Abstract
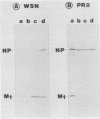
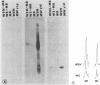
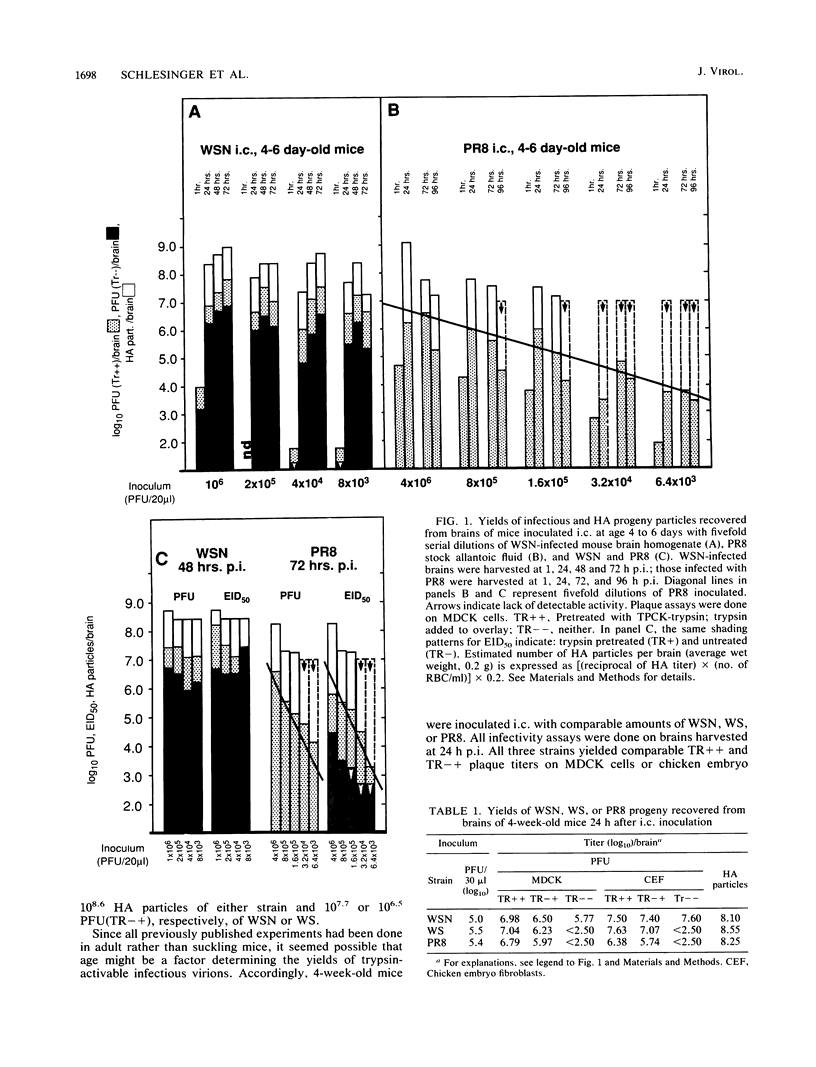
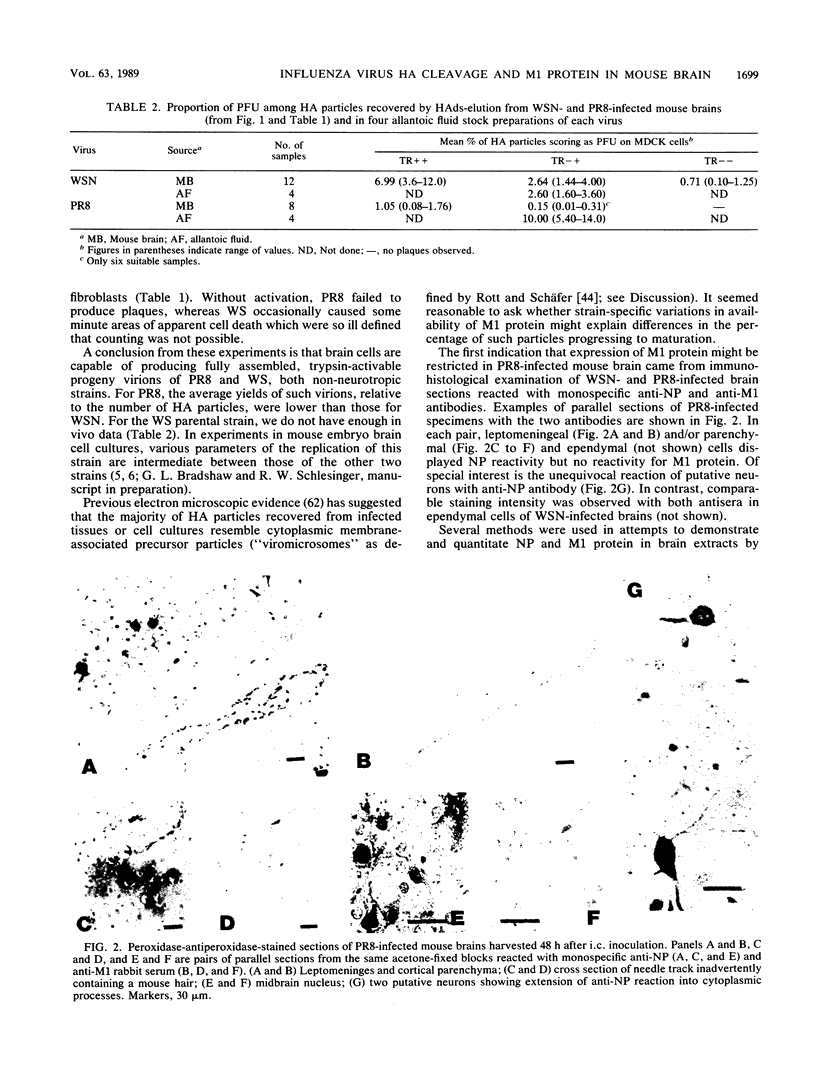
The combined presence of WSN gene segments 6 (neuraminidase), 7 (M1 and M2), and 8 (NS1 and NS2) in reassortants of WSN with A/Aichi/2/68 (H3N2) has been found by others to be necessary for full expression of neurovirulence in mice. We are examining the expression of the analogous three gene segments in brains of mice after intracerebral infection with non-neuroadapted strains A/WS/33 (WS) (from which WSN was derived) and A/PR/8/34 (PR8). Our aim is to determine possible mechanisms by which one or more of the five gene products may restrict replication of these strains in mouse brain cells to a single cycle, yielding noninfectious hemagglutinating particles (incomplete growth cycle). We found that minority subsets of such particles did produce plaques, provided they were activated by trypsin (analogous to other abortive systems producing virions with uncleaved HA), a step obviated for some WSN virions by indirect promotion of hemagglutinin cleavage by the neuraminidase of that strain. The percentage of such potentially infectious virions, relative to total hemagglutinating particles, was significantly lower in WS- or PR8-infected than in WSN-infected brains, suggesting possible defects in synthesis or function of M1 protein in the former. Cells in immunostained sections and appropriate bands in Western blots (immunoblots) of viral proteins electrophoretically separated from lysates of PR8-infected brains reacted with antibody to nucleoprotein but not to M1 protein. Either method revealed the presence of both proteins in WSN-infected brains. In contrast, Western blot analyses of particles concentrated from PR8-, WS-, or WSN-infected brains by hemadsorption, elution, and pelleting did reveal NP and M1 bands with comparable relative peroxidase-antiperoxidase staining intensities. The findings suggest that availability of M1 protein is a factor influencing the extent or rate of assembly of potentially infectious (i.e., trypsin-activated) progeny virions in mouse brains and that in this respect the two non-neurovirulent strains differ from WSN quantitatively rather than qualitatively.
Full text
PDF


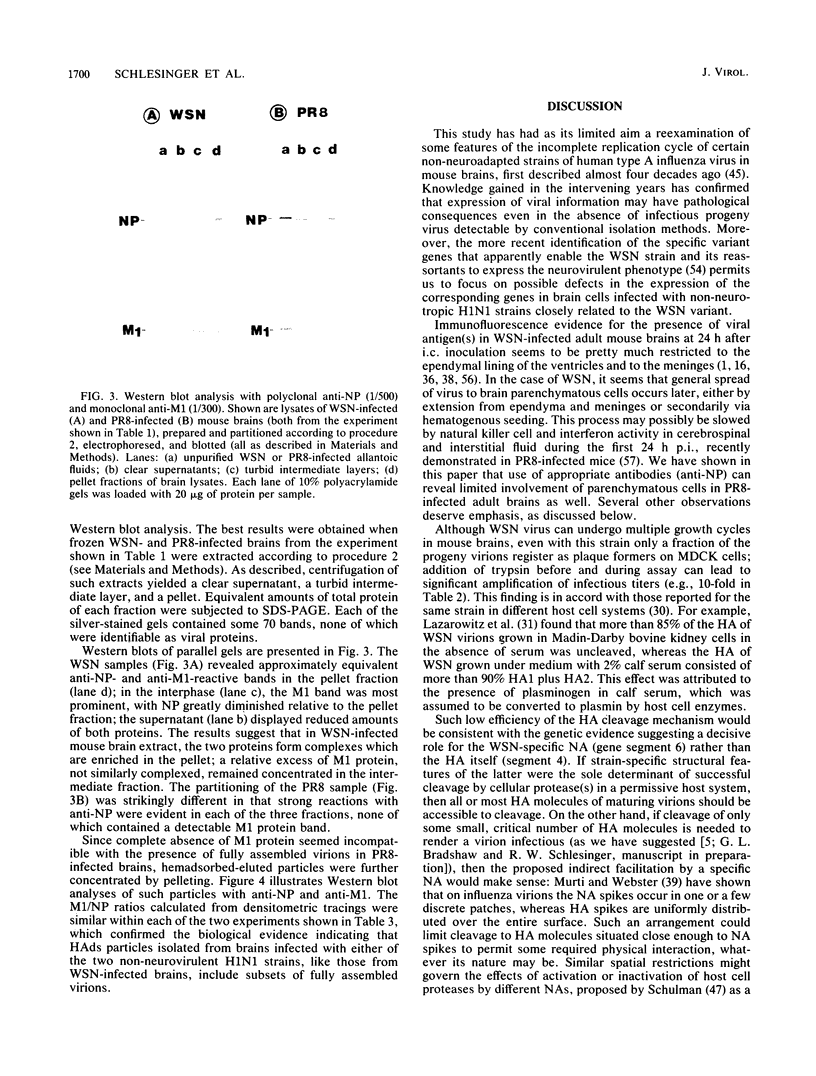


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell T. M., Narang H. K., Field E. J. Influenzal encephalitis in mice. A histopathological and electron microscopical study. Arch Gesamte Virusforsch. 1971;34(2):157–167. doi: 10.1007/BF01241717. [DOI] [PubMed] [Google Scholar]
- Bonin J., Scholtissek C. Mouse neurotropic recombinants of influenza A viruses. Arch Virol. 1983;75(4):255–268. doi: 10.1007/BF01314891. [DOI] [PubMed] [Google Scholar]
- Bradshaw G. L., Schlesinger R. W., Schwartz C. D. Effects of cell differentiation on replication of A/WS/33, WSN, and A/PR/8/34 influenza viruses in mouse brain cell cultures: biological and immunological characterization of products. J Virol. 1989 Apr;63(4):1704–1714. doi: 10.1128/jvi.63.4.1704-1714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler-White A. J., Naeve C. W., Murphy B. R. Characterization of a gene coding for M proteins which is involved in host range restriction of an avian influenza A virus in monkeys. J Virol. 1986 Feb;57(2):697–700. doi: 10.1128/jvi.57.2.697-700.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS H. J. F. The growth of influenza viruses and Newcastle disease virus in mouse brain. Br J Exp Pathol. 1951 Apr;32(2):110–117. [PMC free article] [PubMed] [Google Scholar]
- CAIRNS H. J. The limited growth of influenza viruses in the mouse brain. Aust J Exp Biol Med Sci. 1954 Feb;32(1):123–128. doi: 10.1038/icb.1954.14. [DOI] [PubMed] [Google Scholar]
- Collins A. R., Knobler R. L., Powell H., Buchmeier M. J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell--cell fusion. Virology. 1982 Jun;119(2):358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti G., Valcavi P., Natali A., Schito G. C. Different patterns of replication in influenza virus-infected KB cells. Arch Virol. 1980;66(4):309–320. doi: 10.1007/BF01320627. [DOI] [PubMed] [Google Scholar]
- DONALD H. B., ISAACS A. Counts of influenza virus particles. J Gen Microbiol. 1954 Jun;10(3):457–464. doi: 10.1099/00221287-10-3-457. [DOI] [PubMed] [Google Scholar]
- DUVOISIN R. C., YAHR M. D. ENCEPHALITIS AND PARKINSONISM. Arch Neurol. 1965 Mar;12:227–239. doi: 10.1001/archneur.1965.00460270003001. [DOI] [PubMed] [Google Scholar]
- FRASER K. B., NAIRN R. C., McENTEGART M. G., CHADWICK C. S. Neurotropic and non-neurotropic influenza-A infection of mouse brain studied with fluorescent antibody. J Pathol Bacteriol. 1959 Oct;78:423–433. doi: 10.1002/path.1700780209. [DOI] [PubMed] [Google Scholar]
- Hiti A. L., Nayak D. P. Complete nucleotide sequence of the neuraminidase gene of human influenza virus A/WSN/33. J Virol. 1982 Feb;41(2):730–734. doi: 10.1128/jvi.41.2.730-734.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C., Brown C. M. Differences in the control of virus mRNA splicing during permissive or abortive infection with influenza A (fowl plague) virus. J Gen Virol. 1984 Jan;65(Pt 1):153–164. doi: 10.1099/0022-1317-65-1-153. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Johnson R. T. Granular ependymitis. Occurrence in myxovirus infected rodents and prevalence in man. Am J Pathol. 1972 Jun;67(3):511–526. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus as a sequela of experimental myxovirus infections. Exp Mol Pathol. 1969 Feb;10(1):68–80. doi: 10.1016/0014-4800(69)90049-5. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Krystal M., Elliott R. M., Benz E. W., Jr, Young J. F., Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Goldberg A. R., Choppin P. W. Proteolytic cleavage by plasmin of the HA polypeptide of influenza virus: host cell activation of serum plasminogen. Virology. 1973 Nov;56(1):172–180. doi: 10.1016/0042-6822(73)90296-1. [DOI] [PubMed] [Google Scholar]
- Lohmeyer J., Talens L. T., Klenk H. D. Biosynthesis of the influenza virus envelope in abortive infection. J Gen Virol. 1979 Jan;42(1):73–88. doi: 10.1099/0022-1317-42-1-73. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. An analysis of the toxicity for mice of influenza virus. I. Intracerebral toxicity. Br J Exp Pathol. 1960 Dec;41:586–592. [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. Intracerebral injections and the growth of viruses in the mouse brain. Br J Exp Pathol. 1960 Feb;41:52–59. [PMC free article] [PubMed] [Google Scholar]
- Mayer V., Schulman J. L., Kilbourne E. D. Nonlinkage of neurovirulence exclusively to viral hemagglutinin or neuraminidase in genetic recombinants of A-NWS (HON1) influenza virus. J Virol. 1973 Feb;11(2):272–278. doi: 10.1128/jvi.11.2.272-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K., Wolf A., Harter D. H., Duffy P. E., Gamboa E. T., Hsu K. C. Murine influenza virus encephalomyelitis. I. Neuropathological and immunofluorescence findings. J Neuropathol Exp Neurol. 1973 Jan;32(1):51–71. doi: 10.1097/00005072-197301000-00004. [DOI] [PubMed] [Google Scholar]
- Murti K. G., Webster R. G. Distribution of hemagglutinin and neuraminidase on influenza virions as revealed by immunoelectron microscopy. Virology. 1986 Feb;149(1):36–43. doi: 10.1016/0042-6822(86)90084-x. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Sugiura A. Neurovirulence of influenza virus in mice. II. Mechanism of virulence as studied in a neuroblastoma cell line. Virology. 1980 Mar;101(2):450–457. doi: 10.1016/0042-6822(80)90458-4. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Polypeptide synthesis in simian virus 5-infected cells. J Virol. 1977 Jul;23(1):177–187. doi: 10.1128/jvi.23.1.177-187.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenholt R. T., Foege W. H. 1918 influenza, encephalitis lethargica, parkinsonism. Lancet. 1982 Oct 16;2(8303):860–864. doi: 10.1016/s0140-6736(82)90820-0. [DOI] [PubMed] [Google Scholar]
- Reinacher M., Bonin J., Narayan O., Scholtissek C. Pathogenesis of neurovirulent influenza A virus infection in mice. Route of entry of virus into brain determines infection of different populations of cells. Lab Invest. 1983 Dec;49(6):686–692. [PubMed] [Google Scholar]
- SCHLESINGER R. W. Incomplete growth cycle of influenza virus in mouse brain. Proc Soc Exp Biol Med. 1950 Jul;74(3):541–548. doi: 10.3181/00379727-74-17966. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER R. W. The relation of functionally deficient forms of influenza virus to viral development. Cold Spring Harb Symp Quant Biol. 1953;18:55–59. doi: 10.1101/sqb.1953.018.01.012. [DOI] [PubMed] [Google Scholar]
- Schulman J. L., Palese P. Virulence factors of influenza A viruses: WSN virus neuraminidase required for plaque production in MDBK cells. J Virol. 1977 Oct;24(1):170–176. doi: 10.1128/jvi.24.1.170-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Waterfield M. D. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Hay A. J. Replication of the influenza virus genome. Virology. 1982 Apr 15;118(1):96–108. doi: 10.1016/0042-6822(82)90323-3. [DOI] [PubMed] [Google Scholar]
- Sugiura A., Ueda M. Neurovirulence of influenza virus in mice. I. Neurovirulence of recombinants between virulent and avirulent virus strains. Virology. 1980 Mar;101(2):440–449. doi: 10.1016/0042-6822(80)90457-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER R. R. A pantropic strain of influenza virus: generalized infection and viremia in the infant mouse. Virology. 1955 Dec;1(5):497–515. doi: 10.1016/0042-6822(55)90039-8. [DOI] [PubMed] [Google Scholar]
- WERNER G. H., SCHLESINGER R. W. Morphological and quantitative comparison between infectious and non-infectious forms of influenza virus. J Exp Med. 1954 Aug 1;100(2):203–216. doi: 10.1084/jem.100.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabuke-Bunoti M. A., Bennink J. R., Plotkin S. A. Influenza virus-induced encephalopathy in mice: interferon production and natural killer cell activity during acute infection. J Virol. 1986 Dec;60(3):1062–1067. doi: 10.1128/jvi.60.3.1062-1067.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Geraci J., Petursson G., Skirnisson K. Conjunctivitis in human beings caused by influenza A virus of seals. N Engl J Med. 1981 Apr 9;304(15):911–911. doi: 10.1056/NEJM198104093041515. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Bean W. J., Van Wyke K. L., Geraci J. R., St Aubin D. J., Petursson G. Characterization of an influenza A virus from seals. Virology. 1981 Sep;113(2):712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Schild G. C. Molecular mechanisms of variation in influenza viruses. Nature. 1982 Mar 11;296(5853):115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]