Abstract
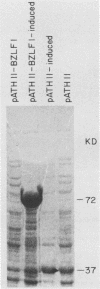
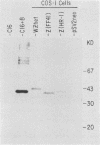
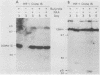
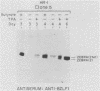
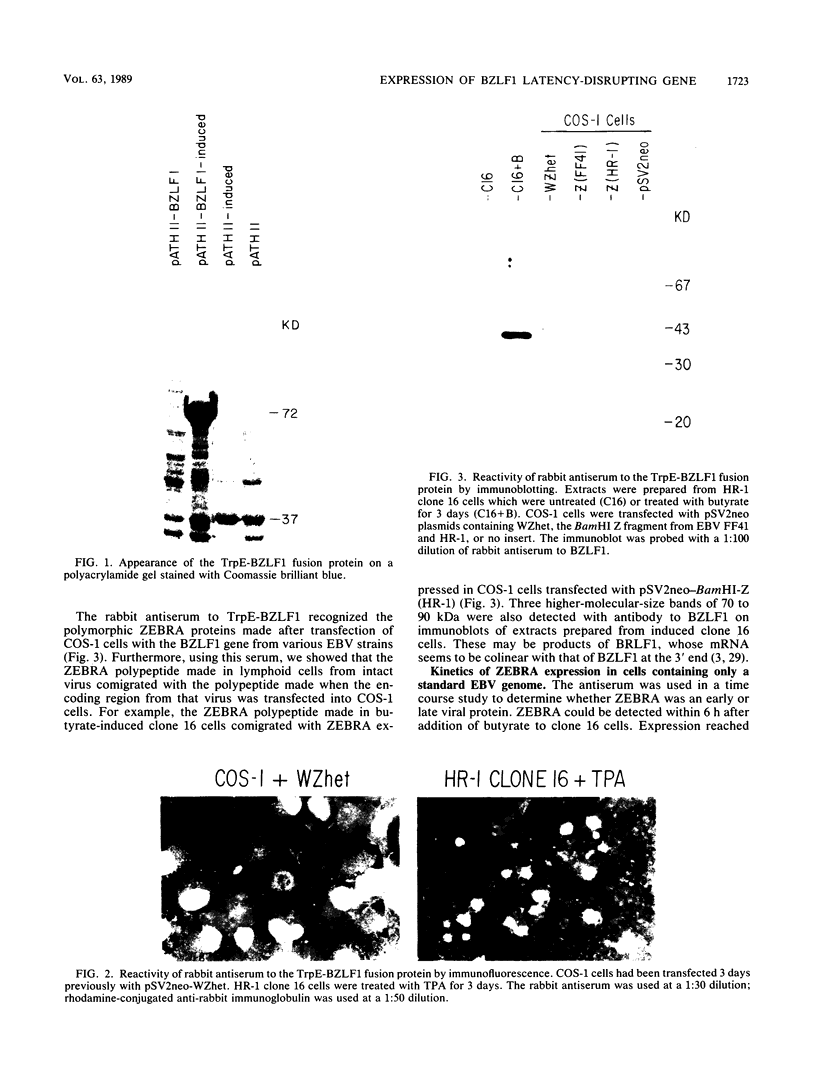
Previous experiments using gene transfer of plasmids with heterologous promoters identified an Epstein-Barr virus (EBV) gene (BZLF1) whose product (ZEBRA) switches the virus from a latent to a replicative state. We have now studied expression of ZEBRA in lymphoid cells harboring either standard virus or a mixture of standard and defective (heterogeneous [het]) viruses. A high-titer rabbit antiserum to a TrpE-BZLF1 fusion protein was used to identify ZEBRA expressed from standard and het EBV DNA. These ZEBRA proteins could be distinguished from each other on the basis of their electrophoretic mobilities. ZEBRA could not be detected in cells latently infected with standard EBV. However, within 6 h after induction of replication by sodium butyrate, ZEBRA appeared and persisted long thereafter. Synthesis of ZEBRA was insensitive to phosphonoacetic acid or acycloguanosine, behavior characteristic of an early replicative protein. ZEBRA was constitutively expressed in cells containing both defective and standard EBV genomes. ZEBRA was made predominantly from the het genome but also from the standard genome. Control of BZLF1 expression appears to occur at the transcriptional level. No BZLF1-specific transcript was detected in cells containing only standard latent EBV. BZLF1 transcripts could be detected in these cells if virus replication was induced by treatment with butyrate. Cells bearing both standard and het genomes did not require addition of an exogenous inducing agent to transcribe the BZLF1 gene. The experiments suggest that regulation of transcription of the BZLF1 gene is a pivotal event in the control of EBV replication.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bauer G., Birnbaum U., Höfler P., Heldin C. H. EBV-inducing factor from platelets exhibits growth-promoting activity for NIH 3T3 cells. EMBO J. 1985 Aug;4(8):1957–1961. doi: 10.1002/j.1460-2075.1985.tb03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M., Bodescot M., Perricaudet M., Farrell P. Epstein-Barr virus gene expression in P3HR1-superinfected Raji cells. J Virol. 1987 Oct;61(10):3120–3132. doi: 10.1128/jvi.61.10.3120-3132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier-Greco A., Manet E., Chavrier P., Mosnier C., Daillie J., Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986 Dec 1;5(12):3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cho M. S., Bornkamm G. W., zur Hausen H. Structure of defective DNA molecules in Epstein-Barr virus preparations from P3HR-1 cells. J Virol. 1984 Jul;51(1):199–207. doi: 10.1128/jvi.51.1.199-207.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Jenson H., Seibl R., Wolf H., Miller G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J Virol. 1987 Dec;61(12):3672–3679. doi: 10.1128/jvi.61.12.3672-3679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
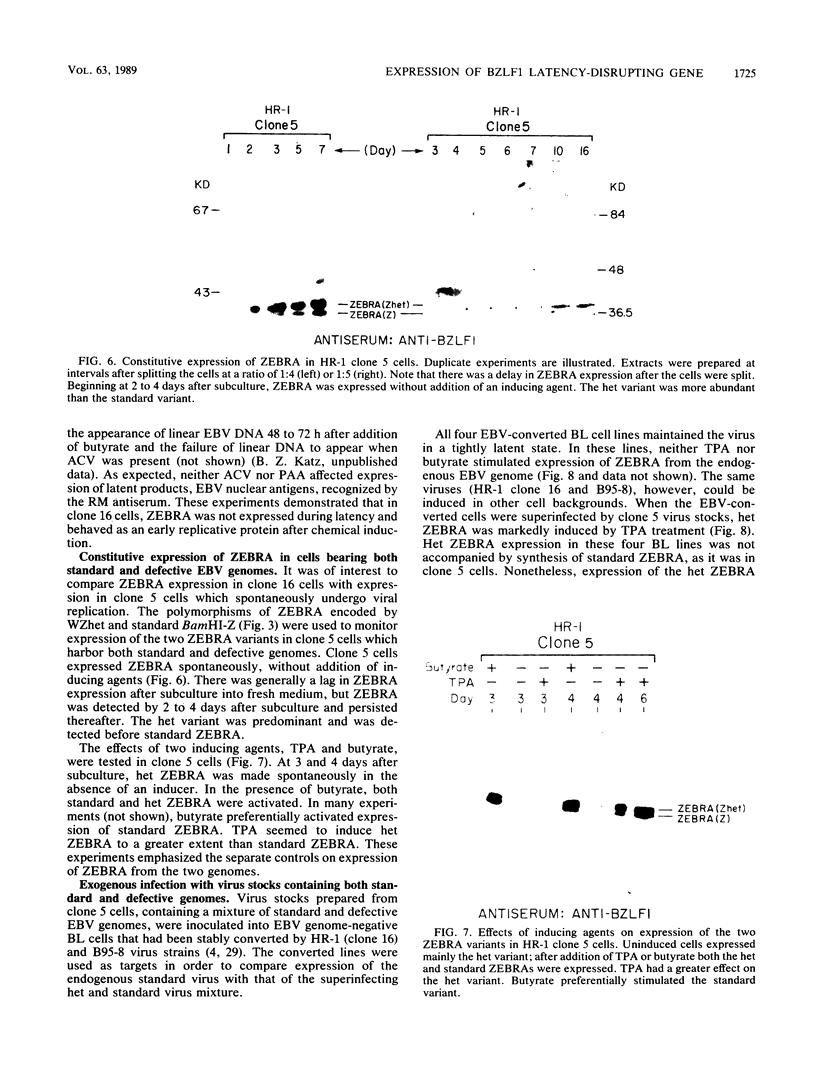
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hardwick J. M., Lieberman P. M., Hayward S. D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988 Jul;62(7):2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Heston L., Rabson M., Brown N., Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982 Jan 14;295(5845):160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- Jenson H. B., Farrell P. J., Miller G. Sequences of the Epstein-Barr Virus (EBV) large internal repeat form the center of a 16-kilobase-pair palindrome of EBV (P3HR-1) heterogeneous DNA. J Virol. 1987 May;61(5):1495–1506. doi: 10.1128/jvi.61.5.1495-1506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson H. B., Miller G. Polymorphisms of the region of the Epstein-Barr virus genome which disrupts latency. Virology. 1988 Aug;165(2):549–564. doi: 10.1016/0042-6822(88)90599-5. [DOI] [PubMed] [Google Scholar]
- Jenson H. B., Rabson M. S., Miller G. Palindromic structure and polypeptide expression of 36 kilobase pairs of heterogeneous Epstein-Barr virus (P3HR-1) DNA. J Virol. 1986 May;58(2):475–486. doi: 10.1128/jvi.58.2.475-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux G., Freese U. K., Fischer R., Polack A., Kofler E., Bornkamm G. W. TPA-inducible Epstein-Barr virus genes in Raji cells and their regulation. Virology. 1988 Feb;162(2):503–507. doi: 10.1016/0042-6822(88)90496-5. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Heston L., Countryman J. P3HR-1 Epstein-Barr virus with heterogeneous DNA is an independent replicon maintained by cell-to-cell spread. J Virol. 1985 Apr;54(1):45–52. doi: 10.1128/jvi.54.1.45-52.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Rabson M., Heston L. Epstein-Barr virus with heterogeneous DNA disrupts latency. J Virol. 1984 Apr;50(1):174–182. doi: 10.1128/jvi.50.1.174-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Heston L., Miller G. Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2762–2766. doi: 10.1073/pnas.80.9.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C., Taylor N., Countryman J., Jenson H., Kolman J., Miller G. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9801–9805. doi: 10.1073/pnas.85.24.9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Farrell P. J., Miller G. Novel nuclear antigens recognized by human sera in lymphocytes latently infected by Epstein-Barr virus. Virology. 1987 Jan;156(1):153–162. doi: 10.1016/0042-6822(87)90446-6. [DOI] [PubMed] [Google Scholar]
- Seibl R., Motz M., Wolf H. Strain-specific transcription and translation of the BamHI Z area of Epstein-Barr Virus. J Virol. 1986 Dec;60(3):902–909. doi: 10.1128/jvi.60.3.902-909.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Shimizu N., Sakuma S., Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986 Mar;57(3):1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Bornkamm G. W., Schmidt R., Hecker E. Tumor initiators and promoters in the induction of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):782–785. doi: 10.1073/pnas.76.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]