Abstract
HIV-1 replication is inhibited by the incorporation of chain-terminating nucleotides at the 3′ end of the growing DNA chain. Here we show a nucleotide-dependent reaction catalyzed by HIV-1 reverse transcriptase that can efficiently remove the chain-terminating residue, yielding an extendible primer terminus. Radioactively labeled 3′-terminal residue from the primer can be transferred into a product that is resistant to calf intestinal alkaline phosphatase and sensitive to cleavage by snake venom phosphodiesterase. The products formed from different nucleotide substrates have unique electrophoretic migrations and have been identified as dinucleoside tri- or tetraphosphates. The reaction is inhibited by dNTPs that are complementary to the next position on the template (Ki ≈ 5 μM), suggesting competition between dinucleoside polyphosphate synthesis and DNA polymerization. Dinucleoside polyphosphate synthesis was inhibited by an HIV-1 specific non-nucleoside inhibitor and was absent in mutant HIV-1 reverse transcriptase deficient in polymerase activity, indicating that this activity requires a functional polymerase active site. We suggest that dinucleoside polyphosphate synthesis occurs by transfer of the 3′ nucleotide from the primer to the pyrophosphate moiety in the nucleoside di- or triphosphate substrate through a mechanism analogous to pyrophosphorolysis. Unlike pyrophosphorolysis, however, the reaction is nucleotide-dependent, is resistant to pyrophosphatase, and produces dinucleoside polyphosphates. Because it occurs at physiological concentrations of ribonucleoside triphosphates, this reaction may determine the in vivo activity of many nucleoside antiretroviral drugs.
DNA synthesis by HIV-1 reverse transcriptase (RT) is inhibited by ddNTPs and triphosphorylated forms of other nucleoside analogs that lack a free 3′ hydroxyl group. These compounds [most notably 3′-azido-3′-deoxythymidine (AZT), 2′,3′-dideoxyinosine (ddI), 2′,3′-dideoxycytidine (ddC), 2′,3′-didehydro-2′,3′-dideoxythymidine (d4T), and 2′,3′-dideoxy-3′-thiacytidine (3TC)] are used in therapy for HIV-related disease and AIDS (1). The triphosphate derivatives of these compounds are competitive inhibitors of RT and function as alternative substrates in DNA synthesis (2–7). Under conditions of excess RT and limiting viral genome concentration, as is usually the case in HIV-infected cells, viral replication is sensitive to incorporation of a single chain-terminating nucleotide that blocks all further elongation of that DNA chain. Activities that could remove incorporated chain-terminating nucleotides from the HIV-1 genome, and thus rescue it, have not been studied extensively; however, they may play an important role in the sensitivity of HIV-1 to these compounds. Retroviral RTs lack 3′ exonuclease proofreading activity (8, 9) but are capable of pyrophosphorolysis, the reversal of polymerization. A terminal dideoxynucleoside monophosphate thus can be transferred to PPi releasing an unblocked, extendible DNA chain and ddNTP (10–12); however, concentrations of intracellular PPi required for efficient pyrophosphorolysis by HIV-1 RT (10) are much higher than those reported in mammalian cells (13, 14).
As an extension of studies of nucleotide-dependent interactions between HIV-1 RT and chain-terminated primer-template (15), the effects of adding mismatched dNTPs and ribonucleotides to the enzyme–primer–template complex were tested. An unexpected nucleotide-dependent removal of dideoxynucleotide from the primer terminus was observed. In the present report, we show that this reaction is catalyzed by the polymerase active site of HIV-1 RT and occurs through a pyrophosphorolysis-related mechanism. The two phosphate groups of a nucleoside diphosphate or the β and γ phosphate groups of a nucleoside triphosphate are used as a pyrophosphate analog to attack and cleave the 3′ phosphate bond of the primer, producing an unblocked primer shortened by one base and a dinucleoside tri- or tetraphosphate containing the dideoxynucleoside monophosphate from the primer terminus linked through its phosphate group to the distal phosphate of the free nucleoside di- or triphosphate. The dinucleoside polyphosphate synthesis activity provides HIV-1 RT with an efficient means of rescuing blocked primer termini. In addition, the dinucleoside polyphosphates are of potential physiological importance. They belong to a class of compounds implicated in diverse metabolic processes in both prokaryotic and eukaryotic cells (16–22) that may contribute to metabolic events that occur in HIV-infected cells.
MATERIALS AND METHODS
Expression and Purification of HIV-1 RT.
His-tagged HIV-1 RT was prepared by inserting a short, double-stranded oligonucleotide segment (plus strand, 5′-CATGCATCACCATCACCATCA-3′; minus strand, 5′-CATGTGATGGTGATGGTGATG-3′) into the NcoI site in the HIV-1 RT expression vector pKRT2 (23) (obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, Rockville, MD). The active site double-mutant (D185N/D186N) of HIV-1 RT was constructed by the megaprimer PCR method (24) by using Pwo polymerase (Boehringer Mannheim) and the oligonucleotide 5′-CTATTTCTAAGTCGGATCCTACATACAAATTATTCATGTATTGATAG-3′ and was subcloned into the his-tagged HIV-1 RT-encoding plasmid. The mutant expression clone was confirmed by DNA sequence analysis. His-tagged HIV-1 RT and mutant enzymes were expressed in JM109 Escherichia coli and were purified to apparent homogeneity by metal affinity chromatography by using His-Bind Resin (Novagen). RNA-dependent DNA polymerase activity and RNase H activity were assayed as described (25, 26). The specific RNA-dependent DNA polymerase activity of the wild-type enzyme was 20,000 units/mg of protein, where one unit is the amount of enzyme required for incorporation of 1.0 nmol of [3H]TMP in 10 min at 37°C using poly(rA)/oligo(dT) as substrate.
3′- and 5′-Labeled Oligonucleotide Primer.
L32 primer (5′-CTACTAGTTTTCTCCATCTAGACGATACCAGA-3′) was annealed with excess WL50 template (5′-GAGTGCTGAGGTCTTCATTCTGGTATCGTCTAGATGGAGAAAACTAGTAG-3′) and 16 pmol of primer/template was incubated with 50 μCi (15 pmol) of [α32P]ddATP (Amersham) and 40 pmol of HIV-1 RT in 300 μl of RB buffer (40 mM Hepes, pH 7.5/20 mM MgCl2/60 mM KCl/1 mM DTT/2.5% glycerol/80 μg/ml BSA) for 30 min at 37°C to add a labeled chain-terminating nucleotide to the primer 3′ terminus. Unlabeled ddAMP-terminated primer was prepared by incubating 200 pmol of L32 primer with 100 μM ddATP and 45 units of terminal transferase (Pharmacia). L32-ddAMP primer was 5′-labeled by using T4 polynucleotide kinase (GIBCO/BRL) and [γ32P]ATP (Dupont/NEN) and was annealed with 4-fold excess unlabeled WL50 template after heat inactivation of the T4 polynucleotide kinase.
Primer Modification Assay.
3′-labeled ddAMP-terminated L32 primer (5 nM) annealed to WL50 template was incubated with HIV-1 RT, Moloney murine leukemia virus (M-MuLV) RT (GIBCO/BRL), or avian myeloblastosis virus (AMV) RT (Boehringer Mannheim) and pyrophosphatase-treated nucleotides, as indicated, in 10 μl of RB buffer containing 80 μg/ml BSA for 3–60 min at 37°C (42°C for AMV RT). The reaction was terminated by heating (90°C for 3 min), followed by addition of an equal volume of loading buffer (16 M urea/180 mM Tris/58 mM taurine/1 mM EDTA/0.5% bromphenol blue/0.5% xylene cyanol). Samples were reheated (90°C for 3 min) and separated by electrophoresis through a 20% polyacrylamide gel containing 8 M urea followed by autoradiography or quantitation by phosphorimaging (Molecular Dynamics).
Primer Rescue Assay.
5′-32P-labeled, ddAMP-terminated L32 primer (5 nM) annealed to WL50 template was incubated with HIV-1 RT (200 nM) and pyrophosphatase-treated nucleotides, as indicated, in 10 μl of RB buffer for 10 min at 37°C. The reaction was terminated by heating at 90°C for 5 min. The samples were placed on ice for 5 min, followed by addition of 3 μl of RB buffer containing exonuclease-free Klenow fragment of E. coli DNA polymerase 1 (United States Biochemical) (0.1 units/μl) and dNTPs (500 μM each of dATP, dCTP, dGTP, and dTTP). The samples were incubated at 37°C for 30 min. The reaction was terminated by heating at 90°C for 3 min, followed by addition of equal volume of loading buffer. The samples were separated by electrophoresis through a 20% polyacrylamide gel containing 8 M urea.
Kinetic Constants.
Kinetic constants for formation of reaction products were obtained under conditions of saturating amounts of RT in which the concentration of primer-template bound to RT was assumed to be equal to the primer concentration. The rate of synthesis of dinucleoside polyphosphates is limited by the intrinsic synthesis activity of the RT/template-primer complex and the nucleotide concentration. The apparent kcat and Km were determined from the rate of specific product formation as a function of nucleotide concentration by using sigmaplot 2.0 (Jandel, San Rafael, CA). The Ki for dTTP was calculated by using the equation kcatapp/Kmapp = kcat/[Km(1+I/Ki)], where kcatapp and Kmapp are kinetic constants obtained in the presence of inhibitor concentration, I.
Synthesis of Radioactive Dinucleoside Polyphosphates.
[α32P]ddATP (20 μCi, Amersham) was incubated for 24 h at 30°C with 25 μg of firefly luciferase (Sigma) in a reaction mixture of 50 μl containing 50 mM Hepes⋅KOH (pH 7.4), 5 mM MgCl2, 1 mg/ml BSA, 0.05 units of inorganic pyrophosphatase (Boehringer Mannheim), 0.1 mM d-Luciferin (Sigma), and either 2 mM ATP or 2 mM GTP, to produce [32P]AP4ddA or [32P]GP4ddA, respectively (27).
Enzyme Digestion of Primer Modification Products.
Parallel digestions were carried out with 2 units of calf intestinal alkaline phosphatase (CIP; New England Biolabs) or 4 μg of snake venom phosphodiesterase (SVPD; Boehringer Mannheim) in 20 μl of RB buffer on (i) labeled primer cleavage products formed after incubation of 2.5 nM [32P]ddAMP-terminated L32 primer annealed to WL50 template with 100 nM HIV-1 RT and 3.2 mM GTP for 5 min at 37°C followed by heat-inactivation (90°C for 5 min) of the RT; (ii) 32P-labeled Gp4ddA synthesized by firefly luciferase as described above, heat-inactivation (90°C for 5 min) of the firefly luciferase and dilution of the radioactive products to 2 nCi/μl; or (iii) control DNA, 5′-labeled ddAMP-terminated L32 oligonucleotide (2.5 nM) annealed with unlabeled WL50 template. GTP was added to adjust all three specimen types to 3.2 mM. After incubation at 37°C, the digestion was terminated by heating at 90°C for 5 min and by addition of equal volume of loading buffer. The samples were separated by electrophoresis through a 20% polyacrylamide gel containing 8 M urea.
RESULTS
Characteristics of a Nucleotide-Dependent Primer Modifying Activity of HIV-1 RT.
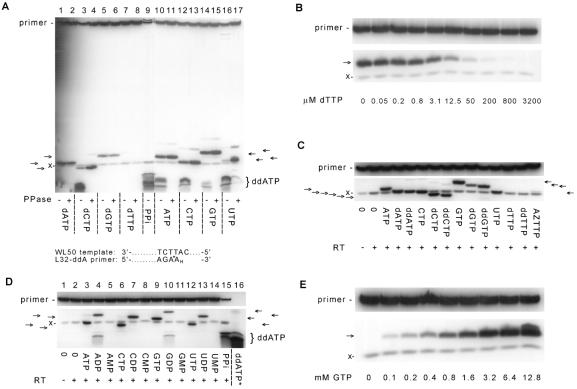
Incubation of 3′-labeled, chain-terminated primer/template with HIV-1 RT in the presence of dNTPs or NTPs gave rise to multiple radiolabeled cleavage products (Fig. 1A). Some of the products were nucleotide-specific with distinct electrophoretic mobilities (indicated by arrows) whereas others migrated in the region of ddATP. Formation of labeled ddATP can be explained by pyrophosphorolysis caused by PPi contamination of the commercial nucleotide preparations whereas the nucleotide-specific products indicate an unexpected reaction of free nucleotide with the labeled primer terminus. Pretreatment of the nucleotides with pyrophosphatase to remove the PPi (Fig. 1A, lanes marked “+PPase”) prevented the appearance of ddATP; however, the nucleotide-specific products still were observed and, in several cases, were made in increased amounts (for example, compare the amount of the UTP-dependent product in Fig. 1A, lanes 16 and 17). It is noteworthy that no nucleotide-specific product was generated by dTTP (Fig. 1A, lanes 7 and 8), and addition of dTTP to the reaction inhibited the synthesis of the GTP-specific primer cleavage product (Fig. 1B). Because dTTP is complementary to the next position on the template, it is recognized as a substrate for the polymerase activity of HIV-1 RT and can induce a conformational change in the enzyme–primer–template complex. This results in formation of a stable complex as detected by a gel electrophoretic mobility retardation assay (15). The apparent kd of dTTP for stable complex formation with HIV-1 RT and L32-ddAMP/WL50 primer/template is 3.8 ± 1.4 μM (data not shown), which is comparable to the Ki of dTTP of 5.5 ± 2.0 μM as an inhibitor of the primer modification reaction (mixed inhibition), obtained by varying the dTTP concentration in experiments similar to that shown in Fig. 1B. These results suggest that formation of a stable complex with chain-terminated primer/template in the presence of the next complementary dNTP and nucleotide-dependent primer modification are mutually exclusive reactions of HIV-1 RT.
Figure 1.
Nucleotide-dependent primer modification by HIV-1 RT. [32P]ddAMP-terminated L32 primer (5 nM), annealed to WL50 template, was incubated with excess HIV-1 RT (200 nM) at 37°C, and the products were separated by electrophoresis through a 20% denaturing polyacrylamide gel. The arrows indicate specific, nucleotide-dependent products formed during the primer modification reaction. The braces indicate ddATP, which migrates as multiple bands. “x” indicates an unidentified labeled product that was independent of added nucleotide. Panels show labeled products formed under the following conditions: (A) fifteen-min incubation in the presence of 3.2 mM of thermostable pyrophosphatase-treated (+PPase) or untreated (−PPase) dNTP, pyrophosphate (PPi), or NTP. A partial sequence of the primer and template is shown. ∗, radioactive 32P. (B) Five-minute incubation with 3.2 mM PPase-treated GTP and indicated concentrations of PPase-treated dTTP. (C) Fifteen-minute incubation with 800 μM PPase-treated NTP, dNTP, or ddNTP, as indicated. (D) Five-minute incubation with 3.2 mM PPase-treated NTP, NDP, or NMP, as indicated. Lanes: 15, 5-min incubation with 3.2 mM PPi; 16, [32P]ddATP as a reference. (E) Five-minute incubation with indicated amounts of PPase-treated GTP.
Unique labeled products were generated by NTPs, dNTPs, and ddNTPs (Fig. 1C) except for deoxythymidine analogs (dTTP, ddTTP, and AZTTP) as discussed previously. Nucleotide-specific products formed by NDPs had slower electrophoretic migration than the products generated by the corresponding NTPs (Fig. 1D) whereas no unique products were formed by nucleoside monophosphates.
Divalent cations were required for synthesis of nucleotide-specific products (Mg2+ optimum 20 mM), and the pH optimum was 7.5 (data not shown). Product formation depended on nucleotide concentration (Fig. 1E and Table 1). The nonhydrolyzable ATP analogs AMP-CPP (α, β,-methylene ATP) and AMP-PCP (β,γ,-methylene ATP) could serve as substrates for this reaction (Table 1), indicating that hydrolysis of the α, β- or β,γ-phosphodiester bonds is not necessary for formation of nucleotide-specific products. For both of these substrates the specificity constant (kcat/Km) was much lower than for ATP, indicating that compounds containing natural phosphodiester bonds in the α, β- or β,γ-linkages are preferred substrates for this reaction. For the other nucleotides tested, the reaction was slow (kcat, 0.08–1.5 per 1,000 s), the Km for nucleotide substrates was between 0.7 and 4.3 mM, and the specificity constant varied over a range of <4-fold (kcat/Km, 0.11–0.39 s−1⋅M−1).
Table 1.
Apparent kinetic parameters for the nucleotide- dependent primer modification reaction catalyzed by HIV-1 RT
| Nucleotide | kcat, s−1 | Km, mM | kcat/Km, s−1⋅M−1 |
|---|---|---|---|
| ATP | 0.42 × 10−3 | 1.6 | 0.26 |
| AMP-CPP | 0.042 × 10−3 | 7.8 | 0.0063 |
| AMP-PCP | 0.25 × 10−3 | 10 | 0.024 |
| ADP | 0.28 × 10−3 | 2.0 | 0.14 |
| dATP | 0.27 × 10−3 | 1.6 | 0.17 |
| CTP | 1.2 × 10−3 | 3.1 | 0.39 |
| dCTP | 1.1 × 10−3 | 3.9 | 0.29 |
| GTP | 1.5 × 10−3 | 4.3 | 0.35 |
| dGTP | 0.08 × 10−3 | 0.74 | 0.11 |
| ddGTP | 0.3 × 10−3 | 1.1 | 0.27 |
| UTP | 0.46 × 10−3 | 2.0 | 0.23 |
Primer modification was tested by using [32P]ddAMP-terminated L32 primer and WL50 template in the presence of the indicated PPase-treated nucleotides (0.1–12.8 mM), as described in the legend of Fig. 1. Values represent the average of duplicate experiments.
Identity of the Primer Modification Products.
The unique electrophoretic mobility of each nucleotide-induced primer cleavage product suggested that the nucleotide moiety was incorporated into the product. The difference in electrophoretic mobility of products formed from nucleoside di- or triphosphates suggested that these products differed in the number of phosphates. In addition, at least one phosphate residue must be derived from the labeled 3′ nucleotide of the primer to account for the presence of radioactivity in the product. These findings, in addition to the requirement for at least two 5′-phosphate residues in the nucleotide substrate, suggest a pyrophosphorolysis-like mechanism for the primer modification reaction. We postulate that HIV-1 RT uses two phosphates of the incoming nucleotide as a pyrophosphate analog to attack and cleave the primer phosphodiester bond, linking the nucleotide monophosphate from the primer terminus to the distal phosphate of the incoming nucleotide. The resulting products are dinucleoside connected by three or four phosphate residues (Np3ddA or Np4ddA) and primer from which the 3′-terminal nucleotide has been removed.
Synthesis of dinucleoside polyphosphates has been reported in a variety of biological systems (21, 28, 29). We used the reaction catalyzed by firefly luciferase (27, 29) with [α-32P]ddATP and unlabeled ATP or GTP to synthesize authentic labeled Ap4ddA (Fig. 2A, lane 2) or Gp4ddA (Fig. 2A, lane 4). These compounds comigrated with the products formed from [32P]ddAMP-terminated primer by HIV-1 RT in the presence of ATP (Fig. 2A, lane 1) or GTP (Fig. 2A, lane 3), supporting the proposed structure of these products. Furthermore, CIP, which can cleave exposed phosphate residues from ribo- and deoxyribonucleotides, released the [32P]phosphate from 5′-labeled DNA primer (Fig. 2B, compare lanes 3 and 6) but not from 3′-labeled ddAMP-terminated primer or the nucleotide-specific primer-derived product produced by HIV-1 RT in the presence of GTP (Fig. 2B, compare lanes 1 and 4) or from 32P-labeled Gp4ddA synthesized by firefly luciferase (Fig. 2B, compare lanes 2 and 5). These results indicated that the primer cleavage product formed by HIV-1 RT in the presence of NTP has no exposed phosphate groups, consistent with the known structure of dinucleoside polyphosphates (Fig. 2C). By contrast, dinucleoside polyphosphates (NpnN′) should be sensitive to cleavage by SVPD. Initial cleavage should produce Np and N′pn−1 or Npn−1 and N′p, and further digestion should convert all products to nucleoside monophosphates (NMP and N′MP) (27, 30). As expected, SVPD digestion of the labeled products formed from 32P-labeled ddATP and GTP by firefly luciferase caused the Gp4ddA band to disappear and caused bands migrating as ddATP and ddAMP to increase (Fig. 2B, compare lanes 2 and 8). Further incubation converted all of the [32P]ddATP to [32P]ddAMP (data not shown). SVPD digestion of the labeled products formed by HIV-RT from [32P]-ddAMP-terminated primer and GTP gave the similar results (Fig. 2B, compare lanes 1 and 7). The unidentified labeled band “x” (Fig. 2B, lane 1), which was not dependent on added nucleotide, has been tentatively identified as ddAMP because it coelectrophoresed with the slower migrating product of SVPD cleavage of labeled Gp4ddA and was sensitive to digestion by CIP (Fig. 2B, compare lanes 1 and 4). Taken together, the results in Fig. 2 A and B strongly support the conclusion that nucleotide-dependent primer modification by HIV-1 RT involves transfer of the 3′ primer-terminal nucleoside monophosphate to a free nucleoside di- or triphosphate to produce dinucleoside polyphosphate of the form shown in Fig. 2C.
Figure 2.
Properties of labeled products synthesized by HIV-1 RT (A) Products formed after incubation for 5 min at 37°C of [32P]ddAMP-terminated L32-primer annealed to WL50 template in the presence of HIV-1 RT and 3.2 mM PPase-treated ATP (lane 1) or GTP (lane 3). Labeled dinucleoside polyphosphates Ap4ddA (lane 2) or Gp4ddA (lane 4) were synthesized from [32P]ddATP by firefly luciferase (FL). Bands labeled ddATP and “x” are identified in the legend to Fig. 1. The 32P-labeled substrate is indicated at the bottom of the panel by an asterisk. (B) Digestion of labeled products with CIP or SVPD. Lanes: 1, 4, and 7, products formed after incubation of [32P]ddAMP-terminated L32 primer annealed to WL50 template with GTP and HIV-1 RT; 2, 5, and 8, 32P-labeled Gp4ddA synthesized by firefly luciferase; 3, 6, and 9, control DNA, ddAMP-terminated, 5′-32P-labeled L32 primer annealed to unlabeled WL50 template. Portions of each reaction mixture were untreated (lanes 1–3) or digested with CIP (lanes 4–6) or SVPD (lanes 7–9). The expected product of complete SVPD digestion of the 5′-labeled control DNA is [32P]dCMP. (C) Structure of Gp4ddA. ∗, radioactive 32P.
If this hypothesis is correct, the unblocked primer produced by dinucleoside polyphosphate synthesis should permit chain elongation. To address this, we devised a primer rescue assay. First, HIV-1 RT was incubated with 5′-labeled ddAMP-terminated L32 primer annealed to WL50 template and nucleotides to allow the removal of the dideoxynucleotide from the primer terminus through dinucleoside polyphosphate synthesis. The HIV-1 RT was heat-inactivated, and the primer was extended by addition of exonuclease-free Klenow fragment of E. coli DNA polymerase 1 and dNTPs. The higher the concentration of either ATP (Fig. 3, lanes 1–8) or GTP (Fig. 3, lanes 9–16), the more of the primer could be extended. Detectable extension of the primer was observed in the absence of added ATP (Fig. 3, lane 1), possibly because of incomplete chain-termination; however, the ATP-dependent rescue of the chain-terminated primer completely depended on incubation with HIV-1 RT (Fig. 3, compare lanes 1, 7, and 8). The extension of the primer depended on exonuclease-free Klenow fragment of E. coli DNA polymerase 1 (Fig. 3, lane 16), demonstrating that the HIV-1 RT had been inactivated after the initial primer modification step and, therefore, that no primer rescue occurred during the primer elongation step. The apparent kinetic constants of the primer-rescue reaction by HIV-1 RT in the presence of ATP was kcat = 0.74 × 10−3/s, Km, ATP = 4.8 mM, and kcat/Km = 0.16 s−1⋅M−1 (the averages of two experiments). These values are similar to those of the primer modification reaction catalyzed by HIV-1 RT in the presence of ATP (Table 1), suggesting that the blocked primer terminus has been removed through dinucleoside polyphosphate synthesis by HIV-1 RT to produce a fully functional, extendible 3′-terminus on the shortened primer.
Figure 3.
Primer rescue by HIV-1 RT. 5′-32P-labeled, ddAMP-terminated L32 primer annealed to WL50 template was incubated with excess HIV-1 RT and the indicated concentrations of PPase-treated ATP (lanes 1–8) or GTP (lanes 9–16) at 37°C for 10 min. After heat inactivation of the RT, the primer was extended by incubation with exonuclease-free Klenow polymerase and dNTPs for 10 min at 37°C. The products were separated by electrophoresis through a 20% denaturing polyacrylamide gel. The original L32-ddAMP primer is indicated as primer and extended primer products are indicated as ext. primer.
Dinucleoside Polyphosphate Synthesis Is an Inherent Function of Reverse Transcriptase.
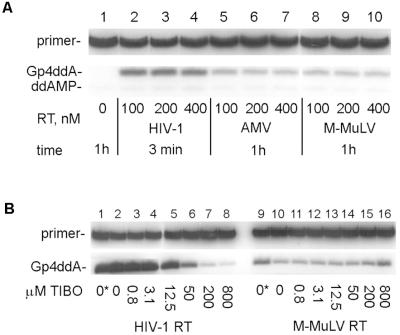
Fig. 4A shows that Gp4ddA synthesis also was catalyzed by RT from AMV and M-MuLV, although to a much lesser extent than with HIV-1 RT. As for HIV-1 RT, the activity was tested under conditions of saturating enzyme concentrations (100, 200, and 400 nM RT produced similar amounts of Gp4ddA), indicating that all of the template/primer was bound by RT and the activity was limited by the intrinsic dinucleoside polyphosphate activity of the RT/primer/template complex and the nucleotide concentration. Apparent kinetic parameters (data not shown) for Gp4ddA synthesis by AMV RT were kcat = 0.052 × 10−3/s, Km = 5.7 mM, kcat/Km = 0.009 s−1⋅M−1; for M-MuLV RT, kcat = 0.023 × 10−3/s, Km = 9 mM, and kcat/Km = 0.003 s−1⋅M−1; versus kcat = 1.5 × 10−3/s, Km = 4 mM, and kcat/Km = 0.3 s−1⋅M−1 for HIV-1 RT.
Figure 4.
Gp4ddA synthesis activity of HIV-1 RT, AMV RT, and M-MuLV RT. (A) Products formed after incubation of 5 nM [32P]ddAMP-terminated L32 primer annealed to WL50 template, 3.2 mM GTP, and no enzyme for 1h at 37°C (lane1); the indicated amounts of HIV-1 RT for 3 min at 37°C (lanes 2–4); AMV RT (Boehringer Mannheim) for 1h at 42°C (lanes 5–7); or M-MuLV RT (GIBCO/BRL) for 1h at 37°C (lanes 8–10). (B) Products formed by the reaction described in A with 200 nM HIV-1 RT and the indicated concentrations of Cl-TIBO for 15 min at 37°C (lanes 1–8), or with 700 nM M-MuLV RT and the indicated concentrations of Cl-TIBO for 1 h at 37°C (lanes 9–16). The highest concentration of Cl-TIBO contained 20% dimethyl sulfoxide, the solvent for this compound. Lanes 1 and 9 (0*) show products formed in the absence of Cl-TIBO but in the presence of 20% dimethyl sulfoxide.
Cl-TIBO [(+)-S-4,5,6,7-tetrahydro-9-chloro-5 methyl-6-(3-methyl-2-butenyl)-imidazo[4,5,1-jk][1,4]-benzodiazepin-2(1H)-thione] is a highly specific non-nucleoside inhibitor of the DNA polymerase activity of HIV-1 RT (31). Gp4ddA synthesis by HIV-1 RT was sensitive to Cl-TIBO (IC50 = 9 μM; Fig. 4B, lanes 1–8), which is comparable to its inhibition of HIV-1 RT RNA-dependent DNA polymerase activity (IC50 = 18.5 ± 4.5 μM, data not shown). By contrast, Gp4ddA synthesis by M-MuLV RT was not inhibited by this drug (Fig. 4B, lanes 9–16). A slight stimulation was observed, which can be accounted for by the solvent (Fig. 4B, compare lanes 9 and 16).
We also compared enzymatic activities between the HIV-1 RT polymerase active site double mutant D185N/D186N and wild-type RT (Table 2). The mutant enzyme retains a large portion of the wild-type RNase H activity but lacks detectable DNA polymerase or Gp4ddA synthesis activity. There is, therefore, a strong correlation between the Gp4ddA synthesis activity and a functional DNA polymerase active site and no correlation with a functional RNaseH active site.
Table 2.
Activities of HIV-1 wild-type RT and D185N/D186N double-mutant RT
| HIV-1 RT | Polymerase activity* | RHaseH activity† | Gp4ddA synthesis activity‡ |
|---|---|---|---|
| Wild-type | 20,000 | 140 | 660 |
| D185N/D186N | <0.5 | 60 | <0.2 |
Nanomoles of dTMP incorporated/10 min/milligram of enzyme.
Nanomoles of AMP released/10 min/milligram of enzyme.
Picomoles of Gp4ddA synthesized/10 min/nanomole of primer-template, assayed in the presence of saturating amounts of enzyme and 12.8 mM GTP.
DISCUSSION
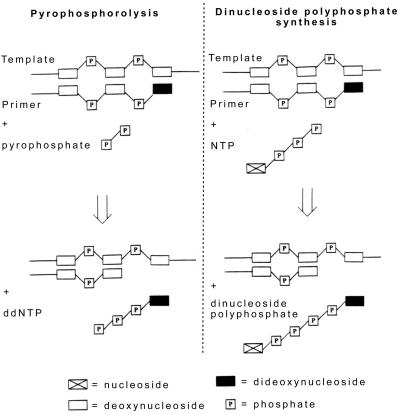
We have shown that HIV-1 RT carries out nucleotide-dependent removal of a dideoxynucleotide residue from the 3′-end of a chain-terminated DNA primer through production of a dinucleoside polyphosphate. This observation, combined with the requirements for at least two phosphate groups in the nucleotide substrate and a functional polymerase active site, has led us to propose a pyrophosphorolysis-related mechanism for this reaction (Fig. 5). In classical pyrophosphorolysis (Fig. 5 Left), the phosphodiester bond in the primer is attacked by PPi whereas in the nucleotide-dependent reaction (Fig. 5 Right), the two distal phosphates of the nucleotide act as an analog of PPi. Similar nucleotide-dependent pyrophosphorolysis-like reactions have been described for aminoacyl-tRNA synthetase (28) and firefly luciferase (29). The reaction described in this paper is notable in that the target of nucleotide attack is a phosphodiester bond in DNA.
Figure 5.
Schematic representation of removal of chain-terminating nucleotide from the primer terminus through either pyrophosphorolysis (Left) or dinucleoside polyphosphate synthesis (Right).
HIV-1 RT catalyzes dinucleoside polyphosphate synthesis in the presence of millimolar concentrations of NTP—concentrations that commonly are found in vivo (32, 33)— suggesting that the reaction may occur in HIV-1-infected cells. Physiological concentrations of dNTPs complementary to the next position on the template inhibit the reaction, presumably through formation of a stable dead-end complex between the chain-terminated primer/template, RT, and the dNTP (15). Thus, we would expect that conditions that favor DNA elongation (high dNTP levels) would restrict removal of chain-terminating nucleotides through dinucleoside polyphosphate synthesis whereas conditions that restrict DNA synthesis (i.e., depletion of dNTP pools) would allow it to occur. The significance of this type of regulation is not clear; however, the synthesis of dinucleoside polyphosphates may have metabolic consequences in infected cells, which will have additional effects on viral replication (16–18, 20–22).
Removal of chain-terminating nucleotides and rescue of terminated DNA chains by this reaction may play a major role in determining the sensitivity of HIV to nucleoside analogs. Drug resistance mutations of HIV-1 may act, in part, through this mechanism. For example, although certain HIV-1 RT mutations confer a 100-fold decrease in the sensitivity to AZT in vivo (34, 35), these mutations have little, if any, effect on the ability of the enzyme to discriminate between AZTTP and dTTP in polymerase reactions in vitro (12, 36–38). This can be explained if the mutations primarily affect removal of the chain terminating AZTMP residue after it has been incorporated. Our preliminary results (P.R.M., unpublished observations) suggest that there is a substantial increase in dinucleoside polyphosphate synthesis in some AZT-resistance mutants in comparison with wild-type HIV-1 RT. In addition, Canard et al. (39) recently have reported increased HIV-1 RT binding to the 3′ end of AZTMP-terminated primers in the AZT-resistant mutants that may facilitate removal of the blocked primer terminus through slow reactions such as pyrophosphorolysis or the activity described in this report.
In the light of the mechanism for rescue of chain-terminated primer described in this paper, incorporation of a chain-terminator into the HIV-1 genome no longer can be considered an irreversible event (which it would be in the absence of an effective removal mechanism), but merely a road-block, where the removal through dinucleoside polyphosphate synthesis becomes the rate-limiting step in HIV-1 replication in the presence of chain-terminating compounds. The presence of a primer rescue activity in HIV-1 RT could account for the relatively modest effects on HIV-1 replication conferred by treatment of infected individuals with most chain-terminating compounds compared with the more pronounced inhibition conferred by HIV-1 protease inhibitors.
Acknowledgments
We thank Dr. Kathleen Downey for critical evaluation of the paper, Drs. Stuart Linn and Murray Deutscher for helpful discussions, and Dr. Gary Tarpley for supplying Cl-TIBO. This work was supported by National Institutes of Health Grants AI 31848 and DK26206.
ABBREVIATIONS
- RT
reverse transcriptase
- PPase
pyrophosphatase
- CIP
calf intestinal alkaline phosphatase
- SVPD
snake venom phosphodiesterase
- AZT
3′-azido-3′-deoxythymidine
- AMV
avian myeloblastosis virus
- M-MuLV
Moloney murine leukemia virus
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Emini E A, Fan H Y. In: Retroviruses. Coffin J M, Hughes S H, Varmus H E, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 637–706. [Google Scholar]
- 2.Furman P A, Fyfe J A, St. Clair M H, Weinhold K, Rideout J L, Freeman G A, Lehrman S, Bolognesi D P, Broder S, et al. Proc Natl Acad Sci USA. 1986;83:8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y-C, Dutschman G E, Bastow K F, Sarngadharan M G, Ting R Y C. J Biol Chem. 1987;262:2187–2189. [PubMed] [Google Scholar]
- 4.St. Clair M H, Richards C A, Spector T, Weinhold K J, Miller W H, Langlois A J, Furman P A. Antimicrob Agents Chemother. 1987;31:1972–1977. doi: 10.1128/aac.31.12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang P, Farquhar D, Plunkett W. J Biol Chem. 1990;265:11914–11918. [PubMed] [Google Scholar]
- 6.Mitsuya H, Yarchoan R, Broder S. Science. 1990;249:1533–1544. doi: 10.1126/science.1699273. [DOI] [PubMed] [Google Scholar]
- 7.Ueno T, Mitsuya H. Biochemistry. 1997;36:1092–1099. doi: 10.1021/bi962393d. [DOI] [PubMed] [Google Scholar]
- 8.Battula N, Loeb L A. J Biol Chem. 1976;251:982–986. [PubMed] [Google Scholar]
- 9.Roberts J D, Bebenek K, Kunkel T A. Science. 1988;242:1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- 10.Reardon J E. J Biol Chem. 1993;268:8743–8751. [PubMed] [Google Scholar]
- 11.Hsieh J-C, Zinnen S, Modrich P. J Biol Chem. 1993;268:24607–24613. [PubMed] [Google Scholar]
- 12.Carroll S S, Geib J, Olsen D B, Stahlhut M, Shafer J A, Kuo L C. Biochemistry. 1994;33:2113–2120. doi: 10.1021/bi00174a018. [DOI] [PubMed] [Google Scholar]
- 13.Russell R G G. Arthritis Rheum. 1976;19:465–478. doi: 10.1002/1529-0131(197605/06)19:3+<465::aid-art1780190722>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Barshop B A, Adamson D T, Vellom D C, Rosen F, Epstein B L, Seegmiller J E. Anal Biochem. 1991;197:266–272. doi: 10.1016/0003-2697(91)90387-9. [DOI] [PubMed] [Google Scholar]
- 15.Tong W, Lu C-D, Sharma S K, Matsuura S, So A G, Scott W A. Biochemistry. 1997;36:5749–5757. doi: 10.1021/bi962410z. [DOI] [PubMed] [Google Scholar]
- 16.Grummt F. Proc Natl Acad Sci USA. 1978;75:371–375. doi: 10.1073/pnas.75.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamecnik P. Anal Biochem. 1983;134:1–10. doi: 10.1016/0003-2697(83)90255-5. [DOI] [PubMed] [Google Scholar]
- 18.Varshavsky A. Cell. 1983;34:711–712. doi: 10.1016/0092-8674(83)90526-3. [DOI] [PubMed] [Google Scholar]
- 19.Bochner B R, Lee P C, Wilson S W, Cutler C W, Ames B N. Cell. 1984;37:225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- 20.Bambara R A, Crute J J, Wahl A F. Cancer Invest. 1985;3:473–479. doi: 10.3109/07357908509039809. [DOI] [PubMed] [Google Scholar]
- 21.Baxi M D, Vishwanatha J K. J Pharmacol Toxicol Methods. 1995;33:121–128. doi: 10.1016/1056-8719(94)00127-p. [DOI] [PubMed] [Google Scholar]
- 22.Kisselev L L, Justesen J, Wolfson A D, Frolova L Y. FEBS Lett. 1998;427:157–163. doi: 10.1016/s0014-5793(98)00420-7. [DOI] [PubMed] [Google Scholar]
- 23.D’Aquila R T, Summers W C. J AIDS. 1989;2:579–587. [PubMed] [Google Scholar]
- 24.Sarkar G, Sommer S S. BioTechniques. 1990;8:404–407. [PubMed] [Google Scholar]
- 25.Tan C-K, Zhang J, Li Z-Y, Tarpley G W, Downey K M, So A G. Biochemistry. 1991;30:2651–2655. doi: 10.1021/bi00224a013. [DOI] [PubMed] [Google Scholar]
- 26.Zhan X, Tan C-K, Scott W A, Mian A M, Downey K M, So A G. Biochemistry. 1994;33:1366–1372. doi: 10.1021/bi00172a012. [DOI] [PubMed] [Google Scholar]
- 27.Sillero M A G, Madrid O, Zaera E, Sillero A. Biochim Biophys Acta. 1997;1334:191–199. doi: 10.1016/s0304-4165(96)00092-x. [DOI] [PubMed] [Google Scholar]
- 28.Zamecnik P C, Stephenson M L, Janeway C M, Randerath K. Biochem Biophys Res Commun. 1966;24:91–97. doi: 10.1016/0006-291x(66)90415-3. [DOI] [PubMed] [Google Scholar]
- 29.Guranowski A, Sillero M A G, Sillero A. FEBS Lett. 1990;271:215–218. doi: 10.1016/0014-5793(90)80409-c. [DOI] [PubMed] [Google Scholar]
- 30.Randerath K, Janeway C M, Stephenson M L, Zamecnik P C. Biochem Biophys Res Commun. 1966;24:98–105. doi: 10.1016/0006-291x(66)90416-5. [DOI] [PubMed] [Google Scholar]
- 31.Debyser Z, Pauwels R, Andries K, Desmyter J, Kukla M, Janssen P A J, De Clercq E. Proc Natl Acad Sci USA. 1991;88:1451–1455. doi: 10.1073/pnas.88.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauschka P V. Methods Cell Biol. 1973;7:361–462. doi: 10.1016/s0091-679x(08)61787-2. [DOI] [PubMed] [Google Scholar]
- 33.Gamberucci A, Innocenti B, Fulceri R, Banhegyi G, Giunti R, Pozzan T, Benedetti A. J Biol Chem. 1994;269:23597–23602. [PubMed] [Google Scholar]
- 34.Larder B A, Kemp S D. Science. 1989;246:1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- 35.Larder B A, Kellam P, Kemp S D. AIDS. 1991;5:137–144. doi: 10.1097/00002030-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Lacey S F, Reardon J E, Furfine E S, Kunkel T A, Bebenek K, Eckert K A, Kemp S D, Larder B A. J Biol Chem. 1992;267:15789–15794. [PubMed] [Google Scholar]
- 37.Krebs R, Immendörfer U, Thrall S H, Wöhrl B M, Goody R G. Biochemistry. 1997;36:10292–10300. doi: 10.1021/bi970512z. [DOI] [PubMed] [Google Scholar]
- 38.Kerr S G, Anderson K S. Biochemistry. 1997;36:14064–14070. doi: 10.1021/bi9713862. [DOI] [PubMed] [Google Scholar]
- 39.Canard B, Sarfati S R, Richardson C C. J Biol Chem. 1998;273:14596–14604. doi: 10.1074/jbc.273.23.14596. [DOI] [PubMed] [Google Scholar]