Abstract
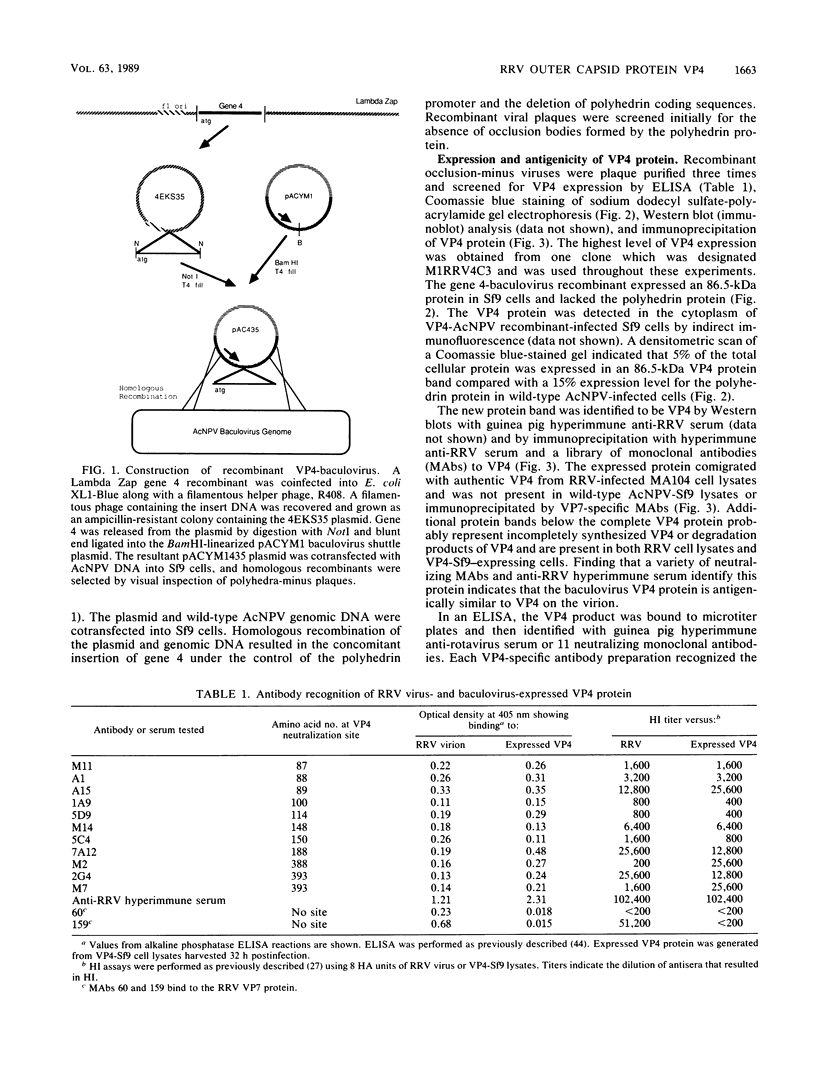
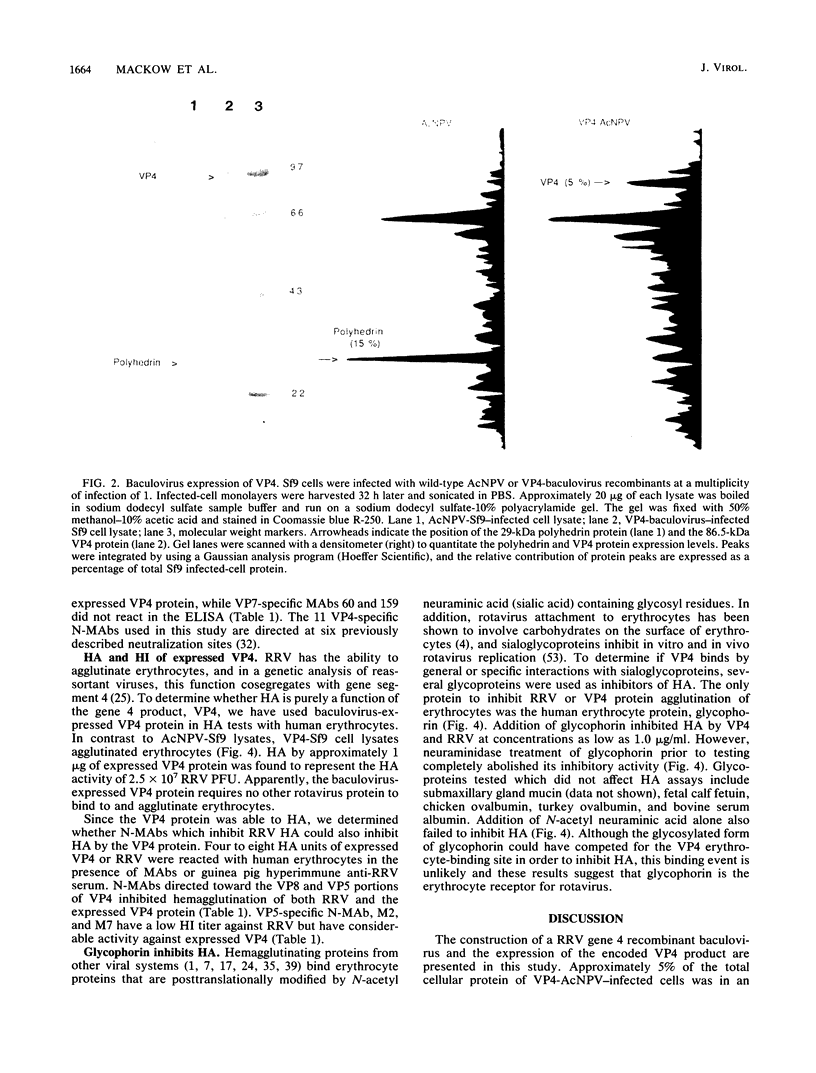
Rhesus rotavirus (RRV) gene 4 was cloned into lambda bacteriophage, inserted into a polyhedrin promoter shuttle plasmid, and expressed in Sf9 cells by a recombinant baculovirus. The baculovirus-expressed VP4 protein made up approximately 5% of the Spodoptera frugiperda-infected cell protein. Monoclonal antibodies that neutralize the virus bound to the expressed VP4 polypeptide, indicating that the expressed VP4 protein was antigenically indistinguishable from viral VP4. In addition, we have determined that the baculovirus-expressed VP4 protein bound to erythrocytes and functions as the RRV hemagglutinin. The endogenous hemagglutinating activity of the VP4 protein, like the virus, was inhibited by guinea pig antirotavirus hyperimmune serum and by VP4-specific neutralizing monoclonal antibodies. The human erythrocyte protein, glycophorin, also inhibited hemagglutination by RRV or the expressed VP4 protein and appears to be the rotavirus erythrocyte receptor. The baculovirus-expressed VP4 protein was conserved functionally and antigenically in the absence of other outer or inner capsid rotavirus components and represents a logical candidate for future immunological studies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Bond C. W. Biological properties of mengovirus: characterization of avirulent, hemagglutination-defective mutants. Arch Virol. 1987;93(1-2):31–49. doi: 10.1007/BF01313892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C. F., Lizano M., López S. Synthesis in Escherichia coli and immunological characterization of a polypeptide containing the cleavage sites associated with trypsin enhancement of rotavirus SA11 infectivity. J Gen Virol. 1987 Mar;68(Pt 3):633–642. doi: 10.1099/0022-1317-68-3-633. [DOI] [PubMed] [Google Scholar]
- Arias C. F., López S., Espejo R. T. Gene protein products of SA11 simian rotavirus genome. J Virol. 1982 Jan;41(1):42–50. doi: 10.1128/jvi.41.1.42-50.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastardo J. W., Holmes I. H. Attachment of SA-11 rotavirus to erythrocyte receptors. Infect Immun. 1980 Sep;29(3):1134–1140. doi: 10.1128/iai.29.3.1134-1140.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., Zakai N., Loyter A. Active function of membrane receptors for enveloped viruses. I. Specific requirement for liposome-associated sialoglycolipids, but not sialoglycoproteins, to allow lysis of phospholipid vesicles by reconstituted Sendai viral envelopes. Exp Cell Res. 1986 Oct;166(2):279–294. doi: 10.1016/0014-4827(86)90477-5. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Lazdins I., Tregear G. W., Holmes I. H. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc Natl Acad Sci U S A. 1986 May;83(10):3465–3468. doi: 10.1073/pnas.83.10.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Myslinski J., Kalica A. R., Greenberg H. B., Wyatt R. G., Kapikian A. Z., Chanock R. M. In vitro transcription of two human rotaviruses. J Virol. 1982 Sep;43(3):1032–1037. doi: 10.1128/jvi.43.3.1032-1037.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T., Ishida N. Evidence for endocytosis-independent infection by human rotavirus. Arch Virol. 1987;97(1-2):93–99. doi: 10.1007/BF01310737. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Lapidot M., Zakai N., Loyter A. Protein blot analysis of virus receptors: identification and characterization of the Sendai virus receptor. Biochim Biophys Acta. 1986 Mar 27;856(1):19–26. doi: 10.1016/0005-2736(86)90004-0. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Saif L. J., Sereno M. M., Chanock R. M., Kapikian A. Z. Infection immunity of piglets to either VP3 or VP7 outer capsid protein confers resistance to challenge with a virulent rotavirus bearing the corresponding antigen. J Virol. 1988 Mar;62(3):744–748. doi: 10.1128/jvi.62.3.744-748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. C., Nestorowicz A. Antigenic determinants of influenza virus hemagglutinin. XI. Conformational changes detected by monoclonal antibodies. Virology. 1985 Aug;145(1):72–83. doi: 10.1016/0042-6822(85)90202-8. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., James J. D., Jr, Kapikian A. Z. Hemagglutination by simian rotavirus. J Clin Microbiol. 1978 Mar;7(3):314–315. doi: 10.1128/jcm.7.3.314-315.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaljot K. T., Shaw R. D., Rubin D. H., Greenberg H. B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988 Apr;62(4):1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Benfield D. A., Greenberg H. B. Characterization of homotypic and heterotypic VP7 neutralization sites of rhesus rotavirus. Virology. 1988 Aug;165(2):511–517. doi: 10.1016/0042-6822(88)90595-8. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Inouye S. Purification of an outer capsid glycoprotein of neonatal calf diarrhea virus and preparation of its antisera. Infect Immun. 1983 Jan;39(1):155–158. doi: 10.1128/iai.39.1.155-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Nagata L., Masri S. A., Pon R. T., Lee P. W. Analysis of functional domains on reovirus cell attachment protein sigma 1 using cloned S1 gene deletion mutants. Virology. 1987 Sep;160(1):162–168. doi: 10.1016/0042-6822(87)90056-0. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. W., Lee P. W. Glycophorin is the reovirus receptor on human erythrocytes. Virology. 1987 Jul;159(1):94–101. doi: 10.1016/0042-6822(87)90351-5. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Wang G. J., Clerx J. P., Chiu W. Three-dimensional structure of rotavirus. J Mol Biol. 1988 Jan 20;199(2):269–275. doi: 10.1016/0022-2836(88)90313-0. [DOI] [PubMed] [Google Scholar]
- Rohrmann G. F. Polyhedrin structure. J Gen Virol. 1986 Aug;67(Pt 8):1499–1513. doi: 10.1099/0022-1317-67-8-1499. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Mackow E. R., Dyall-Smith M. L., Lazdins I., Holmes I. H., Greenberg H. B. Serotypic analysis of VP3 and VP7 neutralization escape mutants of rhesus rotavirus. J Virol. 1988 Sep;62(9):3509–3512. doi: 10.1128/jvi.62.9.3509-3512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Stoner-Ma D. L., Estes M. K., Greenberg H. B. Specific enzyme-linked immunoassay for rotavirus serotypes 1 and 3. J Clin Microbiol. 1985 Aug;22(2):286–291. doi: 10.1128/jcm.22.2.286-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kitaoka S., Konno T., Sato T., Ishida N. Two modes of human rotavirus entry into MA 104 cells. Arch Virol. 1985;85(1-2):25–34. doi: 10.1007/BF01317003. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kitaoka S., Sato T., Konno T., Iwasaki Y., Numazaki Y., Ishida N. Further investigation on the mode of entry of human rotavirus into cells. Arch Virol. 1986;91(1-2):135–144. doi: 10.1007/BF01316734. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Hoshino Y., Nishikawa K., Green K. Y., Maloy W. L., Morita Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Cross-reactive and serotype-specific neutralization epitopes on VP7 of human rotavirus: nucleotide sequence analysis of antigenic mutants selected with monoclonal antibodies. J Virol. 1988 Jun;62(6):1870–1874. doi: 10.1128/jvi.62.6.1870-1874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Maloy W. L., Nishikawa K., Green K. Y., Hoshino Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Identification of cross-reactive and serotype 2-specific neutralization epitopes on VP3 of human rotavirus. J Virol. 1988 Jul;62(7):2421–2426. doi: 10.1128/jvi.62.7.2421-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Schiff G. M., Hoshino Y., Greenberg H. B. Relative concentrations of serum neutralizing antibody to VP3 and VP7 proteins in adults infected with a human rotavirus. J Virol. 1988 May;62(5):1543–1549. doi: 10.1128/jvi.62.5.1543-1549.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Willoughby R., Wee S. B., Miskuff R., Vonderfecht S. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J Clin Invest. 1987 Jan;79(1):148–154. doi: 10.1172/JCI112775. [DOI] [PMC free article] [PubMed] [Google Scholar]