognosis are breast128, prostate129, head and neck130,131, thyroid132, non-small cell lung133 and hepatocellular134 carcinomas (TABLE 1).
Increased BSP expression in primary breast135 and prostate65 carcinoma is also associated with tumour progression. In non-small cell lung carcinoma, BSP expression is associated with bone metastasis development and could be useful in identifying high-risk patients who could benefit from novel modalities of surveillance and preventive treatment136. Expression of other SIBLINGs might also correlate with tumour progression. DSPP expression correlates with aggressiveness in human prostate cancers2 and in oral cancer119. unique among the SIBLINGs, DMP1 expression was inversely associated with progression in human breast cancer121. The positive prognostic value of DMP1 for breast cancer patients has only been reported in one study and awaits confirmation. Small interfering RNA (siRNA)-mediated repression of DMP1 enhances migration of human breast cancer cells in vitro121. Thus, it can be speculated that the expression of DMP1 alters cancer cell motility and hence reduces local invasion and metastatic spreading. This effect could be achieved through a competition of DMP1 with BSP and/or OPN for their binding to the cell membrane integrin receptors and the subsequent activation of their corresponding MMP partner. Such putative mechanisms urge investigations of whether modulation of SIBLING expression might differentially affect cancer cell behaviour.
SIBLINGs can also be detected in the blood, and it is therefore not surprising that several studies have established a correlation between blood levels of OPN and BSP and the presence of a malignant tumour. However, the high affinity interaction of CFH with several SIBLINGs, including OPN and BSP84, masks all known antibodybinding sites and therefore interferes with accurate direct measurement of these proteins in the serum137. Interestingly, this masking implies that the biological activities of OPN and BSP might be limited to autocrine and/or paracrine effects as the abundant (0.5 mg/ml in blood) complement protein will quickly bind and inactivate them as they diffuse away from their sites of secretion and action. OPN, however, is interesting because 5–10% of the total amount of this SIBLING in the blood escapes the masking by CFH by mechanisms that are currently unknown. Through careful preparation of plasma, this fraction of OPN has been successfully used in many studies, as mentioned below, but when serum is analysed this fraction is masked by CFH. One confounding factor for the use of blood OPN levels is that inflammation also increases OPN levels138. In patients with bone metastases, increased plasma (not serum) levels of OPN have been suggested to be the result of both cancer cell secretion and accelerated bone turnover34. Similar to increased expression in tumour cells themselves, increased OPN plasma levels appears to be a marker for metastatic progression and poor survival in patients with breast139, prostate140, renal cell141, lung142, gastric143, head and neck144, hepatocellular145, cervical127 and pancreatic cancers146. Serum BSP (after disruption of the SIBLING–CFH complex) is significantly increased in patients with colon, breast, prostate and lung cancer137. BSP blood level also predicts bone metastasis development in patients with breast cancer147; predicts tumour burden, neoplastic bone involvement and prognosis in multiple myeloma148; and is an independent prognostic factor for human prostate cancer-related death149. No data on the blood levels of DMP1, DSPP or MEPE in human malignancies are available. Although detection of increased blood levels of OPN and BSP bear obvious diagnostic and prognostic value, such tests are not yet available to clinicians.
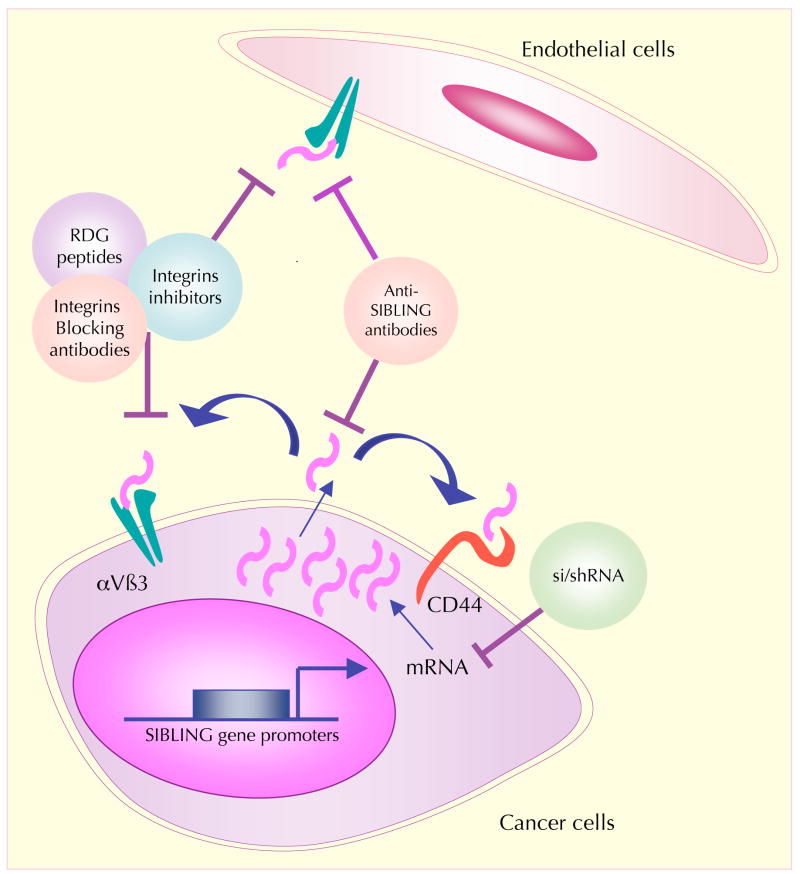
SIBLINGs as therapeutic targets Because of the plausible roles of SIBLINGs in cancer as pro-oncogenic, pro-metastatic and pro-angiogenic molecules, these proteins also have the potential to be valuable targets for cancer therapy (FIG. 4).
Figure 4. SIBLINGs and their cell receptors are potential therapeutic targets for cancer therapy.
Suppression of the expression of small integrin-binding ligand N-linked glycoproteins (SIBLINGs) in cancer cells at the level of mRNA can be accomplished through RNA interference by the use of specific small interfering RNAs (siRNAs) or short hairpin RNAs (shRNAs). Blocking of tumour-derived SIBLINGs at the protein level by specific blocking peptides and antibodies reduces tumour progression and metastatic dissemination because it affects interactions of SIBLINGs with their receptors on the cancer cell surface. This might result in the dysfunction of signalling pathways that affect tumour cell proliferation and survival. Because SIBLINGs exert their effects principally through integrins and CD44, antibody-mediated interference with these receptor– ligand interactions or suppression of associated signal transduction events are other potential means to restrain tumour progression. Inhibition of the binding of SIBLINGs to endothelial cell surface integrin receptors triggers endothelial cell apoptosis and can thereby decrease tumour-associated angiogenesis.
Several studies have already demonstrated the value of OPN and BSP as therapeutic targets in preclinical animal models. Silencing these SIBLINGs using siRNA and short hairpin RNA (shRNA) technology, or interfering with their activities using specific antibodies, inhibits or reduces tumour progression and the development of metastases. Decreasing expression of OPN in human squamous oesophagus carcinoma using shRNA reduces tumour growth and lymph node metastases in vivo150. Similar experiments performed on murine colon cancer cells led to the inhibition of tumour growth and the formation of liver metastases151. Silencing of OPN or BSP using specific antisense oligonucleotides in a human breast cancer cell line resulted in a significant decrease of osteolytic bone metastases in nude rats62. Although the use of RNA interference for therapy is attractive, much work remains before such therapeutics become available to patients152.
Antibody-based anticancer therapies, such as antibodies directed against vascular endothelial growth factor, have recently met with clinical success and are now becoming available to cancer patients153. Antibodies directed against OPN were effective in inhibiting development of lung metastases in nude mice inoculated with human hepatocellular carcinoma cells. In this case, OPN, which has been identified as a major gene in the signature for hepatocellular carcinoma, acts as both a diagnostic marker and therapeutic target for metastatic dissemination154. Anti-BSP antibodies also have therapeutic potential particularly for the prevention and treatment of breast cancer bone metastases, as suggested by the significant reduction of osteolytic lesion size in a nude rat model of human breast cancer bone metastases155.
Both integrins and CD44 have well-established roles in tumour progression. Therefore, interfering with these receptor–ligand interactions by controlling receptor cell surface expression, blocking receptor–ligand binding or suppressing associated signal transduction are promising ways to block both tumour development and metastatic dissemination (FIG. 4). CD44 has been targeted by diverse therapeutic strategies, including cytotoxic and immunotherapeutic approaches156. Because of its involvement in many processes that accompany tumour development and metastatic dissemination of cancers, αvβ3 integrin has long been a candidate target for cancer therapy by specific antibodies, peptide inhibitors and non-peptide antagonists that mimic the binding domain of physiological ligands156,157. Proof of principle that such strategies block angiogenesis, as well as tumour growth and dissemination, has been obtained in several animal models and small molecule inhibitors of this receptor are under study as drug candidates.
Finally, the ectopic expression of BSP in osteotropic neoplasms recently inspired a gene therapy protocol in bladder cancer using a conditional-replicating adenovirus. A truncated IBSP promoter controlling the E1A/B lytic-regulating sequence was used to construct the adenovirus AD-BSP-E1A. This virus had lytic activity on human bladder cancer cell lines and significantly reduced the size of bladder tumours in an orthotopic mouse model, opening a promising new strategy for the treatment of aggressive yet sensitive bladder tumours158.
From the results so far, one can speculate that SIBLINGs (particularly OPN and BSP) are viable targets that seem likely to form the basis of new anticancer therapies in the future.
Conclusions and future directions
The aim of this Review is to present an overview of some of the data that implicate the SIBLING family at several steps of cancer development and progression. Although OPN is the family member for which there is the most abundant and convincing data of its role as a key player at most of the critical steps in the evolution of malignancies, studies have revealed that BSP holds the same multifunctional role in cancer biology. Such activities are expected to be associated with DSPP and DMP1 also.
Much remains to be learned about the involvement of SIBLINGs in cancer progression, and we hope this Review will inspire cancer researchers to look more closely at this family. Future investigations should validate the use of SIBLINGs as prognostic markers in large population studies, determine their value as surrogate markers for the prediction of metastases in cancer patients and explore their potential as predictive indicators of the patient response to a given therapy (chemotherapy and/or radiotherapy). Studies are also needed to test the potential value of SIBLINGs as markers for the difficult diagnosis of precancerous lesions such as those found in breast, prostate, colon and oral tumours. Refining our knowledge of the mechanisms involved in how SIBLINGs modulate MMPs (in the absence and/or presence of natural or synthetic MMP inhibitors) could lead to the development of potential biomimetics for use in interventions. It is also of interest to determine the biological relevance of the interactions between SIBLINGs and CFH. For therapeutic strategies, the potential synergy of the combined repression of two or more SIBLINGs should also be tested.
SIBLINGs have the biological plausibility to have active roles in tumour cell adhesion, proliferation, invasion, matrix degradation, immune functions (inflammation and complement evasion), angiogenesis and metastasis. Based on the data accumulated so far, it is tempting to speculate that one of the major roles for SIBLINGs and their proteolytic fragments is to orchestrate, in the near proximity of cancer cells, a dynamically changing microenvironment that supports the key local steps that need to be temporally and spatially coordinated for successful invasion. These include adhesion through interactions with specific integrins, matrix degradation through localization and perhaps activation of MMPs, and migration through activation of selective signalling pathways. Such multifunctional activities are possible thanks to the abilities of SIBLINGs to bind multiple proteins. It is also likely that SIBLINGs are crucial for tumour growth and metastasis because they may participate in angiogenesis. The possible collaboration and/or competition between SIBLINGs during these processes remain to be elucidated by future research. Although acquisition of new data is crucial, the results to date make a case for the future of SIBLINGs as prominent molecular tools for diagnostic, prognostic and therapeutic applications in cancer.
At a glance
Small integrin-binding ligand N-linked glycoproteins (SIBLINGs) are a family of glycophosphoproteins comprising osteopontin (OPN), bone sialoprotein (BSP), dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP) and matrix extracellular phosphoglycoprotein (MEPE).
The genes encoding the SIBLINGs are located within a cluster on chromosome 4 and encode soluble, hydrophilic proteins sharing common functional motifs and domains, including an Arg–Gly–Asp (RGD) motif that binds αvβ3 integrin.
SIBLINGs were initially described as mineralized tissue-associated glycophosphoproteins and were thought to be functionally restricted to these tissues. Recent results show that they are more widely distributed and are expressed in nonmineralized normal tissue, such as metabolically active ductal epithelial cells.
Some SIBLINGs activate specific metalloproteinases (MMPs; BSP activates MMP2, OPN, MMP3 and DMP1, MMP9). These three SIBLINGs also bind complement factor H and prevent complement attack.
SIBLINGs are overexpressed in many cancers. OPN and, less so, BSP are by far the more widely studied to date and their levels of expression are correlated with tumour aggressiveness. SIBLINGs can be detected in the blood and their level of expression is associated with prognosis.
Among SIBLINGs, OPN is involved in almost all steps of tumour progression, including invasion, metastasis and angiogenesis.
In vitro and in vivo experimental models demonstrated that interference with SIBLINGs, such as small interfering RNA selective knockdown, has potential anticancer therapeutic value.
Identifying the specific roles of SIBLINGs in cancer–stroma interactions and signalling cascades involving growth factor–growth factor receptor and cell–matrix interactions could result in the development of additional and refined strategies for the prevention and treatment of metastases.
Acknowledgments
The work of A.B. and V.C. is supported by grants from the National Fund for Scientific Research (Belgium), the Centre Anti-Cancéreux of the University of Liège and the European Commission through METABRE Contract CEE LSHC-CT-2004-503049. The work of K.O. is supported by National Institute of Dental and Craniofacial Research/National Institutes of Health (NIH) Grant K23 017791- 01A1, Medical College of Georgia Research Institute Grant STP 00105W005, and the Wendy Will Case Cancer Fund. The research of L.W.F. is supported by the Intramural Research Program of the NIH, Department of Health and Human Services. The work of N.S.F. is supported by grants from the National Cancer Institute (R21CA87311 and R01 CA113865) and from the Department of Defense Congressionally Directed Medical Research Program (W81XWH-04-1-0844 and BC010478).
Glossary
- Integrin
Integrins are a large family of heterodimeric cell surface adhesion receptors that bind extracellular matrix and cell surface ligands. They promote stable interactions between cells and their environment and mediate intracellular signalling
- Dentin
The main, calcareous part of a tooth, beneath the enamel and surrounding the pulp chamber and root canals
- RGD motif
A tripeptide, Arg–Gly–Asp (RGD), found in numerous proteins that support cell adhesion. A subset of the integrins recognize the RGD motif within their ligands, the binding of which mediates both cell–substratum and cell–cell interactions
- Hydroxyapatite crystals
The principal inorganic constituent of bone matrix and teeth, imparting rigidity to these structures, and consisting of hydrated calcium phosphate, Ca5(PO4)3OH
- Metabolically active normal duct
Epithelia, such as that of the kidney nephrons, that alter the tonicity of the fluid they process in the course of normal physiology. The kidney nephrons process isotonic urine into the voided hypotonic urine
- Type 0 introns
Introns that disrupt an open reading frame between codon junctions and therefore permit any splicing combination to other type 0 exons without causing frameshifts
- Metabolically passive normal duct
Epithelia, such as that of the lacrimal gland ducts, that do not alter the tonicity of the fluid they process in the course of normal physiology. Tears from the lacrimal acini are isotonic and are secreted unchanged through the duct system
- CD44
A family of cell surface signal transducing glycoproteins involved in cell–cell interactions, cell adhesion and migration. CD44s bind hyaluronan, a high-molecular mass polysaccharide found in the extracellular matrix, and a variety of extracellular as well as cell surface ligands. CD44 exists in multiple spliced forms and shows a high variability in glycosylation
- Transglutaminases
A family of enzymes that catalyse the crosslinking of proteins at a glutamine in one chain with lysine in another chain. Although the family members have different structures, they share an active site (Tyr–Gly–Gln–Cys–Trp) and strict Ca2+ dependence
- Osteoclast
A cell that breaks down mineralized bone and is responsible for bone resorption
- Dental pulp cells
Cells that comprise the soft tissue forming the inner structure of a tooth and containing nerves and blood vessels as well as possibly dentin stem cells
- Osteotropic
Describes tumours that metastasize preferentially to the skeleton
- Bone lesions
Lytic lesions are areas of the bone marked by destruction, whereas sclerotic lesions are areas of the bone marked by thickening or hardening. A mixed lytic and sclerotic lesion exhibits facets of both resorption (destruction) and thickening (formation)
- Opsonization
The process whereby opsonins (antibodies or complement proteins) make an invading cell or microorganism more susceptible to phagocytosis by binding to its surface
- Chicken chorioallantoic membrane assay
A biological assay using the well-vascularized chorioallantoic membrane of the chicken egg to evaluate the biological activity of pro- and anti-angiogenic factors
- Renal osteodystrophy
A bone disease characterized by softening and fibrous degeneration of bone and the formation of cysts in bone tissue, caused by chronic renal failure
- Eccrine sweat ducts
These ducts transport sweat to the surface of the skin and are involved in evaporative cooling
- Oncogenic hypophosphataemic osteomalacia
Osteomalacia (softening of the bones) resulting from renal phosphate wasting and low serum 1,25-dihydroxy vitamin D secondary to the presence of a tumour of which complete resection results in rapid resolution of the symptoms and signs
Contributor Information
Akeila Bellahcène, Metastasis Research Laboratory, University of Liège, Belgium.
Vincent Castronovo, Metastasis Research Laboratory, University of Liège, Belgium.
Kalu U. E. Ogbureke, Department of Oral Biology and Maxillofacial Pathology, Medical College of Georgia, Augusta, GA, USA
Larry W. Fisher, Craniofacial and Skeletal Diseases Branch, DIR, NIDCR, NIH, Bethesda, MD, USA
Neal S. Fedarko, Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
References
- 1.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. Like many other proteins that have multiple binding partners, BSP and OPN are shown by NMR to be unstructured and flexible in solution. The SIBLING family is first defined here based on common exon and intron elements. [DOI] [PubMed] [Google Scholar]
- 2.Chaplet M, et al. Expression of dentin sialophosphoprotein in human prostate cancer and its correlation with tumor aggressiveness. Int J Cancer. 2006;118:850–856. doi: 10.1002/ijc.21442. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, et al. Bone sialoprotein promotes tumor cell migration in both in vitro and in vivo models. Connect Tissue Res. 2003;44(Suppl 1):279–284. [PubMed] [Google Scholar]
- 4.El-Tanani MK, et al. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–474. doi: 10.1016/j.cytogfr.2006.09.010. This is a detailed overview of OPN-mediated cell signalling in relation to cancer progression. [DOI] [PubMed] [Google Scholar]
- 5.Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001;1:621–632. doi: 10.2174/1566524013363339. [DOI] [PubMed] [Google Scholar]
- 6.Sung V, Stubbs JT, 3rd, Fisher L, Aaron AD, Thompson EW. Bone sialoprotein supports breast cancer cell adhesion proliferation and migration through differential usage of the αvβ3 and αvβ5 integrins. J Cell Physiol. 1998;176:482–494. doi: 10.1002/(SICI)1097-4652(199809)176:3<482::AID-JCP5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Tatusova TA, Madden TL. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. [DOI] [PubMed] [Google Scholar]
- 8.Fisher LW, Whitson SW, Avioli LV, Termine JD. Matrix sialoprotein of developing bone. J Biol Chem. 1983;258:12723–12727. [PubMed] [Google Scholar]
- 9.George A, Sabsay B, Simonian PA, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- 10.Feng JQ, et al. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem. 1998;273:9457–9464. doi: 10.1074/jbc.273.16.9457. [DOI] [PubMed] [Google Scholar]
- 11.Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg–Gly–Asp cell-binding sequence. Proc Natl Acad Sci USA. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317:59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellahcene A, Castronovo V. Expression of bone matrix proteins in human breast cancer: potential roles in microcalcification formation and in the genesis of bone metastases. Bull Cancer. 1997;84:17–24. [PubMed] [Google Scholar]
- 14.Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–670. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- 15.Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs) Kidney Int. 2005;68:155–166. doi: 10.1111/j.1523-1755.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 16.Ogbureke KU, Fisher LW. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J Histochem Cytochem. 2007;55:403–409. doi: 10.1369/jhc.6A7075.2007. [DOI] [PubMed] [Google Scholar]
- 17.Furger KA, et al. β3 integrin expression increases breast carcinoma cell responsiveness to the malignancy-enhancing effects of osteopontin. Mol Cancer Res. 2003;1:810–819. [PubMed] [Google Scholar]
- 18.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Teramoto H, et al. Autocrine activation of an osteopontin-CD44–Rac pathway enhances invasion and transformation by H-RasV12. Oncogene. 2005;24:489–501. doi: 10.1038/sj.onc.1208209. [DOI] [PubMed] [Google Scholar]
- 20.Agnihotri R, et al. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276:28261–28267. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- 21.Prince CW, Dickie D, Krumdieck CL. Osteopontin, a substrate for transglutaminase and factor XIII activity. Biochem Biophys Res Commun. 1991;177:1205–1210. doi: 10.1016/0006-291x(91)90669-x. [DOI] [PubMed] [Google Scholar]
- 22.Higashikawa F, Eboshida A, Yokosaki Y. Enhanced biological activity of polymeric osteopontin. FEBS Lett. 2007;581:2697–2701. doi: 10.1016/j.febslet.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Valverde P, Tu Q, Chen J. BSP and RANKL induce osteoclastogenesis and bone resorption synergistically. J Bone Miner Res. 2005;20:1669–1679. doi: 10.1359/JBMR.050511. [DOI] [PubMed] [Google Scholar]
- 24.Hur EM, et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 25.Iczkiewicz J, Jackson MJ, Smith LA, Rose S, Jenner P. Osteopontin expression in substantia nigra in MPTP-treated primates and in Parkinson’s disease. Brain Res. 2006;1118:239–250. doi: 10.1016/j.brainres.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 26.Rice J, Courter DL, Giachelli CM, Scatena M. Molecular mediators of αvβ3-induced endothelial cell survival. J Vasc Res. 2006;43:422–436. doi: 10.1159/000094884. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura K, et al. Differentiation, maturation, and survival of dendritic cells by osteopontin regulation. Clin Diagn Lab Immunol. 2005;12:206–212. doi: 10.1128/CDLI.12.1.206-212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JL, et al. Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: OPN–CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007;67:2089–2097. doi: 10.1158/0008-5472.CAN-06-3625. [DOI] [PubMed] [Google Scholar]
- 29.Brakebusch C, Hirsch E, Potocnik A, Fassler R. Genetic analysis of β1 integrin function: confirmed, new and revised roles for a crucial family of cell adhesion molecules. J Cell Sci. 1997;110:2895–2904. doi: 10.1242/jcs.110.23.2895. [DOI] [PubMed] [Google Scholar]
- 30.Katagiri YU, et al. CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine– glycine–aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- 31.Christensen B, et al. Cell type-specific posttranslational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem. 2007;282:19463–19472. doi: 10.1074/jbc.M703055200. [DOI] [PubMed] [Google Scholar]
- 32.Pecheur I, et al. Integrin αvβ3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 2002;16:1266–1268. doi: 10.1096/fj.01-0911fje. [DOI] [PubMed] [Google Scholar]
- 33.van der Pluijm G, et al. Bone sialoprotein peptides are potent inhibitors of breast cancer cell adhesion to bone. Cancer Res. 1996;56:1948–1955. [PubMed] [Google Scholar]
- 34.Standal T, et al. Osteopontin is an adhesive factor for myeloma cells and is found in increased levels in plasma from patients with multiple myeloma. Haematologica. 2004;89:174–182. [PubMed] [Google Scholar]
- 35.Kulkarni GV, Chen B, Malone JP, Narayanan AS, George A. Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1. Arch Oral Biol. 2000;45:475–484. doi: 10.1016/s0003-9969(00)00010-8. [DOI] [PubMed] [Google Scholar]
- 36.Sharp JA, Waltham M, Williams ED, Henderson MA, Thompson EW. Transfection of MDA-MB-231 human breast carcinoma cells with bone sialoprotein (BSP) stimulates migration and invasion in vitro and growth of primary and secondary tumors in nude mice. Clin Exp Metastasis. 2004;21:19–29. doi: 10.1023/b:clin.0000017167.17065.61. [DOI] [PubMed] [Google Scholar]
- 37.Khodavirdi AC, et al. Increased expression of osteopontin contributes to the progression of prostate cancer. Cancer Res. 2006;66:883–888. doi: 10.1158/0008-5472.CAN-05-2816. [DOI] [PubMed] [Google Scholar]
- 38.Angelucci A, et al. Osteopontin enhances the cell proliferation induced by the epidermal growth factor in human prostate cancer cells. Prostate. 2004;59:157–166. doi: 10.1002/pros.20008. [DOI] [PubMed] [Google Scholar]
- 39.Lecrone V, Li W, Devoll RE, Logothetis C, Farach-Carson MC. Calcium signals in prostate cancer cells: specific activation by bone-matrix proteins. Cell Calcium. 2000;27:35–42. doi: 10.1054/ceca.1999.0083. [DOI] [PubMed] [Google Scholar]
- 40.Bell WR. The fibrinolytic system in neoplasia. Semin Thromb Hemost. 1996;22:459–478. doi: 10.1055/s-2007-999047. [DOI] [PubMed] [Google Scholar]
- 41.Price JT, Thompson EW. Mechanisms of tumour invasion and metastasis: emerging targets for therapy. Expert Opin Ther Targets. 2002;6:217–233. doi: 10.1517/14728222.6.2.217. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi C, et al. Serum osteopontin, an enhancer of tumor metastasis to bone, promotes B16 melanoma cell migration. J Cell Biochem. 2007;101:979–986. doi: 10.1002/jcb.21298. [DOI] [PubMed] [Google Scholar]
- 43.Khan SA, et al. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: novel post-transcriptional, post-translational regulation. Clin Exp Metastasis. 2005;22:663–673. doi: 10.1007/s10585-006-9007-0. [DOI] [PubMed] [Google Scholar]
- 44.Caers J, et al. The involvement of osteopontin and its receptors in multiple myeloma cell survival, migration and invasion in the murine 5T33MM model. Br J Haematol. 2006;132:469–477. doi: 10.1111/j.1365-2141.2005.05886.x. This paper describes possible roles of OPN interactions with integrins and CD44 variants in survival, increasing cell proliferation, inhibiting apoptosis and promoting cell migration, as well as metalloproteinase activity in multiple myeloma cells. It used blocking antibodies (against CD44 and/or αvβ3) to block migration. [DOI] [PubMed] [Google Scholar]
- 45.Kolb A, et al. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol Ther. 2005;4:740–746. doi: 10.4161/cbt.4.7.1821. [DOI] [PubMed] [Google Scholar]
- 46.Hu Z, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res. 2005;11:4646–4652. doi: 10.1158/1078-0432.CCR-04-2013. [DOI] [PubMed] [Google Scholar]
- 47.Tuck AB, et al. Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene. 1999;18:4237–4246. doi: 10.1038/sj.onc.1202799. [DOI] [PubMed] [Google Scholar]
- 48.Mi Z, Guo H, Wai PY, Gao C, Kuo PC. Integrin-linked kinase regulates osteopontindependent MMP-2 and uPA expression to convey metastatic function in murine mammary epithelial cancer cells. Carcinogenesis. 2006;27:1134–1145. doi: 10.1093/carcin/bgi352. [DOI] [PubMed] [Google Scholar]
- 49.Das R, Mahabeleshwar GH, Kundu GC. Osteopontin induces AP-1-mediated secretion of urokinase-type plasminogen activator through c-Src-dependent epidermal growth factor receptor transactivation in breast cancer cells. J Biol Chem. 2004;279:11051–11064. doi: 10.1074/jbc.M310256200. [DOI] [PubMed] [Google Scholar]
- 50.Teti A, et al. Activation of MMP-2 by human GCT23 giant cell tumour cells induced by osteopontin, bone sialoprotein and GRGDSP peptides is RGD and cell shape change dependent. Int J Cancer. 1998;77:82–93. doi: 10.1002/(sici)1097-0215(19980703)77:1<82::aid-ijc14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 51.Rangaswami H, Bulbule A, Kundu GC. Nuclear factor inducing kinase: a key regulator in osteopontininduced MAPK/IκB kinase dependent NF-κB-mediated promatrix metalloproteinase-9 activation. Glycoconj J. 2006;23:221–232. doi: 10.1007/s10719-006-7927-1. [DOI] [PubMed] [Google Scholar]
- 52.Rangaswami H, Kundu GC. Osteopontin stimulates melanoma growth and lung metastasis through NIK/MEKK1-dependent MMP-9 activation pathways. Oncol Rep. 2007;18:909–915. [PubMed] [Google Scholar]
- 53.Fedarko NS, Jain A, Karadag A, Fisher LW. Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J. 2004;18:734–736. doi: 10.1096/fj.03-0966fje. This paper shows that specific SIBLINGs bind MMPs as well as release them from inhibition by TIMPs and low-molecular-weight inhibitors. CFH blocks formation of the SIBLING–MMP complexes and can inactivate previously formed complexes and therefore potentially acts as a negative regulator of many SIBLING functions. [DOI] [PubMed] [Google Scholar]
- 54.Fisher LW, Jain A, Tayback M, Fedarko NS. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10:8501–8511. doi: 10.1158/1078-0432.CCR-04-1072. This paper explores SIBLINGs expression in various cancer types on cancer patient microarray. The degree of correlation between a SIBLING and its partner MMP expression within a given cancer type was also addressed. [DOI] [PubMed] [Google Scholar]
- 55.Karadag A, Ogbureke KU, Fedarko NS, Fisher LW. Bone sialoprotein, matrix metalloproteinase 2, and αvβ3 integrin in osteotropic cancer cell invasion. J Natl Cancer Inst. 2004;96:956–965. doi: 10.1093/jnci/djh169. [DOI] [PubMed] [Google Scholar]
- 56.Karadag A, Fedarko NS, Fisher LW. Dentin matrix protein 1 enhances invasion potential of colon cancer cells by bridging matrix metalloproteinase-9 to integrins and CD44. Cancer Res. 2005;65:11545–11552. doi: 10.1158/0008-5472.CAN-05-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takafuji V, Forgues M, Unsworth E, Goldsmith P, Wang XW. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene. 2007;26:6361–6371. doi: 10.1038/sj.onc.1210463. [DOI] [PubMed] [Google Scholar]
- 58.Mangala LS, Arun B, Sahin AA, Mehta K. Tissue transglutaminase-induced alterations in extracellular matrix inhibit tumor invasion. Mol Cancer. 2005;4:33. doi: 10.1186/1476-4598-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 60.Oates AJ, Barraclough R, Rudland PS. The identification of osteopontin as a metastasis-related gene product in a rodent mammary tumour model. Oncogene. 1996;13:97–104. [PubMed] [Google Scholar]
- 61.Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res. 2004;121:228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 62.Adwan H, Bauerle TJ, Berger MR. Downregulation of osteopontin and bone sialoprotein II is related to reduced colony formation and metastasis formation of MDA-MB-231 human breast cancer cells. Cancer Gene Ther. 2004;11:109–120. doi: 10.1038/sj.cgt.7700659. This was the first paper to demonstrate that downregulating the expression of OPN or BSP in cancer cells inhibits the formation of bone metastases in vivo. [DOI] [PubMed] [Google Scholar]
- 63.Nemoto H, et al. Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J Bone Miner Res. 2001;16:652–659. doi: 10.1359/jbmr.2001.16.4.652. [DOI] [PubMed] [Google Scholar]
- 64.Bellahcene A, Merville MP, Castronovo V. Expression of bone sialoprotein, a bone matrix protein, in human breast cancer. Cancer Res. 1994;54:2823–2826. The first immunohistochemical demonstration of ectopic BSP expression in human breast cancer tumours. [PubMed] [Google Scholar]
- 65.Waltregny D, et al. Prognostic value of bone sialoprotein expression in clinically localized human prostate cancer. J Natl Cancer Inst. 1998;90:1000–1008. doi: 10.1093/jnci/90.13.1000. [DOI] [PubMed] [Google Scholar]
- 66.Bellahcene A, et al. Expression of bone sialoprotein in human lung cancer. Calcif Tissue Int. 1997;61:183–188. doi: 10.1007/s002239900320. [DOI] [PubMed] [Google Scholar]
- 67.Bellahcene A, et al. Bone sialoprotein mRNA and protein expression in human multiple myeloma cell lines and patients. Br J Haematol. 2000;111:1118–1121. doi: 10.1046/j.1365-2141.2000.02506.x. [DOI] [PubMed] [Google Scholar]
- 68.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 69.Zhang JH, et al. Over-expression of bone sialoprotein enhances bone metastasis of human breast cancer cells in a mouse model. Int J Oncol. 2003;23:1043–1048. [PubMed] [Google Scholar]
- 70.Zhang JH, et al. Bone sialoprotein promotes bone metastasis of a non-bone-seeking clone of human breast cancer cells. Anticancer Res. 2004;24:1361–1368. [PubMed] [Google Scholar]
- 71.Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39:246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. A detailed study of the ‘osteomimicry hypothesis’ to explain the propensity of prostate cancer cells to grow and establish in the bone microenvironment. [DOI] [PubMed] [Google Scholar]
- 72.Zayzafoon M, Abdulkadir SA, McDonald JM. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem. 2004;279:3662–3670. doi: 10.1074/jbc.M308158200. [DOI] [PubMed] [Google Scholar]
- 73.Bellahcene A, et al. Transcriptome analysis reveals an osteoblast-like phenotype for human osteotropic breast cancer cells. Breast Cancer Res Treat. 2007;101:135–148. doi: 10.1007/s10549-006-9279-8. [DOI] [PubMed] [Google Scholar]
- 74.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 75.McKee MD, Nanci A. Secretion of Osteopontin by macrophages and its accumulation at tissue surfaces during wound healing in mineralized tissues: a potential requirement for macrophage adhesion and phagocytosis. Anat Rec. 1996;245:394–409. doi: 10.1002/(SICI)1097-0185(199606)245:2<394::AID-AR19>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 76.O’Regan AW, et al. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro. J Immunol. 1999;162:1024–1031. [PubMed] [Google Scholar]
- 77.Weber GF, Cantor H. The immunology of −1/osteopontin. Cytokine Growth Factor Rev. 1996;7:241–248. doi: 10.1016/s1359-6101(96)00030-5. [DOI] [PubMed] [Google Scholar]
- 78.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- 79.Koh A, et al. Role of osteopontin in neutrophil function. Immunology. 2007;122:466–475. doi: 10.1111/j.1365-2567.2007.02682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wai PY, et al. Osteopontin inhibits macrophage nitric oxide synthesis to enhance tumor proliferation. Surgery. 2006;140:132–140. doi: 10.1016/j.surg.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Shiba H, et al. Macrophage inflammatory protein-3α and β-defensin-2 stimulate dentin sialophosphoprotein gene expression in human pulp cells. Biochem Biophys Res Commun. 2003;306:867–871. doi: 10.1016/s0006-291x(03)01075-1. [DOI] [PubMed] [Google Scholar]
- 82.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Volanakis JE. In: The Human Complement System in Health and Disease. Volankis JE, Frank MM, editors. Marcel Dekker; New York: 1998. pp. 9–32. [Google Scholar]
- 84.Fedarko NS, Fohr B, Robey PG, Young MF, Fisher LW. Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. J Biol Chem. 2000;275:16666–16672. doi: 10.1074/jbc.M001123200. [DOI] [PubMed] [Google Scholar]
- 85.Jain A, Karadag A, Fohr B, Fisher LW, Fedarko NS. Three SIBLINGs (small integrin-binding ligand, N-linked glycoproteins) enhance factor H’s cofactor activity enabling MCP-like cellular evasion of complement-mediated attack. J Biol Chem. 2002;277:13700–13708. doi: 10.1074/jbc.M110757200. [DOI] [PubMed] [Google Scholar]
- 86.Nam JS, et al. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor β in a mouse model of breast cancer. Cancer Res. 2006;66:6327–6335. doi: 10.1158/0008-5472.CAN-06-0068. This paper reports a direct association between TGFβ pro-metastatic activity and BSP expression in breast cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 88.Cui R, et al. Abrogation of the interaction between osteopontin and αvβ3 integrin reduces tumor growth of human lung cancer cells in mice. Lung Cancer. 2007;57:302–310. doi: 10.1016/j.lungcan.2007.03.019. A demonstration in a mouse model that disruption of the interaction of osteopontin with the αvβ3 intergin has therapeutic potential to diminish tumour progression. [DOI] [PubMed] [Google Scholar]
- 89.Tang H, et al. Inhibition of osteopontin would suppress angiogenesis in gastric cancer. Biochem Cell Biol. 2007;85:103–110. doi: 10.1139/o06-208. [DOI] [PubMed] [Google Scholar]
- 90.Bellahcene A, et al. Bone sialoprotein mediates human endothelial cell attachment and migration and promotes angiogenesis. Circ Res. 2000;86:885–891. doi: 10.1161/01.res.86.8.885. The first study to show BSP has angiogenesispromoting activity using in vitro and in vivo assays. [DOI] [PubMed] [Google Scholar]
- 91.Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factordependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152–161. doi: 10.1158/0008-5472.CAN-07-2126. [DOI] [PubMed] [Google Scholar]
- 92.Robey PG. Vertebrate mineralized matrix proteins: structure and function. Connect Tissue Res. 1996;35:131–136. doi: 10.3109/03008209609029183. [DOI] [PubMed] [Google Scholar]
- 93.Giachelli CM. Inducers and inhibitors of biomineralization: lessons from pathological calcification. Orthod Craniofac Res. 2005;8:229–231. doi: 10.1111/j.1601-6343.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 94.Carlinfante G, et al. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin Exp Metastasis. 2003;20:437–444. doi: 10.1023/a:1025419708343. [DOI] [PubMed] [Google Scholar]
- 95.Tunio GM, Hirota S, Nomura S, Kitamura Y. Possible relation of osteopontin to development of psammoma bodies in human papillary thyroid cancer. Arch Pathol Lab Med. 1998;122:1087–1090. [PubMed] [Google Scholar]
- 96.Steitz SA, et al. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99:1233–1239. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- 98.Sato M, et al. Transcriptional regulation of osteopontin gene in vivo by PEBP2αA/CBFA1 and ETS1 in the skeletal tissues. Oncogene. 1998;17:1517–1525. doi: 10.1038/sj.onc.1202064. [DOI] [PubMed] [Google Scholar]
- 99.Javed A, et al. Runt homology domain transcription factors (RUNX, CBFA, and AML) mediate repression of the bone sialoprotein promoter: evidence for promoter context-dependent activity of Cbfa proteins. Mol Cell Biol. 2001;21:2891–2905. doi: 10.1128/MCB.21.8.2891-2905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fen JQ, et al. Dentin matrix protein 1, a target molecule for CBFA1 in bone, is a unique bone marker gene. J Bone Miner Res. 2002;17:1822–1831. doi: 10.1359/jbmr.2002.17.10.1822. [DOI] [PubMed] [Google Scholar]
- 101.Chen S, et al. Differential regulation of dentin sialophosphoprotein expression by RUNX2 during odontoblast cytodifferentiation. J Biol Chem. 2005;280:29717–29727. doi: 10.1074/jbc.M502929200. [DOI] [PubMed] [Google Scholar]
- 102.Blyth K, Cameron ER, Neil JC. The RUNX genes: gain or loss of function in cancer. Nature Rev Cancer. 2005;5:376–387. doi: 10.1038/nrc1607. [DOI] [PubMed] [Google Scholar]
- 103.Pratap J, et al. Regulatory roles of RUNX2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev. 2006;25:589–600. doi: 10.1007/s10555-006-9032-0. [DOI] [PubMed] [Google Scholar]
- 104.Inman CK, Shore P. The osteoblast transcription factor RUNX2 is expressed in mammary epithelial cells and mediates osteopontin expression. J Biol Chem. 2003;278:48684–48689. doi: 10.1074/jbc.M308001200. [DOI] [PubMed] [Google Scholar]
- 105.Barnes GL, et al. Osteoblast-related transcription factors RUNX2 (CBFA1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 2003;63:2631–2637. [PubMed] [Google Scholar]
- 106.Colla S, et al. Human myeloma cells express the bone regulating gene RUNX2/CBFA1 and produce osteopontin that is involved in angiogenesis in multiple myeloma patients. Leukemia. 2005;19:2166–2176. doi: 10.1038/sj.leu.2403976. [DOI] [PubMed] [Google Scholar]
- 107.Agrawal D, et al. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst. 2002;94:513–521. doi: 10.1093/jnci/94.7.513. [DOI] [PubMed] [Google Scholar]
- 108.Wai PY, et al. ETS-1 and RUNX2 regulate transcription of a metastatic gene, osteopontin, in murine colorectal cancer cells. J Biol Chem. 2006;281:18973–18982. doi: 10.1074/jbc.M511962200. [DOI] [PubMed] [Google Scholar]
- 109.El-Tanani MK, et al. BRCA1 suppresses osteopontin-mediated breast cancer. J Biol Chem. 2006;281:26587–26601. doi: 10.1074/jbc.M604403200. [DOI] [PubMed] [Google Scholar]
- 110.Packer L, et al. Osteopontin is a downstream effector of the PI3-kinase pathway in melanomas that is inversely correlated with functional PTEN. Carcinogenesis. 2006;27:1778–1786. doi: 10.1093/carcin/bgl016. [DOI] [PubMed] [Google Scholar]
- 111.Kim MS, et al. Hyaluronic acid induces osteopontin via the phosphatidylinositol 3-kinase/Akt pathway to enhance the motility of human glioma cells. Cancer Res. 2005;65:686–691. [PubMed] [Google Scholar]
- 112.Samant RS, et al. Breast cancer metastasis suppressor 1 (BRMS1) inhibits osteopontin transcription by abrogating NFκB activation. Mol Cancer. 2007;6:6. doi: 10.1186/1476-4598-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Senger D, Asch B, Smith B, Perruzzi C, Dvorak H. A secreted phosphoprotein marker for neoplastic transformation of both epithelial and fibroblastic cells. Nature. 1983;302:714–715. doi: 10.1038/302714a0. [DOI] [PubMed] [Google Scholar]
- 114.Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16:885–893. doi: 10.1016/0092-8674(79)90103-x. [DOI] [PubMed] [Google Scholar]
- 115.Bellahcene A, et al. Ectopic expression of bone sialoprotein in human thyroid cancer. Thyroid. 1998;8:637–641. doi: 10.1089/thy.1998.8.637. [DOI] [PubMed] [Google Scholar]
- 116.Detry C, et al. Detection of bone sialoprotein in human (pre)neoplastic lesions of the uterine cervix. Calcif Tissue Int. 2003;73:9–14. doi: 10.1007/s00223-002-2108-0. [DOI] [PubMed] [Google Scholar]
- 117.Kayed H, et al. Effects of bone sialoprotein on pancreatic cancer cell growth, invasion and metastasis. Cancer Lett. 2007;245:171–183. doi: 10.1016/j.canlet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 118.Riminucci M, et al. Coexpression of bone sialoprotein (BSP) and the pivotal transcriptional regulator of osteogenesis, CBFA1/RUNX2, in malignant melanoma. Calcif Tissue Int. 2003;73:281–289. doi: 10.1007/s00223-002-2134-y. [DOI] [PubMed] [Google Scholar]
- 119.Ogbureke KU, et al. Up-regulation of SIBLING proteins and correlation with cognate MMP expression in oral cancer. Oral Oncol. 2007;43:920–932. doi: 10.1016/j.oraloncology.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 120.Chaplet M, et al. Dentin matrix protein 1 is expressed in human lung cancer. J Bone Miner Res. 2003;18:1506–1512. doi: 10.1359/jbmr.2003.18.8.1506. [DOI] [PubMed] [Google Scholar]
- 121.Bucciarelli E, et al. Low dentin matrix protein 1 expression correlates with skeletal metastases development in breast cancer patients and enhances cell migratory capacity in vitro. Breast Cancer Res Treat. 2007;105:95–104. doi: 10.1007/s10549-006-9436-0. [DOI] [PubMed] [Google Scholar]
- 122.Rowe PS, et al. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 123.Agrawal D, et al. Osteopontin identified as colon cancer tumor progression marker. C R Biol. 2003;326:1041–1043. doi: 10.1016/j.crvi.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 124.Rohde F, et al. Expression of osteopontin, a target gene of de-regulated Wnt signaling, predicts survival in colon cancer. Int J Cancer. 2007 doi: 10.1002/ijc.22868. [DOI] [PubMed] [Google Scholar]
- 125.Bao LH, Sakaguchi H, Fujimoto J, Tamaya T. Osteopontin in metastatic lesions as a prognostic marker in ovarian cancers. J Biomed Sci. 2007;14:373–381. doi: 10.1007/s11373-006-9143-1. [DOI] [PubMed] [Google Scholar]
- 126.Matusan K, Dordevic G, Stipic D, Mozetic V, Lucin K. Osteopontin expression correlates with prognostic variables and survival in clear cell renal cell carcinoma. J Surg Oncol. 2006;94:325–331. doi: 10.1002/jso.20447. [DOI] [PubMed] [Google Scholar]
- 127.Sakaguchi H, Fujimoto J, Hong BL, Tamaya T. Clinical implications of osteopontin in metastatic lesions of uterine cervical cancers. Cancer Lett. 2007;247:98–102. doi: 10.1016/j.canlet.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 128.Rudland PS, et al. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62:3417–3427. This paper demonstrates on a large collection of breast cancer specimens that OPN expression levels are an independent prognostic indicator that correlates with reduced patient survival. [PubMed] [Google Scholar]
- 129.Forootan SS, et al. Prognostic significance of osteopontin expression in human prostate cancer. Int J Cancer. 2006;118:2255–2261. doi: 10.1002/ijc.21619. [DOI] [PubMed] [Google Scholar]
- 130.Bache M, et al. Immunohistochemical detection of osteopontin in advanced head-and-neck cancer: prognostic role and correlation with oxygen electrode measurements, hypoxia-inducible-factor-1a-related markers, and hemoglobin levels. Int J Radiat Oncol Biol Phys. 2006;66:1481–1487. doi: 10.1016/j.ijrobp.2006.07.1376. [DOI] [PubMed] [Google Scholar]
- 131.Celetti A, et al. Overexpression of the cytokine osteopontin identifies aggressive laryngeal squamous cell carcinomas and enhances carcinoma cell proliferation and invasiveness. Clin Cancer Res. 2005;11:8019–8027. doi: 10.1158/1078-0432.CCR-05-0641. [DOI] [PubMed] [Google Scholar]
- 132.Guarino V, et al. Osteopontin is overexpressed in human papillary thyroid carcinomas and enhances thyroid carcinoma cell invasiveness. J Clin Endocrinol Metab. 2005;90:5270–5278. doi: 10.1210/jc.2005-0271. [DOI] [PubMed] [Google Scholar]
- 133.Donati V, et al. Osteopontin expression and prognostic significance in non-small cell lung cancer. Clin Cancer Res. 2005;11:6459–6465. doi: 10.1158/1078-0432.CCR-05-0541. [DOI] [PubMed] [Google Scholar]
- 134.Pan HW, et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98:119–127. doi: 10.1002/cncr.11487. [DOI] [PubMed] [Google Scholar]
- 135.Bellahcene A, Menard S, Bufalino R, Moreau L, Castronovo V. Expression of bone sialoprotein in primary human breast cancer is associated with poor survival. Int J Cancer. 1996;69:350–353. doi: 10.1002/(SICI)1097-0215(19960822)69:4<350::AID-IJC19>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 136.Papotti M, et al. Bone sialoprotein is predictive of bone metastases in resectable non-small-cell lung cancer: a retrospective case-control study. J Clin Oncol. 2006;24:4818–4824. doi: 10.1200/JCO.2006.06.1952. This study identifies BSP expression as a predictive factor of bone metastasis development in patients with resectable NSCLC. [DOI] [PubMed] [Google Scholar]
- 137.Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7:4060–4066. [PubMed] [Google Scholar]
- 138.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 139.Bramwell VH, et al. Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res. 2006;12:3337–3343. doi: 10.1158/1078-0432.CCR-05-2354. [DOI] [PubMed] [Google Scholar]
- 140.Ramankulov A, Lein M, Kristiansen G, Loening SA, Jung K. Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate. 2007;67:330–340. doi: 10.1002/pros.20540. [DOI] [PubMed] [Google Scholar]
- 141.Ramankulov A, et al. Elevated plasma osteopontin as marker for distant metastases and poor survival in patients with renal cell carcinoma. J Cancer Res Clin Oncol. 2007;133:643–652. doi: 10.1007/s00432-007-0215-z. [DOI] [PubMed] [Google Scholar]
- 142.Chang YS, et al. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer. 2007;57:373–380. doi: 10.1016/j.lungcan.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 143.Wu CY, et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782–789. doi: 10.1136/gut.2006.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Petrik D, et al. Plasma osteopontin is an independent prognostic marker for head and neck cancers. J Clin Oncol. 2006;24:5291–5297. doi: 10.1200/JCO.2006.06.8627. [DOI] [PubMed] [Google Scholar]
- 145.Zhang H, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:709–717. doi: 10.1007/s00432-006-0119-3. [DOI] [PubMed] [Google Scholar]
- 146.Koopmann J, et al. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:487–491. [PubMed] [Google Scholar]
- 147.Diel IJ, et al. Serum bone sialoprotein in patients with primary breast cancer is a prognostic marker for subsequent bone metastasis. Clin Cancer Res. 1999;5:3914–3919. [PubMed] [Google Scholar]
- 148.Woitge HW, et al. Serum bone sialoprotein as a marker of tumour burden and neoplastic bone involvement and as a prognostic factor in multiple myeloma. Br J Cancer. 2001;84:344–351. doi: 10.1054/bjoc.2000.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jung K, et al. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J Cancer. 2004;111:783–791. doi: 10.1002/ijc.20314. [DOI] [PubMed] [Google Scholar]
- 150.Ito T, et al. An inducible short-hairpin RNA vector against osteopontin reduces metastatic potential of human esophageal squamous cell carcinoma in vitro and in vivo. Clin Cancer Res. 2006;12:1308–1316. doi: 10.1158/1078-0432.CCR-05-1611. [DOI] [PubMed] [Google Scholar]
- 151.Wai PY, et al. Osteopontin silencing by small interfering RNA suppresses in vitro and in vivo CT26 murine colon adenocarcinoma metastasis. Carcinogenesis. 2005;26:741–751. doi: 10.1093/carcin/bgi027. [DOI] [PubMed] [Google Scholar]
- 152.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nature Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Handb Exp Pharmacol. 2008;181:131–150. doi: 10.1007/978-3-540-73259-4_6. [DOI] [PubMed] [Google Scholar]
- 154.Ye QH, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nature Med. 2003;9:416–423. doi: 10.1038/nm843. This paper combines the demonstration that OPN is a diagnostic and prognostic marker for hepatocellular carcinoma and works as an effective target in antibody-based treatment. [DOI] [PubMed] [Google Scholar]
- 155.Bauerle T, et al. Characterization of a rat model with site-specific bone metastasis induced by MDA-MB-231 breast cancer cells and its application to the effects of an antibody against bone sialoprotein. Int J Cancer. 2005;115:177–186. doi: 10.1002/ijc.20840. [DOI] [PubMed] [Google Scholar]
- 156.Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta. 2001;1552:61–85. doi: 10.1016/s0304-419x(01)00037-3. [DOI] [PubMed] [Google Scholar]
- 157.Brooks PC, et al. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 158.Melquist JJ, et al. Conditionally replicating adenovirus-mediated gene therapy in bladder cancer: an orthotopic in vivo model. Urol Oncol. 2006;24:362–371. doi: 10.1016/j.urolonc.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 159.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 160.Sreenath T, et al. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 161.Senger DR, Perruzzi CA, Papadopoulos A, Tenen DG. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta. 1989;996:43–48. doi: 10.1016/0167-4838(89)90092-7. [DOI] [PubMed] [Google Scholar]
- 162.Patarca R, Wei FY, Singh P, Morasso MI, Cantor H. Dysregulated expression of the T cell cytokine Eta-1 in CD4–8– lymphocytes during the development of murine autoimmune disease. J Exp Med. 1990;172:1177–1183. doi: 10.1084/jem.172.4.1177. An early report on the role of OPN Eta-1 in immune dysregulation. Increased OPN expression was associated with a chronic immune response and autoimmune disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shiraga H, et al. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci USA. 1992;89:426–430. doi: 10.1073/pnas.89.1.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brown LF, et al. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992;3:1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Terasawa M, et al. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Miner Metab. 2004;22:430–438. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- 166.Shen Q, Christakos S. The vitamin D receptor, RUNX2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J Biol Chem. 2005;280:40589–40598. doi: 10.1074/jbc.M504166200. [DOI] [PubMed] [Google Scholar]
- 167.Kim HJ, et al. Okadaic acid stimulates osteopontin expression through de novo induction of AP-1. J Cell Biochem. 2002;87:93–102. doi: 10.1002/jcb.10280. [DOI] [PubMed] [Google Scholar]
- 168.Roca H, Phimphilai M, Gopalakrishnan R, Xiao G, Franceschi RT. Cooperative interactions between RUNX2 and homeodomain protein-binding sites are critical for the osteoblast-specific expression of the bone sialoprotein gene. J Biol Chem. 2005;280:30845– 30855. doi: 10.1074/jbc.M503942200. [DOI] [PubMed] [Google Scholar]
- 169.Hassan MQ, et al. HOXA10 controls osteoblastogenesis by directly activating bone regulatory and phenotypic genes. Mol Cell Biol. 2007;27:3337–3352. doi: 10.1128/MCB.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nakayama Y, et al. Insulin-like growth factor-I increases bone sialoprotein (BSP) expression through fibroblast growth factor-2 response element and homeodomain protein-binding site in the proximal promoter of the BSP gene. J Cell Physiol. 2006;208:326–335. doi: 10.1002/jcp.20664. [DOI] [PubMed] [Google Scholar]
- 171.Pang J, et al. Upregulation of dentin matrix protein 1 promoter activities by core binding factor α1 in human dental pulp stem cells. Biochem Biophys Res Commun. 2007;357:505–510. doi: 10.1016/j.bbrc.2007.03.188. [DOI] [PubMed] [Google Scholar]
- 172.Narayanan K, Ramachandran A, Hao J, George A. Transcriptional regulation of dentin matrix protein 1 (DMP1) by AP-1 (c-fos/c-jun) factors. Connect Tissue Res. 2002;43:365–371. doi: 10.1080/03008200290000592. [DOI] [PubMed] [Google Scholar]
- 173.Chen S, et al. Regulation of the cell type-specific dentin sialophosphoprotein gene expression in mouse odontoblasts by a novel transcription repressor and an activator CCAAT-binding factor. J Biol Chem. 2004;279:42182–42191. doi: 10.1074/jbc.M402476200. [DOI] [PubMed] [Google Scholar]
- 174.Oyama Y, Akuzawa N, Nagai R, Kurabayashi M. PPARγ ligand inhibits osteopontin gene expression through interference with binding of nuclear factors to A/T-rich sequence in THP-1 cells. Circ Res. 2002;90:348–355. doi: 10.1161/hh0302.105098. [DOI] [PubMed] [Google Scholar]
- 175.Lamour V, et al. Runx2- and histone deacetylase 3- mediated repression is relieved in differentiating human osteoblast cells to allow high bone sialoprotein expression. J Biol Chem. 2007;282:36240–36249. doi: 10.1074/jbc.M705833200. [DOI] [PubMed] [Google Scholar]
- 176.Galler KM, et al. A novel role for Twist-1 in pulp homeostasis. J Dent Res. 2007;86:951–955. doi: 10.1177/154405910708601007. [DOI] [PubMed] [Google Scholar]
- 177.Chen B, Piel WH, Gui L, Bruford E, Monteiro A. The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics. 2005;86:627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 178.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nature Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 179.Coppola D, et al. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10:184–190. doi: 10.1158/1078-0432.ccr-1405-2. [DOI] [PubMed] [Google Scholar]
- 180.Ang C, Chambers AF, Tuck AB, Winquist E, Izawa JI. Plasma osteopontin levels are predictive of disease stage in patients with transitional cell carcinoma of the bladder. BJU Int. 2005;96:803–805. doi: 10.1111/j.1464-410X.2005.05716.x. [DOI] [PubMed] [Google Scholar]
- 181.Sulzbacher I, Birner P, Trieb K, Lang S, Chott A. Expression of osteopontin and vascular endothelial growth factor in benign and malignant bone tumors. Virchows Arch. 2002;441:345–349. doi: 10.1007/s00428-002-0671-4. [DOI] [PubMed] [Google Scholar]
- 182.Cogan G, et al. Analysis of human bone sialoprotein in normal and pathological tissues using a monoclonal antibody (BSP 1.2 mab) Connect Tissue Res. 2004;45:60–71. doi: 10.1080/03008200490278151. [DOI] [PubMed] [Google Scholar]
- 183.Valabrega G, et al. ErbB2 and bone sialoprotein as markers for metastatic osteosarcoma cells. Br J Cancer. 2003;88:396–400. doi: 10.1038/sj.bjc.6600735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Feng JQ, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nature Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.MacDougall M, Dong J, Acevedo AC. Molecular basis of human dentin diseases. Am J Med Genet A. 2006;140:2536–2546. doi: 10.1002/ajmg.a.31359. [DOI] [PubMed] [Google Scholar]
- 186.Saitoh Y, Kuratsu J, Takeshima H, Yamamoto S, Ushio Y. Expression of osteopontin in human glioma. Its correlation with the malignancy. Lab Invest. 1995;72:55–63. [PubMed] [Google Scholar]
- 187.Bellahcene A, Castronovo V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol. 1995;146:95–100. [PMC free article] [PubMed] [Google Scholar]
- 188.Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1087–1097. doi: 10.1158/1055-9965.EPI-06-1008. [DOI] [PubMed] [Google Scholar]
- 189.Wong YF, et al. Genome-wide gene expression profiling of cervical cancer in Hong Kong women by oligonucleotide microarray. Int J Cancer. 2006;118:2461–2469. doi: 10.1002/ijc.21660. [DOI] [PubMed] [Google Scholar]
- 190.Eschrich S, et al. Molecular staging for survival prediction of colorectal cancer patients. J Clin Oncol. 2005;23:3526–3535. doi: 10.1200/JCO.2005.00.695. [DOI] [PubMed] [Google Scholar]
- 191.Gaumann A, et al. Osteopontin expression in primary sarcomas of the pulmonary artery. Virchows Arch. 2001;439:668–674. doi: 10.1007/s004280100452. [DOI] [PubMed] [Google Scholar]
- 192.Ue T, et al. Co-expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer. 1998;79:127–132. doi: 10.1002/(sici)1097-0215(19980417)79:2<127::aid-ijc5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 193.Matusan K, Dordevic G, Mozetic V, Lucin K. Expression of osteopontin and CD44 molecule in papillary renal cell tumors. Pathol Oncol Res. 2005;11:108–113. doi: 10.1007/BF02893377. [DOI] [PubMed] [Google Scholar]
- 194.Somerman MJ, Berry JE, Khalkhali-Ellis Z, Osdoby P, Simpson RU. Enhanced expression of av integrin subunit and osteopontin during differentiation of HL-60 cells along the monocytic pathway. Exp Cell Res. 1995;216:335–341. doi: 10.1006/excr.1995.1042. [DOI] [PubMed] [Google Scholar]
- 195.Flamant S, et al. Osteopontin is upregulated by BCRABL. Biochem Biophys Res Commun. 2005;333:1378– 1384. doi: 10.1016/j.bbrc.2005.05.203. [DOI] [PubMed] [Google Scholar]
- 196.Chambers AF, et al. Osteopontin expression in lung cancer. Lung Cancer. 1996;15:311–323. doi: 10.1016/0169-5002(95)00595-1. [DOI] [PubMed] [Google Scholar]
- 197.Nagai S, et al. Comprehensive gene expression profile of human activated TH1- and TH2-polarized cells. Int Immunol. 2001;13:367–376. doi: 10.1093/intimm/13.3.367. [DOI] [PubMed] [Google Scholar]
- 198.Saeki Y, et al. Enhanced production of osteopontin in multiple myeloma: clinical and pathogenic implications. Br J Haematol. 2003;123:263–270. doi: 10.1046/j.1365-2141.2003.04589.x. [DOI] [PubMed] [Google Scholar]
- 199.Geissinger E, Weisser C, Fischer P, Schartl M, Wellbrock C. Autocrine stimulation by osteopontin contributes to antiapoptotic signalling of melanocytes in dermal collagen. Cancer Res. 2002;62:4820–4828. [PubMed] [Google Scholar]
- 200.Zhou Y, et al. Osteopontin expression correlates with melanoma invasion. J Invest Dermatol. 2005;124:1044– 1052. doi: 10.1111/j.0022-202X.2005.23680.x. [DOI] [PubMed] [Google Scholar]
- 201.Tiniakos DG, Yu H, Liapis H. Osteopontin expression in ovarian carcinomas and tumors of low malignant potential (LMP) Hum Pathol. 1998;29:1250–1254. doi: 10.1016/s0046-8177(98)90253-2. [DOI] [PubMed] [Google Scholar]
- 202.Tozawa K, et al. Osteopontin expression in prostate cancer and benign prostatic hyperplasia. Urol Int. 1999;62:155–158. doi: 10.1159/000030381. [DOI] [PubMed] [Google Scholar]
- 203.Hotte SJ, Winquist EW, Stitt L, Wilson SM, Chambers AF. Plasma osteopontin: associations with survival and metastasis to bone in men with hormonerefractory prostate carcinoma. Cancer. 2002;95:506–512. doi: 10.1002/cncr.10709. [DOI] [PubMed] [Google Scholar]
- 204.Debucquoy A, et al. Molecular responses of rectal cancer to preoperative chemoradiation. Radiother Oncol. 2006;80:172–177. doi: 10.1016/j.radonc.2006.07.016. [DOI] [PubMed] [Google Scholar]