Abstract
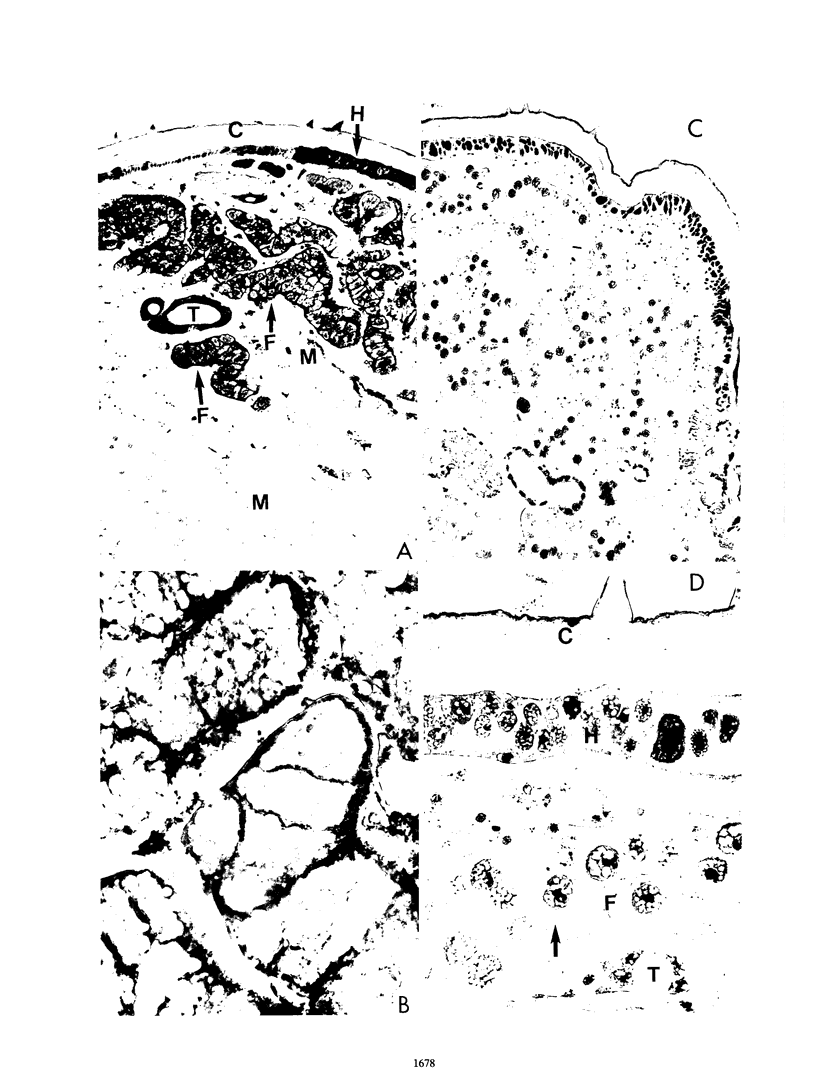
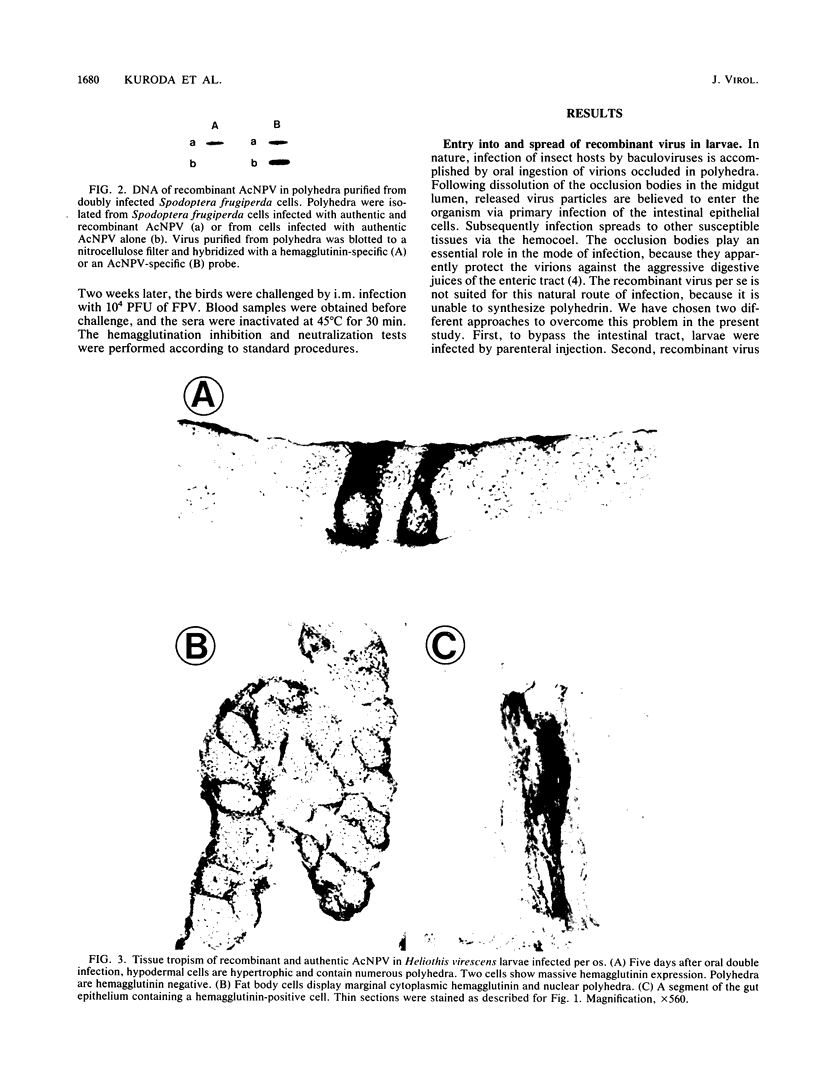
The hemagglutinin of influenza (fowl plague) virus was expressed in larvae of Heliothis virescens by using recombinant Autographa californica nuclear polyhedrosis virus (AcNPV) as a vector. Animals were infected with the recombinant virus either by parenteral injection or by feeding. For oral uptake, recombinant virus occluded in polyhedra obtained from cultured Spodoptera frugiperda cells after coinfection with authentic AcNPV was used. Immunohistological analyses of infected animals revealed that the hemagglutinin was expressed only in those tissues that are also permissive for the replication of authentic AcNPV. These tissues included hypodermis, fat body, and tracheal matrix. After oral infection, hemagglutinin was also detected in individual gut cells. The amount of hemagglutinin synthesized in larvae after parenteral infection was 0.3% of the total protein, compared with 5% obtained in cultured insect cells. The hemagglutinin was transported to the cell surface and expressed in polarized cells only at the apical plasma membrane. It was processed by posttranslational proteolysis into the cleavage products HA1 and HA2. Oligosaccharides were attached by N-glycosidic linkages and were smaller than those found on hemagglutinin obtained from vertebrate cells. Hemagglutinin from larvae expressed receptor binding and cell fusion activities, but quantitation of the hemolytic capacity revealed that it was only about half as active as hemagglutinin from vertebrate or insect cell cultures. Chickens immunized with larval tissues containing hemagglutinin were protected from infection with fowl plague virus. These observations demonstrate that live insects are able to produce a recombinant membrane protein of vertebrate origin in biologically active form.
Full text
PDF


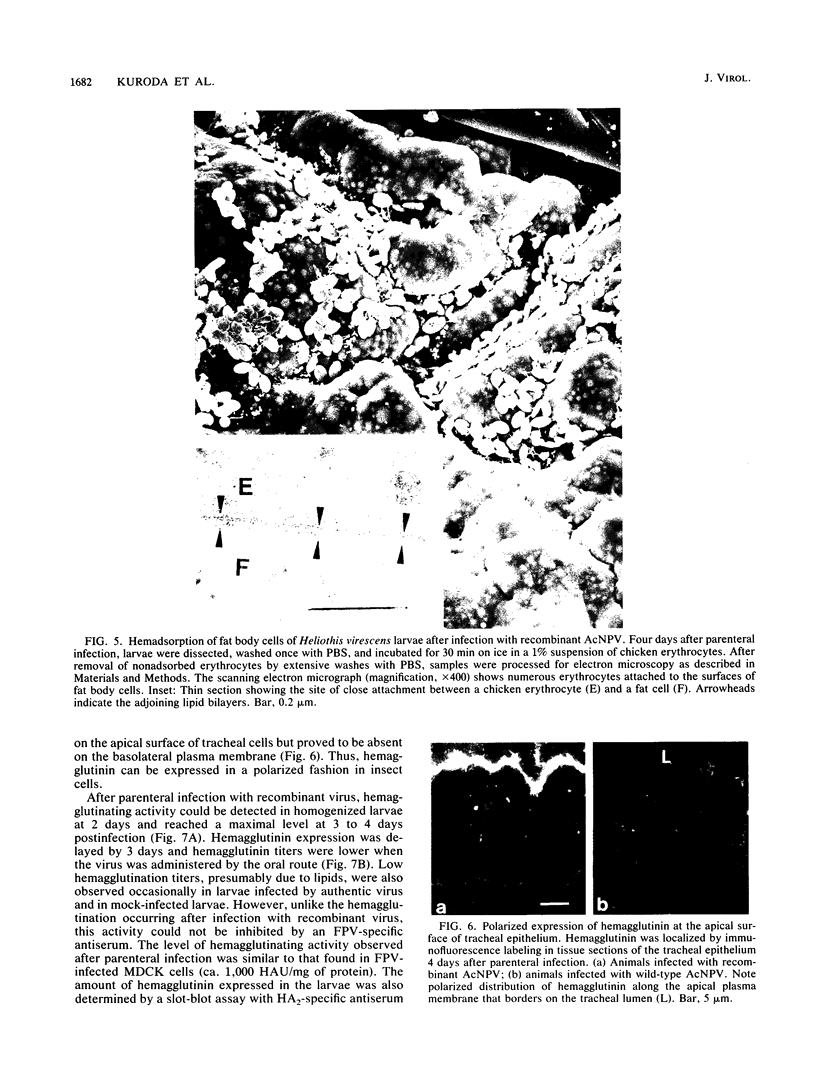

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becht H., Huang R. T., Fleischer B., Boschek C. B., Rott R. Immunogenic properties of the small chain HA2 of the haemagglutinin of influenza viruses. J Gen Virol. 1984 Jan;65(Pt 1):173–183. doi: 10.1099/0022-1317-65-1-173. [DOI] [PubMed] [Google Scholar]
- Carstens E. B., Tjia S. T., Doerfler W. Infection of Spodoptera frugiperda cells with Autographa californica nuclear polyhedrosis virus I. Synthesis of intracellular proteins after virus infection. Virology. 1979 Dec;99(2):386–398. doi: 10.1016/0042-6822(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Franz H. Identification of actin-, alpha-actinin-, and vinculin-containing plaques at the lateral membrane of epithelial cells. J Cell Biol. 1986 May;102(5):1843–1852. doi: 10.1083/jcb.102.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Kosowski S. G., Schaaf K. F. Expression of envelope glycoproteins of human immunodeficiency virus by an insect virus vector. J Virol. 1987 Nov;61(11):3617–3620. doi: 10.1128/jvi.61.11.3617-3620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Becht H. On the structure of the influenza virus envelope. Virology. 1972 Mar;47(3):579–591. doi: 10.1016/0042-6822(72)90547-8. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Hauser C., Rott R., Klenk H. D., Doerfler W. Expression of the influenza virus haemagglutinin in insect cells by a baculovirus vector. EMBO J. 1986 Jun;5(6):1359–1365. doi: 10.1002/j.1460-2075.1986.tb04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Kawai T., Obinata M., Fujiwara H., Horiuchi T., Saeki Y., Sato Y., Furusawa M. Production of human alpha-interferon in silkworm using a baculovirus vector. Nature. 1985 Jun 13;315(6020):592–594. doi: 10.1038/315592a0. [DOI] [PubMed] [Google Scholar]
- Marumoto Y., Sato Y., Fujiwara H., Sakano K., Saeki Y., Agata M., Furusawa M., Maeda S. Hyperproduction of polyhedrin-IGF II fusion protein in silkworm larvae infected with recombinant Bombyx mori nuclear polyhedrosis virus. J Gen Virol. 1987 Oct;68(Pt 10):2599–2606. doi: 10.1099/0022-1317-68-10-2599. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Schreurs J., Otsu K., Kondo A., Arai K., Maeda S. Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene. 1987;58(2-3):273–281. doi: 10.1016/0378-1119(87)90382-9. [DOI] [PubMed] [Google Scholar]
- Possee R. D. Cell-surface expression of influenza virus haemagglutinin in insect cells using a baculovirus vector. Virus Res. 1986 Jul;5(1):43–59. doi: 10.1016/0168-1702(86)90064-x. [DOI] [PubMed] [Google Scholar]
- Rott R., Orlich M., Klenk H. D., Wang M. L., Skehel J. J., Wiley D. C. Studies on the adaptation of influenza viruses to MDCK cells. EMBO J. 1984 Dec 20;3(13):3329–3332. doi: 10.1002/j.1460-2075.1984.tb02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R., Reinacher M., Orlich M., Klenk H. D. Cleavability of hemagglutinin determines spread of avian influenza viruses in the chorioallantoic membrane of chicken embryo. Arch Virol. 1980;65(2):123–133. doi: 10.1007/BF01317323. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Ju G., Ericson B. L., Moschera J., Lahm H. W., Chizzonite R., Summers M. D. Modification and secretion of human interleukin 2 produced in insect cells by a baculovirus expression vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8404–8408. doi: 10.1073/pnas.82.24.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjia S. T., Carstens E. B., Doerfler W. Infection of Spodoptera frugiperda cells with Autographa californica nuclear polyhedrosis virus II. The viral DNA and the kinetics of its replication. Virology. 1979 Dec;99(2):399–409. doi: 10.1016/0042-6822(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Wood H. A. Protease degradation of Autographa californica nuclear polyhedrosis virus proteins. Virology. 1980 Jun;103(2):392–399. doi: 10.1016/0042-6822(80)90198-1. [DOI] [PubMed] [Google Scholar]