Abstract
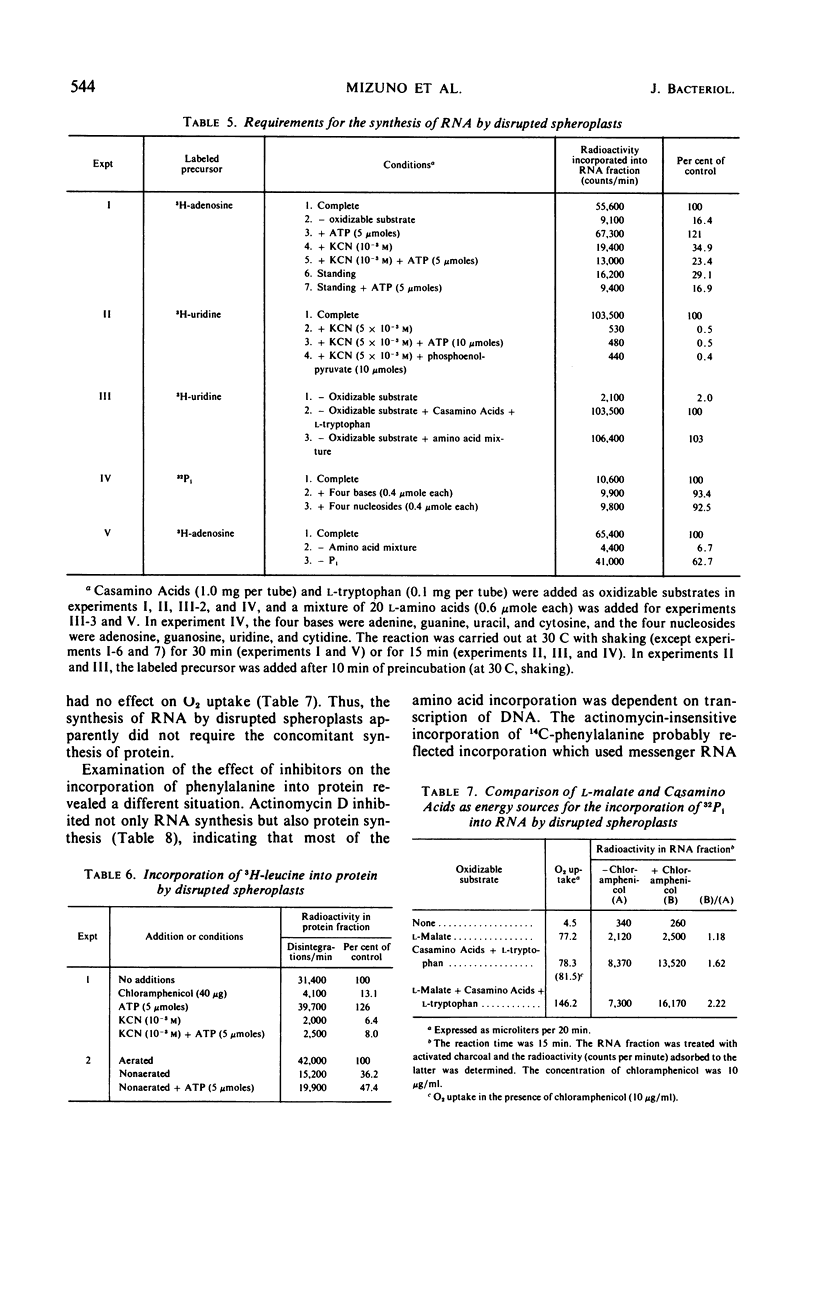
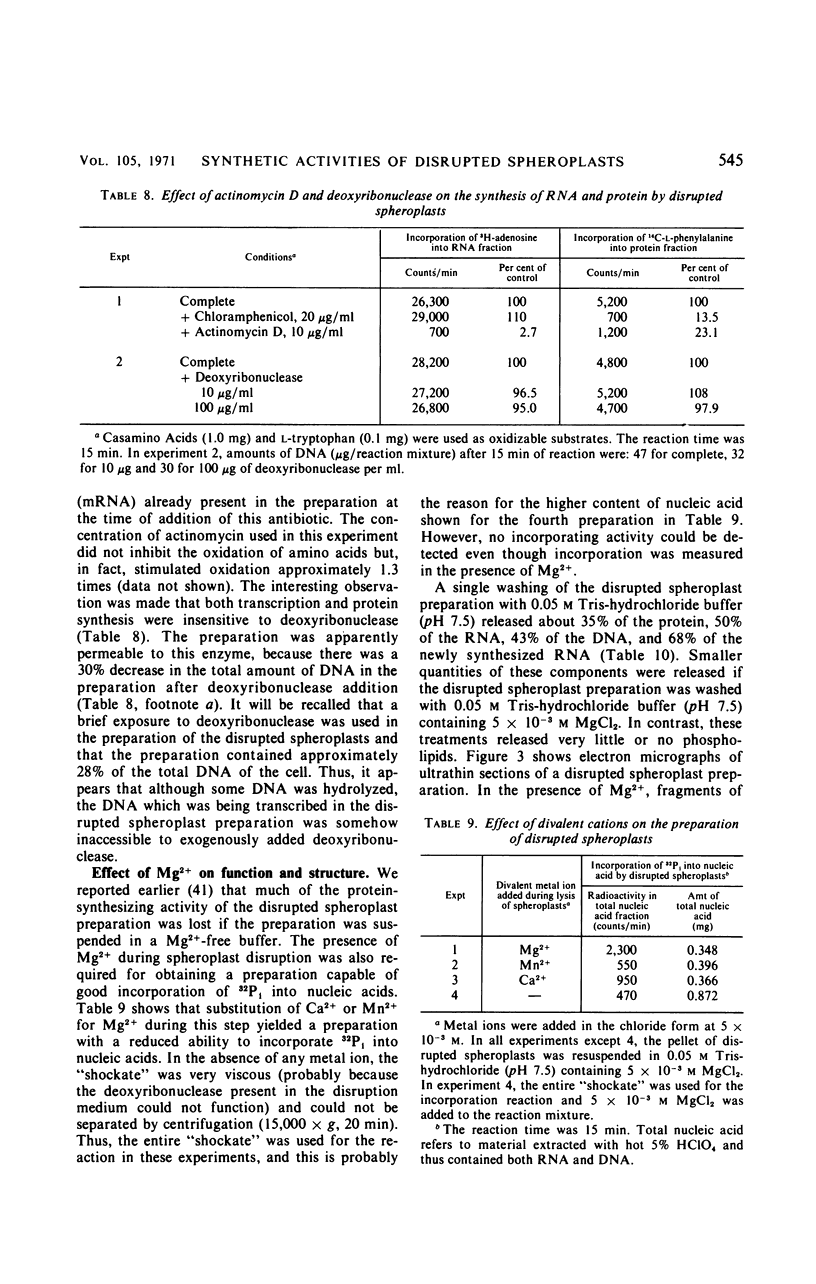
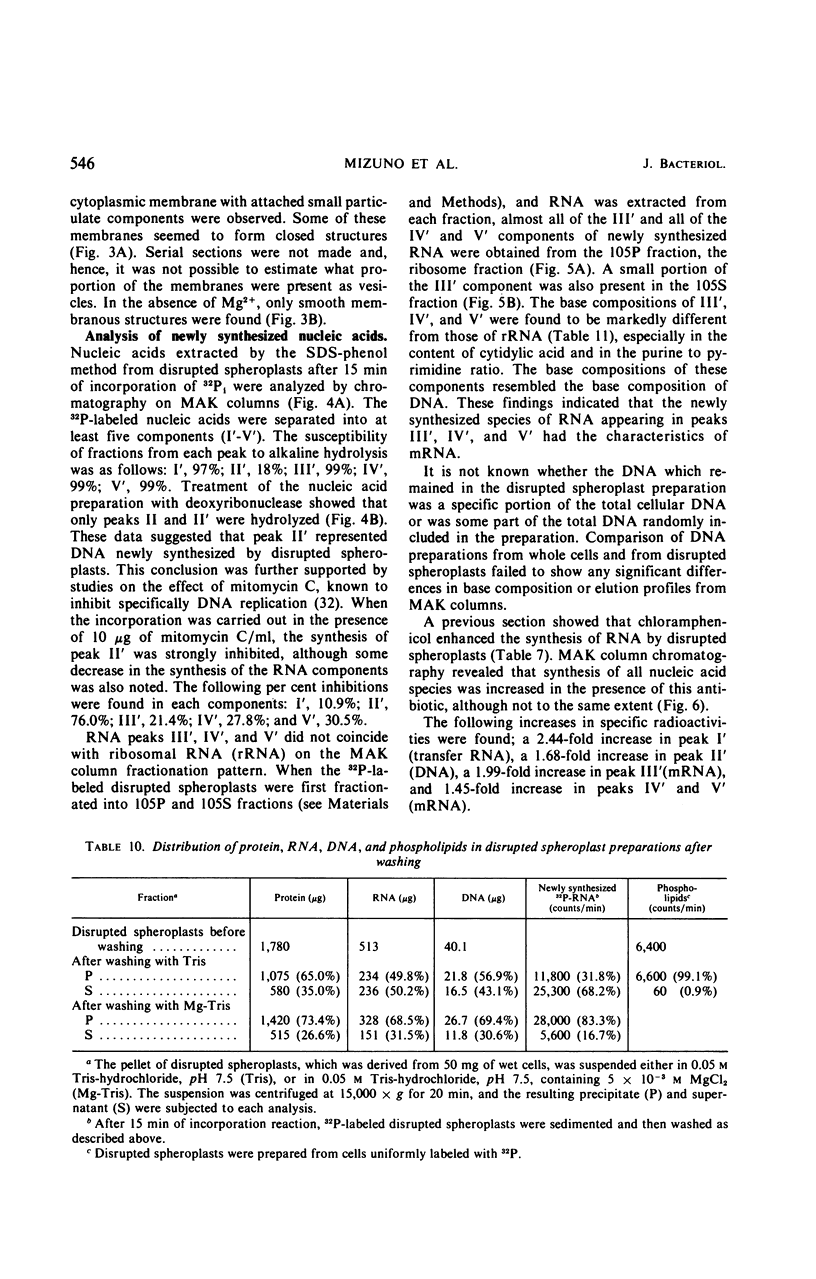
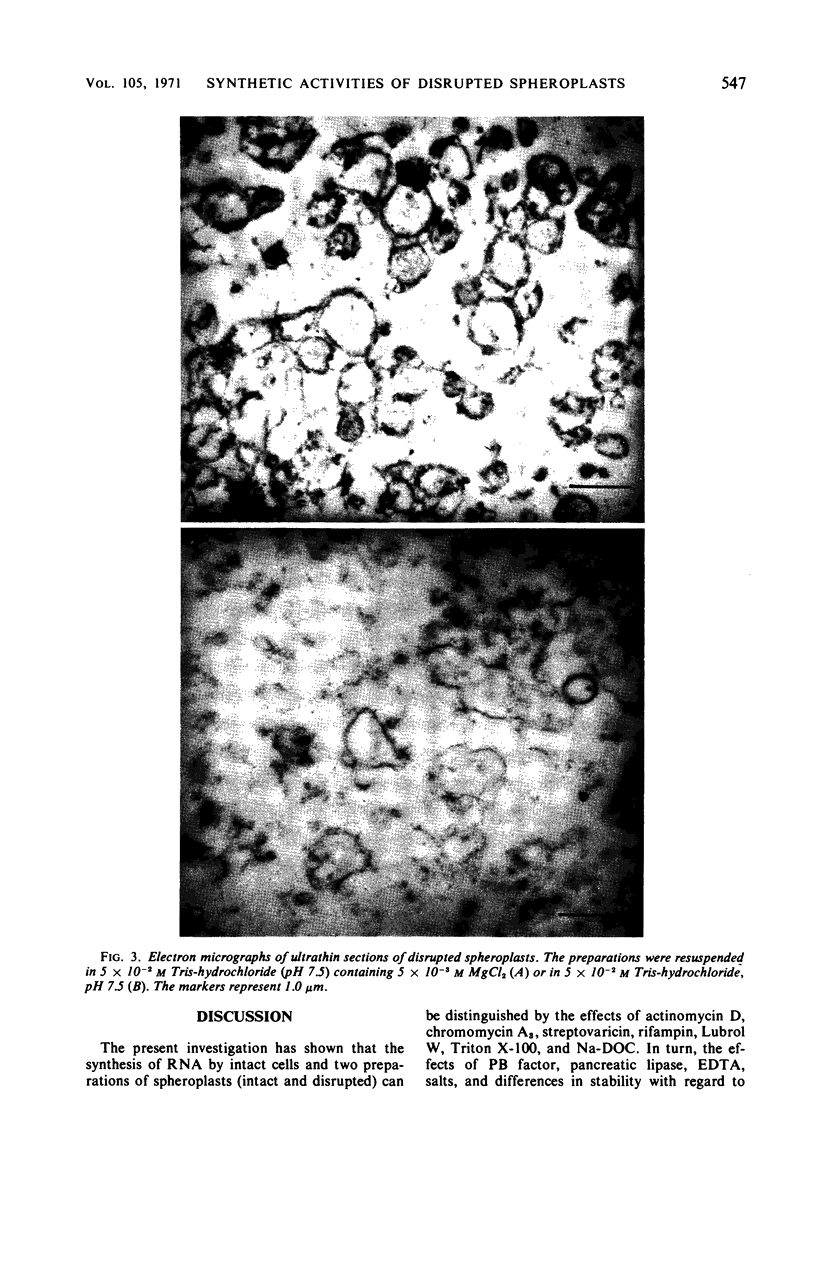
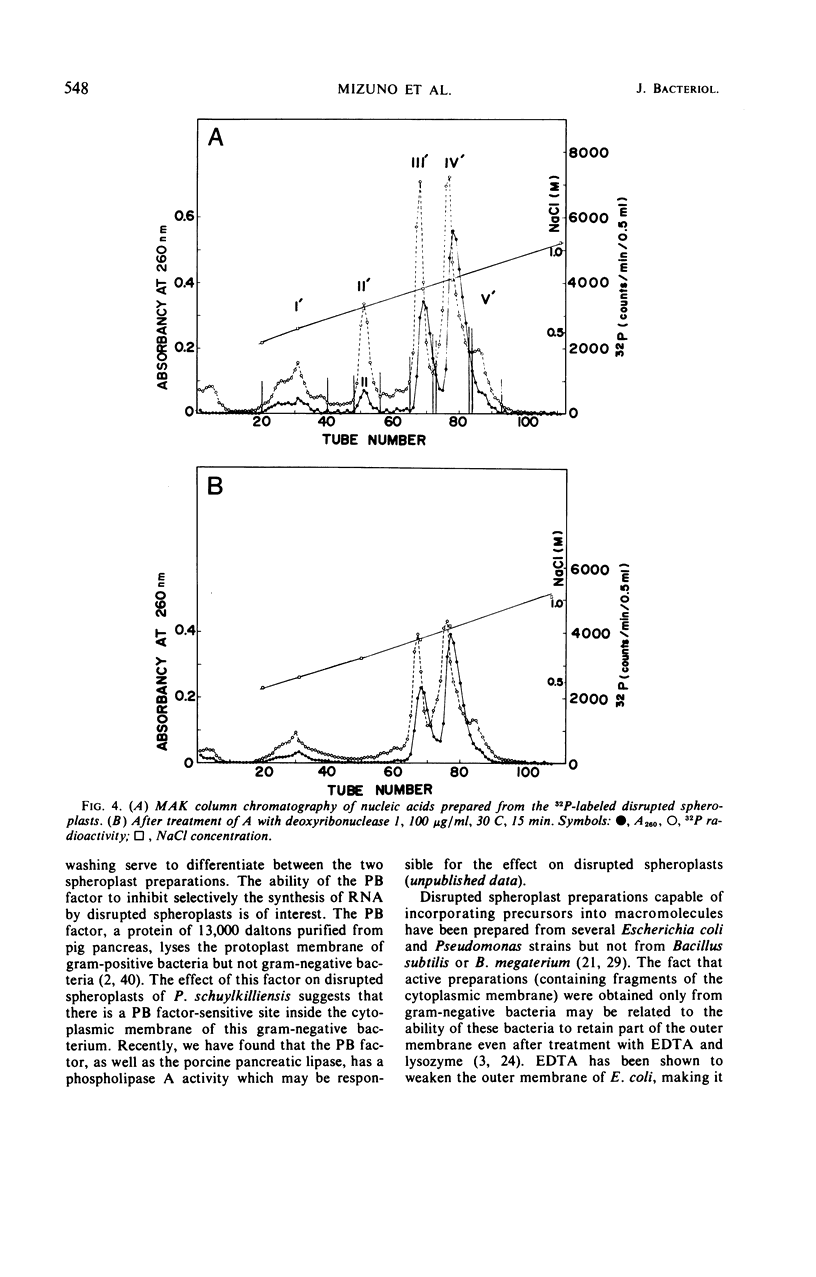
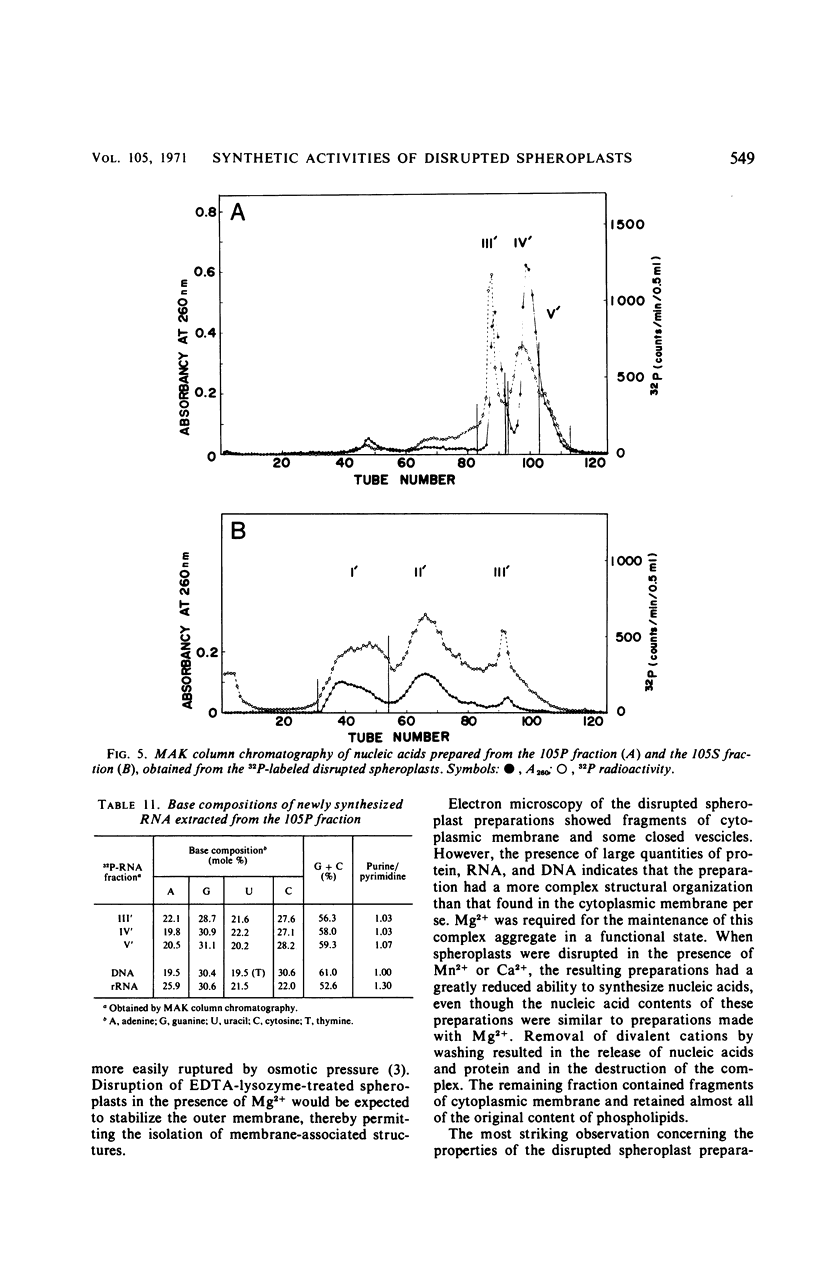
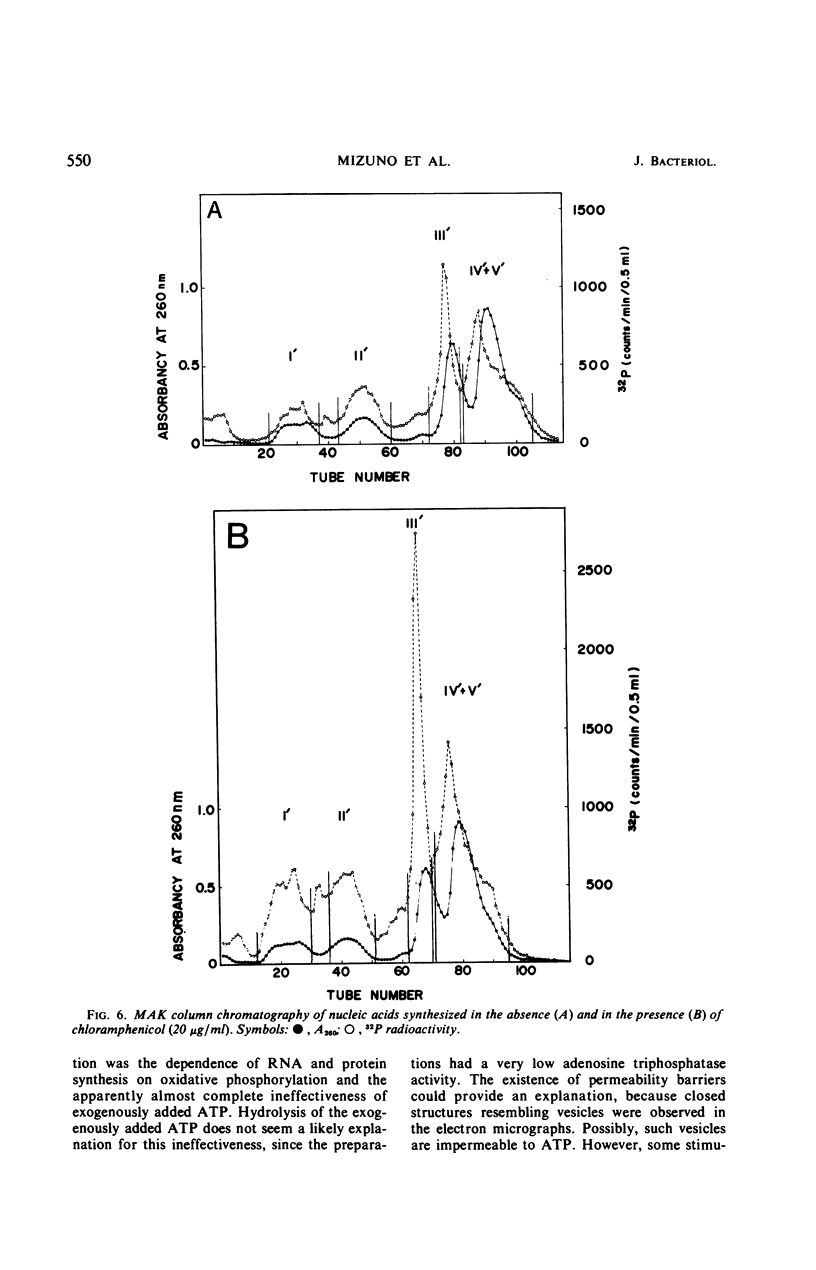
Osmotically shocked spheroplasts obtained from Pseudomonas schuylkilliensis strain P contained about 54, 32, 28, and 82% of the total cellular protein, ribonucleic acid (RNA), deoxyribonucleic acid (DNA), and phospholipid, respectively. This preparation was capable of incorporating 32P-orthophosphate into RNA and DNA, 3H-adenosine or 3H-uridine into RNA, and 3H-leucine or 14C-phenylalanine into protein. These activities were not found in the cytoplasmic fraction which contained most of the glucose-6-phosphate dehydrogenase activity. The synthesis of RNA by intact and disrupted spheroplast preparations was sensitive to actinomycin D, chromomycin A3, streptovaricin, rifampin, Lubrol W, Triton X-100, and sodium deoxycholate, whereas RNA synthesis by intact cells was insensitive to these agents. Ethylenediaminetetraacetic acid, porcine pancreatic lipase, the protoplast-bursting factor, high concentrations of salts, and washing the preparation inhibited the synthesis of RNA by disrupted spheroplasts but had little or no effect on intact spheroplasts. Most of the newly synthesized RNA made by disrupted spheroplasts had the characteristics of messenger RNA. The DNA present in this preparation functioned as a template for RNA synthesis; continued protein synthesis was dependent on concomitant RNA synthesis. An unusual feature of the preparation was the finding that the synthesis of macromolecules was completely dependent on oxidative phosphorylation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED K., JUDAH J. D. Mitochondrial phosphoprotein metabolism. Biochim Biophys Acta. 1963 May 14;71:295–304. doi: 10.1016/0006-3002(63)91084-9. [DOI] [PubMed] [Google Scholar]
- BRIERLEY G. P., BACHMANN E., GREEN D. E. Active transport of inorganic phosphate and magnesium ions by beef heart mitochondria. Proc Natl Acad Sci U S A. 1962 Nov 15;48:1928–1935. doi: 10.1073/pnas.48.11.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRONK J. R. THE NATURE OF THE ENERGY REQUIREMENT FOR AMINO ACID INCORPORATION BY ISOLATED MITOCHONDRIA AND ITS SIGNIFICANCE FOR THYROID HORMONE ACTION. Proc Natl Acad Sci U S A. 1963 Sep;50:524–526. doi: 10.1073/pnas.50.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER J. A., CRATHORN A. R., HUNTER G. D. The site of protein synthesis in Bacillus megaterium. Biochem J. 1958 Aug;69(4):544–553. doi: 10.1042/bj0690544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R., Majumdar C., Weintraub S., Frankel D. M. DNA polymerase and the cell membrane after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:495–500. doi: 10.1101/sqb.1968.033.01.057. [DOI] [PubMed] [Google Scholar]
- GARBUS J., DELUCA H. F., LOOMANS M. E., STRONG F. M. The rapid incorporation of phosphate into mitochondrial lipids. J Biol Chem. 1963 Jan;238:59–63. [PubMed] [Google Scholar]
- GOLDBERG I. H., RABINOWITZ M. Actionmycin D inhibition of deoxyribonucleic acid-dependent synthesis of ribonucleic acid. Science. 1962 Apr 27;136(3513):315–316. doi: 10.1126/science.136.3513.315. [DOI] [PubMed] [Google Scholar]
- Gros F., Gallant J., Weisberg R., Cashel M. Decryptification of RNA polymerase in whole cells of Escherichia coli. J Mol Biol. 1967 May 14;25(3):555–557. doi: 10.1016/0022-2836(67)90206-9. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- KAZIRO Y., KAMIYAMA M. INHIBITION OF RNA POLYMERASE REACTION BY CHROMOMYCIN A3. Biochem Biophys Res Commun. 1965 May 3;19:433–437. doi: 10.1016/0006-291x(65)90142-7. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of ribonucleic acids from mammalian tissues. Biochem J. 1956 Nov;64(3):405–408. doi: 10.1042/bj0640405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MARSH B. B. The estimation of inorganic phosphate in the presence of adenosine triphosphate. Biochim Biophys Acta. 1959 Apr;32:357–361. doi: 10.1016/0006-3002(59)90607-9. [DOI] [PubMed] [Google Scholar]
- MIZUNO S., YOSHIDA E., TAKAHASHI H., MARUO B. Experimental proof of a compartment of "energy-rich"-P in a subcellular system from Pseudomonas. Biochim Biophys Acta. 1961 May 13;49:369–381. doi: 10.1016/0006-3002(61)90136-6. [DOI] [PubMed] [Google Scholar]
- Maruo B., Seto H., Nagata Y. Inducible synthesis of beta-galactosidase in disrupted spheroplast of Escherichia coli. J Bacteriol. 1969 Oct;100(1):209–214. doi: 10.1128/jb.100.1.209-214.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Mizuno S., Yamazaki H., Nitta K., Umezawa H. Inhibition of DNA-dependent RNA polymerase reaction of Escherichia coli by an antimicrobial antibiotic, streptovaricin. Biochim Biophys Acta. 1968 Apr 22;157(2):322–332. doi: 10.1016/0005-2787(68)90086-5. [DOI] [PubMed] [Google Scholar]
- Mizuno S., Yamazaki H., Nitta K., Umezawa H. Inhibition of initiation of DNA-dependent RNA synthesis by an antibiotic B44p. Biochem Biophys Res Commun. 1968 Feb 26;30(4):379–385. doi: 10.1016/0006-291x(68)90755-9. [DOI] [PubMed] [Google Scholar]
- NISMAN B. Incorporation and activation of amino acids by disrupted protoplasts of Escherichia coli. Biochim Biophys Acta. 1959 Mar;32(1):18–31. doi: 10.1016/0006-3002(59)90549-9. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Mizuno S., Maruo B. Preparation and properties of active membrane systems from various species of bacteria. J Biochem. 1966 Apr;59(4):404–410. doi: 10.1093/oxfordjournals.jbchem.a128316. [DOI] [PubMed] [Google Scholar]
- SHIBA S., TERAWAKI A., TAGUCHI T., KAWAMATA J. Selective inhibition of formation of deoxyribonucleic acid in Escherichia coli by mitomycin C. Nature. 1959 Apr 11;183(4667):1056–1057. doi: 10.1038/1831056a0. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Smith M. F., Roseman S. Resolution of a staphylococcal phosphotransferase system into four protein components and its relation to sugar transport. Biochem Biophys Res Commun. 1968 Jun 10;31(5):804–811. doi: 10.1016/0006-291x(68)90634-7. [DOI] [PubMed] [Google Scholar]
- Snyder R. W., Young F. E. Association between the chromosome and the cytoplasmic membrane in Bacillus subtilis. Biochem Biophys Res Commun. 1969 May 8;35(3):354–362. doi: 10.1016/0006-291x(69)90506-3. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]
- Tonomura B., Rabinowitz J. C. An investigation of the induction of beta-galactosidase in a broken spheroplast preparation of Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):177–202. doi: 10.1016/0022-2836(67)90325-7. [DOI] [PubMed] [Google Scholar]
- WACHSMANN J. T., FUKUHARA H., NISMAN B. The activation and incorporation of amino acids by subcellular fractions of Bacillus megaterium strain. M. Biochim Biophys Acta. 1960 Aug 26;42:388–400. doi: 10.1016/0006-3002(60)90816-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Tamura G., Arima K. Properties of protoplast-bursting factor, a substance isolated from pig pancreas. Biochim Biophys Acta. 1967 Jan 18;133(1):134–142. doi: 10.1016/0005-2795(67)90045-1. [DOI] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]